Abstract
Rare, sporadic uterine leiomyomas arise in the setting of severe metabolic aberration due to somatic fumarate hydratase mutation. Germline mutations account for the Hereditary Leiomyomatosis and Renal Cell Carcinoma syndrome, which predisposes for cutaneous and uterine leiomyomas and aggressive renal cell carcinomas. Altered fumarate hydratase leads to fumarate accumulates in affected cells with formation of S-(2-succino)-cysteine, which can be detected with polyclonal antibody. High levels of these modified cysteine residues are found characteristically in fumarate hydratase-deficient cells, but not in normal tissues or tumors unassociated with Hereditary Leiomyomatosis and Renal Cell Carcinoma syndrome. We hypothesized that S-(2-succino)-cysteine-positive leiomyomas, indicating fumarate hydratase aberration, have morphologic features that differ from those without S-(2-succino)-cysteine positivity. Hematoxylin and eosin-stained slides of uterine smooth muscle tumors were prospectively analyzed for features suggesting Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome, such as prominent eosinophilic macronucleoli with perinucleolar halos, yielding 9 cases. Germline genetic testing for fumarate hydratase mutations was performed in 3 cases. A detailed morphological analysis was undertaken, and S-(2-succino)-cysteine immunohistochemistry was performed with controls from a tissue microarray [leiomyomas (19), leiomyosarcomas (29), and endometrial stromal tumors (15)]. Of the 9 study cases, 4 had multiple uterine smooth muscle tumors. All cases had increased cellularity, staghorn vasculature, and fibrillary cytoplasm with pink globules. All cases had inclusion-like nucleoli with perinuclear halos (7 diffuse, 1 focal). All showed diffuse granular cytoplasmic labeling with the S-(2-succino)-cysteine antibody. Two of 3 tested patients had germline fumarate hydratase mutations. Only 1 leiomyoma from the tissue microarray controls was immunohistochemically positive, and it showed features similar to other immunohistochemically positive cases. Smooth muscle tumors with fumarate hydratase aberration demonstrate morphological reproducibility across cases and S-(2-succino)-cysteine immuno-positivity. Although the features described are not specific for germline fumarate hydratase mutation or the Hereditary Leiomyomatosis and Renal Cell Carcinoma syndrome, their presence should suggest fumarate hydratase aberration. Identifying these cases is an important step in the diagnostic workup of patients with possible Hereditary Leiomyomatosis and Renal Cell Carcinoma.
Keywords: uterine leiomyomas, fumarate hydratase, HLRCC (hereditary leiomyomatosis and renal cell carcinoma syndrome), S-(2-succino)-cysteine, succination
Introduction
Uterine leiomyomas display a wide range of morphology, which suggests the presence of associated genetic heterogeneity. Whole-genome sequencing and gene-expression profiling experiments have recently disclosed a repertoire of mutations, chromosomal losses, and rearrangements that characterize these tumors (1). It has been hypothesized that leiomyomas arise in a background of oncogenic activation via MED12 mutation, severe metabolic aberration via fumarate hydratase (FH) mutation, or through other specific chromosomal changes (1). Inspired by work from Merino’s group (2), our group previously reported a case of a uterine leiomyoma with unusual histological features that arose in a patient with renal cell carcinoma and hereditary leiomyomatosis/renal cell carcinoma (HLRCC) syndrome, which is due to germline mutations in FH (3).
In 2001, Launonen et al (4) provided the first description of HLRCC syndrome: members of two families displayed uterine and cutaneous smooth muscle tumors as well as type 2 papillary renal cell carcinoma. This syndrome is now understood to confer an increased risk of renal cell carcinomas and smooth muscle tumors of the skin and uterus (5). Although cutaneous leiomyomas are reportedly the most sensitive and specific clinical marker of this syndrome, uterine leiomyomas are often the first manifestation of the syndrome in women (4, 6, 7). HLRCC exhibits autosomal dominant inheritance, and linkage studies have mapped the gene responsible for HLRCC to the long arm of chromosome 1 on band q42.3-q43, where the FH gene is located (4, 6-9). A variety of germline mutations of the FH gene have been reported in individuals with HLRCC (10). Missense mutations appear the most prevalent, but frameshift, nonsense and splice site mutations have also been reported (11). Heterozygous mutations that characterize HLRCC are typically accompanied by somatic loss of the corresponding allele, indicating that this is probably a tumor suppressor gene (6). Homozygous FH mutation leads to a metabolic disorder with severe encephalopathy, seizures, and poor neurological outcome (12).
FH is one of the key enzymes in the Krebs tricarboxylic acid cycle and catalyzes the conversion of fumarate to malate (13). FH-deficient cells and tissues accumulate high levels of fumarate, which has been proposed to act as an oncometabolite that promotes cancer development (14). Loss of FH activity confers protection from apoptosis in normal human renal cells and fibroblasts (15). Once fumarate accumulates in the cells, by a process termed protein succination, it modifies cysteine residues to S-(2-succino)-cysteine (16, 17). Succination, resulting from FH deficiency, targets and potentially alters the function of multiple proteins and may contribute to dysregulated metabolism (18, 19). Using an antibody against S-(2-succino)-cysteine, Bardella et al. showed that the presence of S-(2-succino)-cysteine positivity by immunohistochemistry predicted genetic alterations in FH in patients referred for genetic testing for HLRCC (20).
There are only approximately 150 families worldwide with HLRCC (21-42) and it has been estimated that the prevalence of germline FH mutation is 1/10,000-1/50,000 (personal communication from Ian Tomlinson, University of Oxford). Somatic FH mutations, on the other hand, are likely to be substantially more common than germline mutations. One group reported that 1.3% of uterine leiomyomas harbor somatic FH mutations (43). Although this represents a small percentage, uterine leiomyomas are very common tumors, indicating that there is a large number of uterine leiomyomas with FH mutations. In this study, we explored associations between uterine leiomyoma morphology with S-(2-succino)-cysteine immunophenotype. We hypothesized that S-(2-succino)-cysteine-positive leiomyomas have morphologic features that differ from those without increased S-(2-succino)-cysteine/succination.
Materials and Methods
Since 2008, we have been prospectively collecting uterine smooth muscle tumors with characteristic morphological features of HLRCC-associated uterine leiomyomas, as originally described by Merino’s group. The cases were retrieved from the archives of the Department of Pathology, Memorial Sloan-Kettering Cancer Center. Hematoxylin and eosin-stained slides of uterine smooth muscle tumors were prospectively analyzed for features suggesting HLRCC, such as prominent eosinophilic macronucleoli with a perinucleolar halo (3, 44), yielding 9 cases, henceforth described as smooth muscle tumors with features suggesting FH aberration (SMT-FHs). Eight of 9 cases were from the consultation files of RAS, of which 6 were initially diagnosed as atypical leiomyoma and 2 as smooth muscle tumors of uncertain malignant potential. Pathological diagnosis led to germline genetic testing in 3 patients. Slides were then re-examined, and the following histologic features were recorded: cellularity, nuclear atypia, nucleolar features, chromatin pattern, nuclear pseudoinclusions, multinucleation, mitotic activity, cytoplasmic features, and vasculature. The number and size of the uterine smooth muscle tumors were also documented. The mean number of slides reviewed per case was 6 (range, 4-24). In addition, clinical characteristics were retrieved from the medical record: age, past medical history including presence of any renal tumor or skin condition, family history, presenting symptoms, number of smooth muscle lesions, and type of procedure performed at time of surgery.
The S-(2-succino)-cysteine antibody was generously provided by Dr Normal Frizzell, one of the co-authors (17). Immunohistochemical staining for S-(2-succino)-cysteine polyclonal antibody was undertaken in all cases, as previously described (20). Briefly, antigen retrieval was performed by microwaving in citrate buffer, pH 6.0, for 15 minutes. The tissues were then blocked for endogenous peroxidase, incubated with S-(2-succino)-cysteine polyclonal antibody (1:5000), washed in PBS (0.1% Tween20), and followed by incubation with anti-rabbit HRP polymer (Envision, Dako) for 30 minutes. Staining was assessed as either absent or present. Renal tumors with confirmed FH mutation were used as positive controls.
To determine the prevalence of S-(2-succino)-cysteine staining in unselected uterine mesenchymal tumors, we subsequently performed S-(2-succino)-cysteine immunohistochemistry on a tissue microarray that included 19 leiomyomas, 29 leiomyosarcomas, and 15 endometrial stromal tumors.
Results
Nine tumors met the criteria for SMT-FH. The mean age was 38 years (range, 25-50). Two patients presented with menorrhagia and one with vaginal discharge. Clinical history was not available for the remaining 6. Two patients had significant past medical histories: one had a high-grade renal cell carcinoma 5 years after uterine leiomyomas, and the other patient had been recently diagnosed with cutaneus leiomyomas. One patient had no remarkable clinical history, and we had no clinical history available for the remaining 6. Five patients had multiple smooth muscle tumors that ranged in size from 0.4 cm to 13 cm, and the remaining patients had one uterine smooth muscle tumor each, measuring 7 cm to 13 cm in greatest dimension. Surgical procedures included total abdominal hysterectomy in 2 patients, myomectomy in 4, and simple hysterectomy in 3 (Table 1).
Table 1.
Patient characteristics.
| Case # |
Age | Symptoms | Other Presentation |
# of Lesions |
Size (cm) | Surgery |
|---|---|---|---|---|---|---|
| 1§* | 32 | Vaginal Discharge |
Renal cell carcinoma |
Multiple | 0.6-5.5 | Total Abdominal Hysterectomy |
| 2§ | 42 | Menorrhagia | Cutaneous leiomyomata |
Multiple | 0.8-7 | Simple Hysterectomy |
| 3 | 47 | N/A | N/A | Single | 10 | Simple Hysterectomy |
| 4 | 25 | N/A | N/A | Single | 13 | Myomectomy |
| 5 | 46 | N/A | N/A | Multiple | 1-4.5 | Simple hysterectomy |
| 6 | 27 | N/A | N/A | Single | 9 | Myomectomy |
| 7 | 37 | Menorrhagia | N/A | Single | 7 | Myomectomy |
| 8 | 50 | N/A | N/A | Multiple | 2.0-13 | Total Abdominal Hysterectomy |
| 9 | 41 | Menorrhagia | None | Multiple | 0.5-4.0 | Myomectomy |
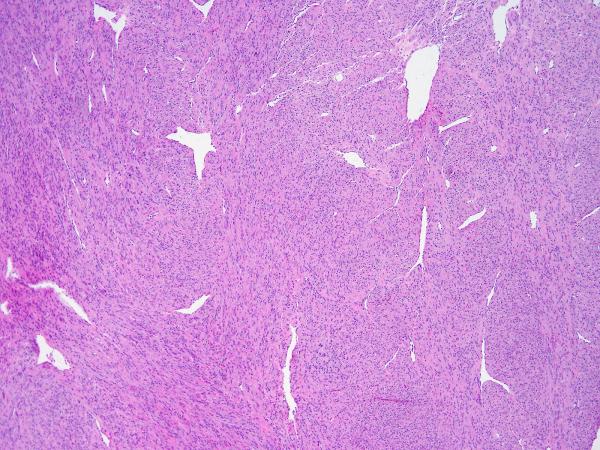
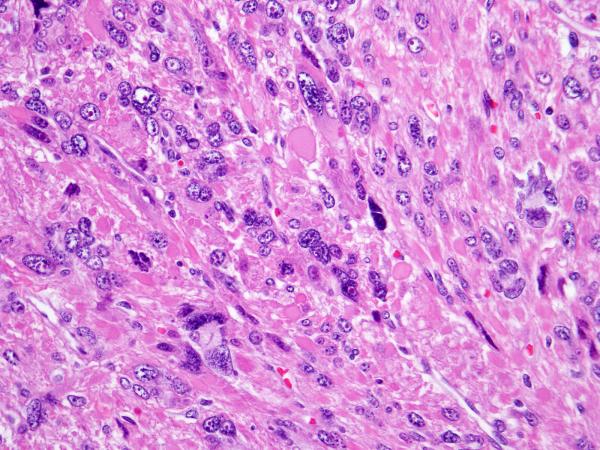
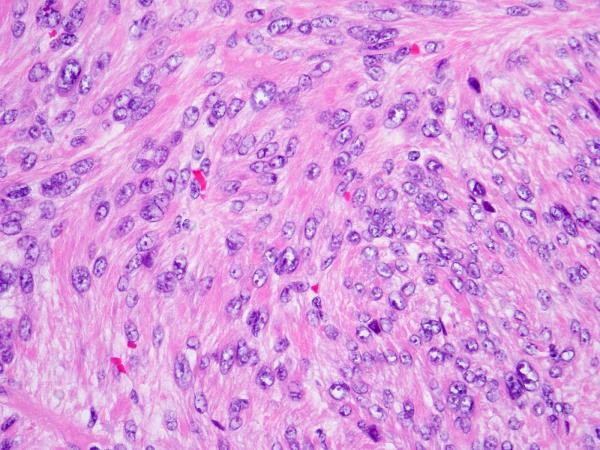
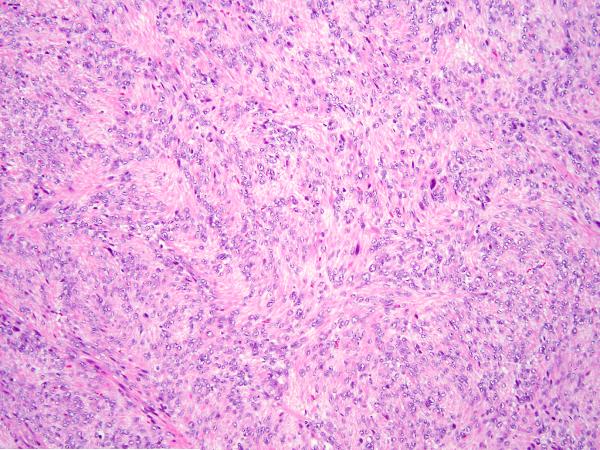
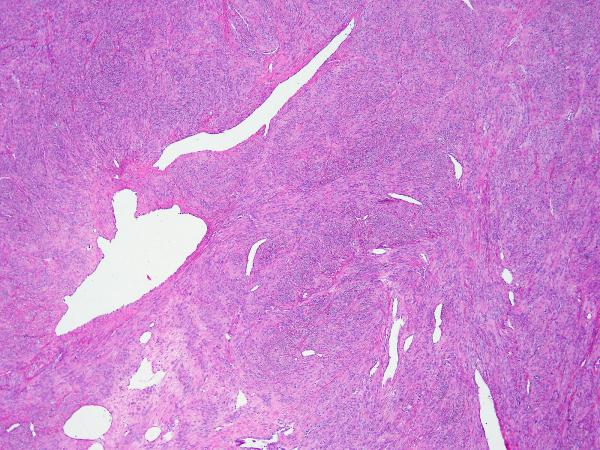
In cases in which normal myometrium was available for comparison, low-power examination revealed that all SMT-FHs showed slightly increased cellularity and a different refractile quality owing to increased fibrillarity. All cases showed staghorn vasculature at low power (Figure 1). Five cases initially gave the impression of a diffusely epithelioid neoplasm owing to the presence of round nuclei, and in 4 cases, this feature was present only focally. Despite the presence of round nuclei, the tumor cells were fusiform in shape, not epithelioid. Nuclear atypia was severe and diffuse in 2 cases, moderate in 3, and mild in the remaining 4. Multinucleation was present in 5 cases, but only focally. The mean mitotic index was 3-4/10 HPF, and only one case showed atypical scattered forms. In all 9 cases, high-power evaluation revealed a characteristic fibrillary cytoplasm that in some areas aggregated to form pink globules (Figure 2). Eight cases showed the characteristic inclusion-like nucleoli, eosinophilic nucleoli with perinuclear halos diffusely throughout the lesion, but this was only evident at 20x magnification and was generally not appreciable at 4x (Figure 3). One case showed these nuclear features only focally. All cases contained tumor cells with round and vesicular nuclei, sometimes resembling “Orphan Annie nuclei” with optical clearing, with or without a small eosinophilic nucleolus. These were more prevalent than cells with macronucleoli and perinucleolar clearing. In patients with multiple leiomyomata and available slides, the leiomyomata did not necessarily appear morphologically similar. Some of the leiomyomata showed more easily appreciated SMT-FH features than others. Three cases showed a “neurilemmoma-like” growth pattern (Figure 4).
Figure 1.
S-(2-succino)-cysteine-positive leiomyoma showing staghorn vasculature and increased cellularity.
Figure 2.
S-(2-succino)-cysteine-positive leiomyoma showing prominent eosinophilic nucleoli with perinucleolar halos and eosinophilic globules.
Figure 3.
S-(2-succino)-cysteine-positive leiomyoma showing prominent eosinophilic nucleoli with perinucleolar halos and fibrillary cytoplasm.
Figure 4.
S-(2-succino)-cysteine-positive leiomyoma showing a neurilemmoma-like growth pattern.
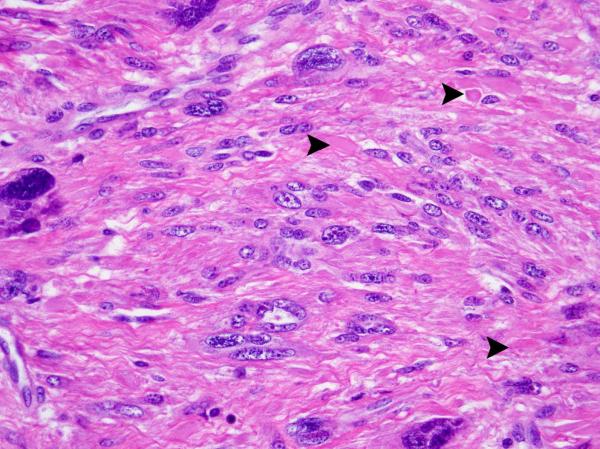
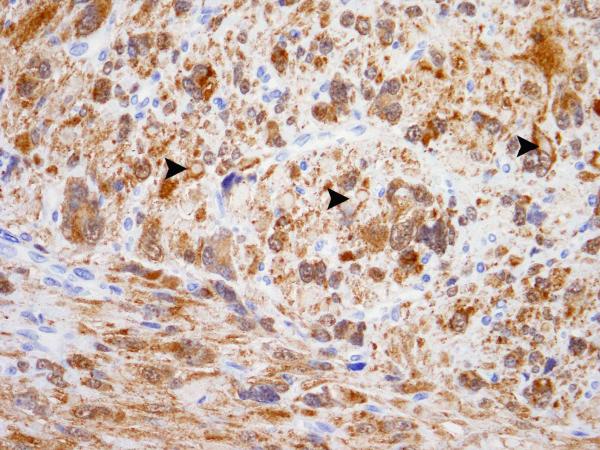
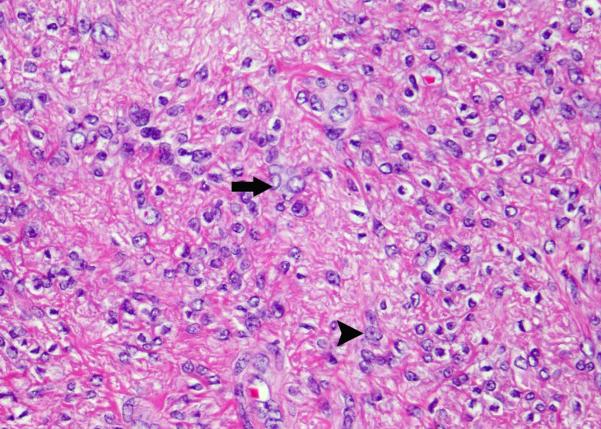
All the lesions demonstrated diffuse granular cytoplasmic labeling with S-(2-succino)-cysteine, but staining was absent in the eosinophilic globules and adjacent normal myometrium, when available for evaluation (Figure 5). S-(2-succino)-cysteine staining of the 63 tissue microarray cases revealed equivocal staining in one leiomyosarcoma, but repeat staining in a complete section of this tumor was negative. Only one leiomyoma was diffusely positive with S-(2-succino)-cysteine. This patient was 44-year-old at presentation. She complained of heavy vaginal bleeding and increasing pelvic pressure. The patient did not report a significant family cancer history and was not noted to have any cutaneous lesions. Hysterectomy revealed multiple leiomyomas, measuring 0.2 cm to 8.5 cm, all with a white and whorled appearance on gross examination. Sections of the largest leiomyoma revealed SMT-FH features, but macronucleoli were not easily found (Figure 6). Sections of several smaller leiomyomas did not reveal SMT-FH features. The remaining 61 cases, including endometrial stromal tumors, leiomyomas, and leiomyosarcomas, were completely negative for S-(2-succino)-cysteine staining. Review of the original slides did not reveal any histologic features suggestive of SMT-FH.
Figure 5.
(A) S-(2-succino)-cysteine-positive leiomyoma showing eosinophilic globules indicated by arrowheads. (B) S-(2-succino)-cysteine immunohistochemistry demonstrates granular cytoplasmic labeling that spares the eosinophilic globules, indicated by arrowheads.
Figure 6.
(A) S-(2-succino)-cysteine-positive leiomyoma from the tissue microarray, showing staghorn vasculature and increased cellularity. (B) Many nuclei show optical clearing (arrow) and small, eosinophilic nucleoli (arrowhead).
Three patients underwent germline genetic testing after our diagnoses suggested the possibility of HLRCC. Two showed a germline mutation in the FH gene. One had a Gly354Arg (1060G to A) and the other had Arg233His (698G to A). The third patient tested negative for germline FH mutation.
Discussion
This study confirms that uterine leiomyomas with FH aberrations, validated with S-(2-succino)-cysteine immunohistochemistry, have characteristic morphological features and that prospective examination for the diagnostic features discussed herein reliably recognizes SMT-FH cases with abnormal succination. Although we were not able to detect the mechanism of FH aberration in most of the reported cases, we confirmed that 2 of 3 tested patients had germline FH mutations and therefore had HLRCC syndrome. The untested cases may harbor either a germline FH mutation or somatic abnormalities that result in diffuse protein succination (19), because S-(2-succino)-cysteine expression appears to be sensitive and specific for FH aberrations. Bardella et al. reported that S-(2-succino)-cysteine positivity was able to predict genetic alterations in FH (19) in cases referred for genetic testing for HLRCC. In the same study, S-(2-succino)-cysteine immunoreactivity was not detected in a survey of 1342 non–HLRCC-related tumors.
Based on the known distribution of FH mutation types and negative germline mutation results for one of our tested SMT-FH patients, SMT-FHs are possibly more commonly due to somatic mutation (43) than to germline mutation (11). In addition to somatic and germline mutations, rearrangements involving chromosome 1 with consequent loss of FH (45) have been reported, as have other mechanisms resulting in whole gene deletion (46). Epigenetic silencing of FH is not currently thought to be an important mechanism for loss of FH function (47). Whether leiomyomas with somatic FH mutations or deleterious rearrangements differ clinically from other leiomyomas is not known, but reported associations between HMGA2 overexpression and multiple, clonally related uterine leiomyomas provide a precedent suggesting that other somatic abnormalities may be linked to particular clinical scenarios (1).
The most obvious application of our results is the use of routine microscopy to identify patients at risk of harboring an occult FH germline mutation, indicating the presence of HLRCC. Surveillance for the development of a syndromic renal cell carcinoma, frequently a very clinically aggressive disease, would then theoretically benefit the patient directly. HLRCC patients typically develop multiple cutaneous and uterine leiomyomas and an aggressive form of renal carcinoma that has been described as part of the spectrum of “type II papillary renal cell carcinoma” (4,6). Cutaneous and uterine leiomyomas are reportedly present in 75% and approximately 80% of patients, respectively (22,23,44) (21), but 40% of patients with cutaneous leiomyomas have only subtle dermatologic manifestations (22,23). The mean age of presentation with cutaneous leiomyomas is 25 years; uterine leiomyomas are characteristically multiple and occur at a mean age of 30 years. Renal cell carcinomas are present in only 20-30% of kindreds (11,48) and occur at an average age of 46 years. HLRCC is therefore an incompletely penetrant syndrome, with a wide spectrum of disease, ranging form asymptomatic to lethal.
Establishing methodology for detection of HLRCC patients will be challenging because of incomplete penetrance and the likelihood that the morphological features of uterine leiomyomas with abnormal succination are not limited to patients with germline FH mutations.
Genetic counseling should be offered to patients that present with obvious cutaneous and/or uterine stigmata typical of HLRCC at a young age (i.e., younger than 30 or 35 years). Regarding the identification of patients at risk of harboring an occult FH germline mutation by examining leiomyoma specimens, we think that it is worthwhile to educate the surgeon about HLRCC and the relative risks of detecting a germline mutation (however sketchy that information currently may be) based on histologic examination alone. The patient and surgeon can therefore make a relatively informed decision about pursuing formal genetics counseling and surveillance for renal cell carcinoma. If a pathologist is inexperienced with the morphology of SMT-FHs or uncertain about the findings in a given case, it is reasonable to seek consultation. It would be preferable to perform S-(2-succino)-cysteine immunohistochemistry instead, but the antibody is not currently available commercially, to our knowledge. The decision to perform germline genetic testing should be a multidisciplinary exercise. The two patients ultimately shown to have germline FH mutations (case numbers 1 and 2, Table 1) had syndromic stigmata other than SMT-FH; one had cutaneous leiomyomata and the other, a 32 year old, had a renal cell carcinoma that was diagnosed previously at another institution. Multiple leiomyomata diagnosed before age 30 or 35 and cutaneous leiomyomata should therefore be as important a trigger for testing as an SMT-FH diagnosis.
Morphologically, although every SMT-FH displays the characteristically prominent nucleoli with perinucleolar clearing, this feature is appreciable mostly only at high-power magnification. Therefore, an appreciation of the low-power appearance of these tumors is at least as important as finding prominent nucleoli. Our work confirms that of previous investigators (3,44) and also contributes new observations. The tumors usually exhibit a fascicular growth pattern with increased cellularity and focal mild nuclear atypia. Despite the presence of tumor cell fascicles, including occasional cases with a “neurolemmoma-like” pattern, many cases display the appearance of an epithelioid neoplasm because of the presence of round or oval nuclei. All cases showed staghorn vasculature, and most cases appeared more fibrillary than the surrounding myometrium. Characteristically and as previously reported by Garg et al (3) and more recently by Sanz-Ortega and colleagues (44), cells have a prominent, eosinophilic nucleolus surrounded by a perinuclear halo. Arguably more striking than the presence of the inclusion-like nucleoli are the ubiquitous eosinophilic cytoplasmic globules. These are displayed in images from recent publications (44).
Uterine smooth muscle tumors may have some of the morphologic characteristics described in the setting of HLRCC. There are a few reports of leiomyomas that contain eosinophilic globules (49,50,51); however, no other morphologic features suggestive of HLRCC were reported. Similarly, atypical leiomyomas may have prominent eosinophilic nucleoli (52) but usually do not show a perinucleolar halo. Staghorn vasculature is very unusual in uterine smooth muscle tumors as well. Therefore, the combination of these unusual features in a given case is responsible for a characteristic appearance not shared by other uterine smooth muscle tumors.
The differential diagnosis of HLRCC-like uterine smooth muscle tumors includes epithelioid smooth muscle tumors, atypical leiomyoma, leiomyosarcoma, endometrial stromal neoplasia, and perivascular epithelioid cell tumors (PEComa). Epithelioid smooth muscle tumors have characteristic round nuclei with eosinophilic cytoplasm, but features such as staghorn vasculature, eosinophilic globules, and a perinucleolar halo are not usually present. Additionally, tumor cells are round, polygonal and epithelioid, not fusiform. Owing to the severe nuclear atypia and variable mitotic index in SMT-FHs, many such tumors are categorized as atypical leiomyomas, which is reasonable provided that criteria for leiomyosarcoma are not met (53). Indeed, a recent publication about atypical leiomyomas included an illustration of a tumor that resembles SMT-FH (54). A diagnosis of leiomyosarcoma was initially considered in some of our cases, but none had necrosis or a high mitotic index. Uterine leiomyosarcoma has been reported in 6 women with FH mutation (55). In all other cases, reports of patients with HLRCC and uterine smooth muscle tumors indicated a benign clinical profile despite the frequent presence of symptoms (7,56). Endometrial stromal tumors are characterized by small blue cells showing perivascular whorls, without the large vessels that are more characteristic of smooth muscle tumors. Although the staghorn vasculature that we describe in our cases can be found in endometrial stromal tumors with or without smooth muscle differentiation, endometrial stromal tumors do not have the cytological features seen in HLRCC-associated leiomyomas. Finally, PEComas can have epitheloid cells and a prominent vasculature. Unlike SMT-FHs, they usually have clear or slightly eosinophilic granular cytoplasm and are often arranged around blood vessels, which range from arborizing capillaries to thicker often hyalinized arterioles (57). We performed immunohistochemical stains for PEComa, namely HMB-45 and Melan A, in one of our study cases, and the results were negative.
Two of the most important weaknesses of this study were lacking clinical data and mutational analysis in many cases. Eight of the 9 cases were second opinion consultation cases where the acquisition of detailed clinical histories was difficult and the decision to perform mutational analysis was left almost entirely to the discretion of the gynecologist. In some cases, we were informed about the presence of multiple leiomyomata, but did not have the opportunity to review slides from all of the leiomyomata. Despite this, we are able to conclude that SMT-FHs demonstrate morphological reproducibility across cases and protein succination with anti- S-(2-succino)-cysteine positivity. The characteristic histological features can be assessed prospectively in unselected cases. Although the features we have described are not specific for germline FH mutation, we believe the features strongly suggest uterine smooth muscle tumors with FH aberration. Identifying these cases is an important first step in the diagnostic workup of patients with possible HLRCC. Instituting surveillance for lethal renal carcinomas may save lives.
Acknowledgement
We thank Dr Patrick J. Pollard for providing data about the prevalence of germline FH mutations.
Footnotes
Disclosure/Conflict of Interest (and Source of Funding)
There are no conflicts of interest. Funded in part by the cancer center core grant P30 CA008748. The core grant provides funding to institutional cores, such as Pathology.
References
- 1.Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43–53. doi: 10.1056/NEJMoa1302736. [DOI] [PubMed] [Google Scholar]
- 2.Sanz J, Teller L, Vockes C, Linehan WM, Sanz-Esponera J, Merino MJ. Genetic alterations of uterine fibroids in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Mod Pathol. 2008;21:223a–223a. [Google Scholar]
- 3.Garg K, Tickoo SK, Soslow RA, Reuter VE. Morphologic features of uterine leiomyomas associated with hereditary leiomyomatosis and renal cell carcinoma syndrome: a case report. Am J Surg Pathol. 2011;35:1235–7. doi: 10.1097/PAS.0b013e318223ca01. [DOI] [PubMed] [Google Scholar]
- 4.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–92. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011;10:397–411. doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 7.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam NA, Bevan S, Churchman M, et al. Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. Am J Hum Genet. 2001;68:1264–9. doi: 10.1086/320124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiuru M, Launonen V, Hietala M, et al. Familial cutaneous leiomyomatosis is a two-hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol. 2001;159:825–9. doi: 10.1016/S0002-9440(10)61757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9:20. doi: 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardie B, Remenieras A, Kattygnarath D, et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J Med Genet. 2011;48:226–234. doi: 10.1136/jmg.2010.085068. [DOI] [PubMed] [Google Scholar]
- 12.Picaud S, Kavanagh KL, Yue WW, et al. Structural basis of fumarate hydratase deficiency. J Inherit Metab Dis. 2011;34:671–6. doi: 10.1007/s10545-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003;3:193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 14.Pollard PJ, Ratcliffe PJ. Cancer. Puzzling patterns of predisposition. Science. 2009;324:192–4. doi: 10.1126/science.1173362. [DOI] [PubMed] [Google Scholar]
- 15.Bardella C, Olivero M, Lorenzato A, et al. Cells lacking the fumarase tumor suppressor are protected from apoptosis through a hypoxia-inducible factor-independent, AMPK-dependent mechanism. Mol Cell Biol. 2012;32:3081–94. doi: 10.1128/MCB.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderson NL, Wang Y, Blatnik M, et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Nagai R, Brock JW, Blatnik M, et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem. 2007;282:34219–28. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 18.Ternette N, Yang M, Laroyia M, et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep. 2013;3:689–700. doi: 10.1016/j.celrep.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Valera V, Sourbier C, et al. A novel fumarate hydratase-deficient HLRCC kidney cancer cell line, UOK268: a model of the Warburg effect in cancer. Cancer Genet. 2012;205:377–90. doi: 10.1016/j.cancergen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardella C, El-Bahrawy M, Frizzell N, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523e6. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95e106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei MH, Toure O, Glenn GM, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18e27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406e10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 25.Alam NA, Rowan AJ, Wortham NC, et al. Germline fumarate hydratase mutations in families with multiple cutaneous and uterine leiomyomata. J Invest Dermatol. 2003;121:741e4. doi: 10.1046/j.1523-1747.2003.12499.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Mir A, Glaser B, Chuang GS, et al. Germline fumarate hydratase mutations in families with multiple cutaneous and uterine leiomyomata. J Invest Dermatol. 2003;121:741e4. doi: 10.1046/j.1523-1747.2003.12499.x. [DOI] [PubMed] [Google Scholar]
- 27.Alam NA, Olpin S, Rowan A, et al. Missense mutations in fumarate hydratase in multiple cutaneous and uterine leiomyomatosis and renal cell cancer. J Mol Diagn. 2005;7:437e43. doi: 10.1016/S1525-1578(10)60574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan I, Wong T, Martinez-Mir A, Christiano AM, McGrath JA. Familial multiple cutaneous and uterine leiomyomas associated with papillary renal cell cancer. Clin Exp Dermatol. 2005;30:75e8. doi: 10.1111/j.1365-2230.2004.01675.x. [DOI] [PubMed] [Google Scholar]
- 29.Matyakhina L, Freedman RJ, Bourdeau I, et al. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90:3773e9. doi: 10.1210/jc.2004-2377. [DOI] [PubMed] [Google Scholar]
- 30.Badeloe S, van Geel M, van Steensel MA, et al. Diffuse and segmental variants of cutaneous leiomyomatosis: novel mutations in the fumarate hydratase gene and review of the literature. Exp Dermatol. 2006;15:735e41. doi: 10.1111/j.1600-0625.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 31.Chuang GS, Martinez-Mir A, Engler DE, Gmyrek RF, Zlotogorski A, Christiano AM. Multiple cutaneous and uterine leiomyomata resulting from missense mutations in the fumarate hydratase gene. Clin Exp Dermatol. 2006;31:118e21. doi: 10.1111/j.1365-2230.2005.01977.x. [DOI] [PubMed] [Google Scholar]
- 32.Pithukpakorn M, Wei MH, Toure O, et al. Fumarate hydratase enzyme activity in lymphoblastoid cells and fibroblasts of individuals in families with hereditary leiomyomatosis and renal cell cancer. J MedGenet. 2006;43:755e62. doi: 10.1136/jmg.2006.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ylisaukko-oja SK, Kiuru M, Lehtonen HJ, et al. Analysis of fumarate hydratase mutations in a population-based series of early onset uterine leiomyosarcoma patients. Int J Cancer. 2006;119:283e7. doi: 10.1002/ijc.21798. [DOI] [PubMed] [Google Scholar]
- 34.Campione E, Terrinoni A, Orlandi A, et al. Cerebral cavernomas in a family with multiple cutaneous and uterine leiomyomas associated with a new mutation in the fumarate hydratase gene. J Invest Dermatol. 2007;127:2271e3. doi: 10.1038/sj.jid.5700851. [DOI] [PubMed] [Google Scholar]
- 35.Lehtonen HJ, Blanco I, Piulats JM, Herva R, Launonen V, Aaltonen LA. Conventional renal cancer in a patient with fumarate hydratase mutation. Hum Pathol. 2007;38:793e6. doi: 10.1016/j.humpath.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Makino T, Nagasaki A, Furuichi M, et al. Novel mutation in a fumalate hydratase gene of a Japanese patient with multiple cutaneous and uterine leiomyomatosis. J Dermatol Sci. 2007;48:151e3. doi: 10.1016/j.jdermsci.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Ahvenainen T, Lehtonen HJ, Lehtonen R, et al. Mutation screening of fumarate hydratase by multiplex ligation-dependent probe amplification: detection of exonic deletion in a patient with leiomyomatosis and renal cell cancer. Cancer Genet Cytogenet. 2008;183:83e8. doi: 10.1016/j.cancergencyto.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Badeloe S, Bladergroen RS, Jonkman MF, et al. Hereditary multiple cutaneous leiomyoma resulting from novel mutations in the fumarate hydratase gene. J Dermatol Sci. 2008;51:139e43. doi: 10.1016/j.jdermsci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet. 2008;9:20. doi: 10.1186/1471-2350-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huter E, Wortham NC, Hartschuh W, Enk A, Jappe U. Single base mutation in the fumarate hydratase gene leading to segmental cutaneous leiomyomatosis. Acta Derm Venereol. 2008;88:63e5. doi: 10.2340/00015555-0341. [DOI] [PubMed] [Google Scholar]
- 41.Onder M, Glenn G, Adisen E, et al. Cutaneous papules, uterine fibroids, and renal cell cancer: one family’s tale. Lancet. 2010;375:170. doi: 10.1016/S0140-6736(09)61499-9. [DOI] [PubMed] [Google Scholar]
- 42.Vahteristo P, Koski TA, Naatsaari L, et al. No evidence for a genetic modifier for renal cell cancer risk in HLRCC syndrome. Fam Cancer. 2010;9:245e51. doi: 10.1007/s10689-009-9312-2. [DOI] [PubMed] [Google Scholar]
- 43.Lehtonen R, Kiuru M, Vanharanta S, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164:17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanz-Ortega J, Vocke C, Stratton P, Linehan WM, Merino MJ. Morphologic and molecular characteristics of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol. 2013;37:74–80. doi: 10.1097/PAS.0b013e31825ec16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross KL, Panhuysen CI, Kleinman MS, et al. Involvement of fumarate hydratase in nonsyndromic uterine leiomyomas: genetic linkage analysis and FISH studies. Genes Chromosomes Cancer. 2004;41:183–90. doi: 10.1002/gcc.20079. [DOI] [PubMed] [Google Scholar]
- 46.Mroch AR, Laudenschlager M, Flanagan JD. Detection of a novel FH whole gene deletion in the propositus leading to subsequent prenatal diagnosis in a sibship with fumarase deficiency. Am J Med Genet A. 2012;158A:155–8. doi: 10.1002/ajmg.a.34344. [DOI] [PubMed] [Google Scholar]
- 47.Dulaimi E, Ibanez de Caceres I, Uzzo RG, et al. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004;10:3972–9. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- 48.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578e85. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 49.Agaimy A, Wunsch PH, Hofstadter F, Schroeder J. Hyaline globules in paucicellular leiomyomas of the gastrointestinal tract are distinct from skeinoid fibers and represent degenerating smooth muscle cells. Pathol Res Pract. 2009;205:417–22. doi: 10.1016/j.prp.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Dundr P, Povysil C, Tvrdik D, Mara M. Uterine leiomyomas with inclusion bodies: an immunohistochemical and ultrastructural analysis of 12 cases. Pathol Res Pract. 2007;203:145–51. doi: 10.1016/j.prp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Matsukuma S, Takeo H, Ohara I, Sakai Y. Endoscopically resected colorectal leiomyomas often containing eosinophilic globules. Histopathology. 2004;45:302–3. doi: 10.1111/j.1365-2559.2004.01863.x. [DOI] [PubMed] [Google Scholar]
- 52.Siti-Aishah MA, Noriah O, Malini MN, Zainul-Rashid MR, Das S. Atypical (symplastic) leiomyoma of the uterus--a case report. Clin Ter. 2011;162:447–50. [PubMed] [Google Scholar]
- 53.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms: A clinicopathologic study of 213 cases. American Journal of Surgical Pathology. 1994;18:535–558. [PubMed] [Google Scholar]
- 54.Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37:643–9. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 55.Ylisaukko-oja SK, Kiuru M, Lehtonen HJ, et al. Analysis of fumarate hydratase mutations in a population-based series of early onset uterine leiomyosarcoma patients. Int J Cancer. 2006;119:283–7. doi: 10.1002/ijc.21798. [DOI] [PubMed] [Google Scholar]
- 56.Grubb RL, 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074–9. doi: 10.1016/j.juro.2007.01.155. discussion 2079-80. [DOI] [PubMed] [Google Scholar]
- 57.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular Epithelioid Cell Neoplasms of Soft Tissue and Gynecologic Origin: A Clinicopathologic Study of 26 Cases and Review of the Literature. Am J Surg Pathol. 2005;29:1558–75. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]