Abstract
The energy-sensing AMP-activated kinase AMPK ensures survival of energy-depleted cells by stimulating ATP production and limiting ATP utilization. Both energy production and energy consumption are profoundly influenced by transport processes across the cell membane including channels, carriers and pumps. Accordingly, AMPK is a powerful regulator of transport across the cell membrane. AMPK regulates diverse K+ channels, Na+ channels, Ca2+ release activated Ca2+ channels, Cl- channels, gap junctional channels, glucose carriers, Na+/H+-exchanger, monocarboxylate-, phosphate-, creatine-, amino acid-, peptide- and osmolyte-transporters, Na+/Ca2+-exchanger, H+-ATPase and Na+/K+-ATPase. AMPK activates ubiquitin ligase Nedd4–2, which labels several plasma membrane proteins for degradation. AMPK further regulates transport proteins by inhibition of Rab GTPase activating protein (GAP) TBC1D1. It stimulates phosphatidylinositol 3-phosphate 5-kinase PIKfyve and inhibits phosphatase and tensin homolog (PTEN) via glycogen synthase kinase 3β (GSK3β). Moreover, it stabilizes F-actin as well as downregulates transcription factor NF-κB. All those cellular effects serve to regulate transport proteins.
Keywords: Ca2+ channels, Cl- channels, K+ channels, Na+ channels, Na+/Ca2+ exchanger, Na+/H+ exchanger, Na+/K+ ATPase, amino acid transporters, glucose carriers, monocarboxylate transporters
Introduction
The ubiquitously expressed adenosine 5′-monophosphate (AMP) -activated protein kinase (AMPK) is composed of a catalytic α-subunit and regulatory β- and γ-subunits.1 The α1 isoform is ubiquitously expressed, whereas the α2 isoform is mainly expressed in skeletal muscle, heart and liver.2 AMPK is activated by increase in the cytosolic AMP/ATP concentration ratio and thus responds to the cellular energy status.3-5 AMPK is further activated by increases in the cytosolic Ca2+ concentration even in energy-replete cells,4,6-9 an effect involving Ca2+/calmodulin-dependent kinase kinase–β- (CaMKKβ) dependent phosphorylation of threonine 172 (Thr-172) residue in the AMPK catalytic α subunit.10,11 Moreover, AMPK is activated by the liver kinase B1 (LKB1) or serine/threonine kinase 11 (STK11),11 the transforming growth factor β-associated kinase 112 and by glucosamine.13
AMPK phosphorylates target proteins at serine or threonine within the consensus sequence Φ(X, β)XXS/TXXXΦ (Φ, hydrophobic; β, basic).14 Phosphorylation modifies the function of target proteins. The cellular functions thus stimulated by AMPK serve in large part to refuel cellular ATP levels.15 AMPK increases ATP generation by stimulating cellular glucose uptake, glycolysis, fatty acid oxidation and the activity of enzymes required for ATP production.5,16-38 It curtails energy expenditure by decreasing protein synthesis, gluconeogenesis and lipogenesis.5,15,17,39-41 AMPK thus protects cells against detrimental effects of energy depletion.12,15,42-44 However, AMPK may trigger suicidal death of energy-depleted cells.45 It further inhibits cell proliferation,46 counteracts hypertrophy,47 fosters phagocytosis,48 and stimulates autophagy.49,50
The orchestration of cell survival during energy depletion involves regulation of transport across the cell membrane. Avoidance of Ca2+ overflow requires regulation of Ca2+ transport. The present brief review thus compiles the effects of AMPK on channels, carriers, and pumps. Some examples are provided of how AMPK-sensitive transport could counteract energy depletion and/or Ca2+ overflow. The reader is encouraged to consult excellent earlier reviews on similar topics.51,52
AMPK-Regulated Ion Channels
AMPK regulates a wide variety of membrane transport proteins.51,53-72 K+ channels downregulated by AMPK include Ca2+-activated potassium channels such as KCa3.1,51,73 inwardly rectifying potassium channels such as Kir1.1 (ROMK),77 and Kir2.1,54 the voltage-gated K+ channels Kv1.5,74 Kv2.1,76 Kv7.1,56,58,59 and Kv11.1 (hERG),75 as well as two-P potassium channels such as K2P2.1 (TREK-1),71 K2P9.1 (TASK-3),69 and K2P10.1 (TREK-2).71
AMPK has been shown to inhibit ATP-sensitive Kir6.x channels,78,79 whereas other studies reported a stimulatory effect on cardiac ATP-sensitive Kir6.2,72,79 and on Kir6.2 in β-cells.80 The large Ca2+-activated K+ channel KCa1.1 has similarly been shown to be up-81 or downregulated82 by AMPK.
K+ channels are the most important channels maintaining the cell membrane potential.83 Reduced K+ fluxes through inhibited K+ channels depolarize whereas enhanced K+ fluxes through activated K+ channels hyperpolarize energy-depleted cells. Depolarization following downregulation of K+ channels decreases the electical driving force for electrogenic Na+-coupled transport thus curtailing Na+ entry and the subsequent requirement for costly Na+ extrusion by Na+/K+ ATPase in epithelia such as the proximal renal tubule.83
Depolarization following inhibition of K+ channels decreases the electrical driving force for HCO3- exit and thus favors alkanization of cells with HCO3- permeable channels or Na+-coupled HCO3- cotransport.84 Similarly, depolarization alkalinizes the cytosol of cells expressing Cl- channels in parallel to Cl-/ HCO3- exchange, as it decreases the electrical driving force for Cl- exit thus increasing the cytosolic Cl- concentration and via Cl-/HCO3- exchange the cytosolic HCO3- concentration. A depolarization by 18 mV doubles the cytosolic equilibrium Cl- concentration, doubles the equilibrium HCO3- concentration and thus increases cytosolic pH at equilibrium by 0.3 pH units. The alkalinization fosters glycolytic flux and thus ATP generation from glucose85 without the requirement of energy-consuming extrusion of H+ by either H+ ATPase or Na+/H+ exchanger (see below).
A depolarization further leads to decreased store-operated Ca2+ entry (SOCE) through Ca2+ release activated Ca2+ channels (Fig. 1), which show a prominent inward rectification.86 In contrast, depolarization activates voltage-gated Ca2+ channels87 with subsequent Ca2+ entry and energy-consuming excitation in excitable cells. Thus, hyperpolarization rather than depolarization results in a reduction of energy consumption of excitable cells. The impact of K+ channel activity on cytosolic Ca2+ activity and energy consumption hence depends on the expression and regulation of the Ca2+ channel types in the energy-depleted cell.
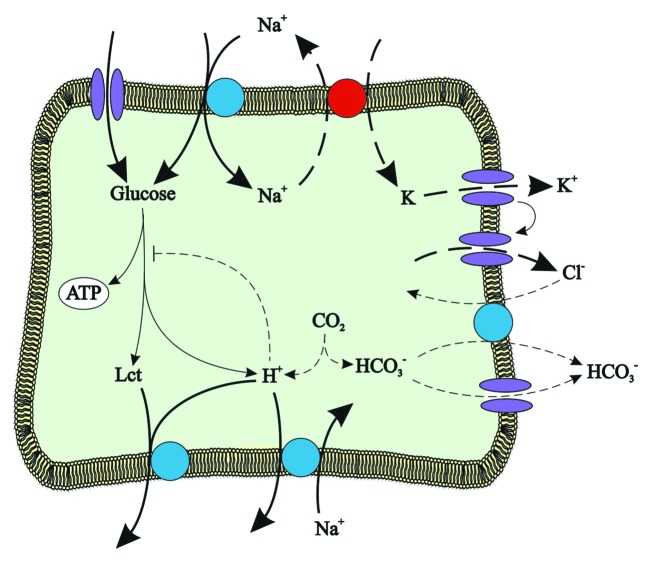
Figure 1. Tentative model illustrating AMPK-sensitive regulation of glucose uptake, lactate release and cytosolic pH. AMPK-stimulated processes are shown in bold solid lines, AMPK-inhibited processes in bold dashed lines.
Cardiac repolarization is expected to be delayed by the AMPK-induced downregulation of the cardiac K+ channels Kv11.175 and Kv7.1.59 This mechanism may participate in the events causing arrhythmia following cardiac ischemia. Mutations in the AMPK γ2 subunit are associated with potentially fatal cardiac arrhythmias.51,75 Whether regulation of K+ channels contributes to the underlying mechanisms, has, however, remained ill defined.
AMPK activity decreases the frequency of evoked action potentials in cultured hippocampal neurons thus decreasing the energy consumption of those cells.76 The increase in KATP current in cardiomyocytes by AMPK activity contributes to hypoxia-induced preconditioning of the heart protecting against myocardial infarction.72
AMPK-dependent stimulation of KATP channel activity inhibits and AMPK-dependent inhibition of KATP channel activity stimulates insulin release.78-80,88-90
The decrease of KCa1.1,70,91 K2P9.1,69,91 K2P2.171 and/or K2P10.171 activity by AMPK presumably contributes to oxygen sensing within type I cells of the carotid body.51 Hypoxia leads to K+ channel inhibition with subsequent cell membrane depolarization, Ca2+ entry and degranulation in those cells.82,92
AMPK-sensitive stimulation of KCa1.1 channel activity contributes to the protection of outer hair cells (OHC) of the inner ear against acoustic trauma.81 Accordingly, recovery from hearing loss following acoustic overexposure is significantly delayed in AMPKα1-deficient mice.81
AMPK participates in the regulation of Cl- secretion. It reduces the activity of some Cl- channel proteins implicated in epithelial transport and cell volume regulation.51,93-96 In particular, AMPK inhibits the Cl- channel CFTR (cystic transmembran transport regulator), which is expressed in the apical cell membrane of Cl--secreting epithelial cells.51,93-96
AMPK activity slows the inactivation of the voltage-gated cardiac Na+ channel Nav1.5 and shifts the voltage activation curve toward hyperpolarized values, an effect which could prolong the action potential.97 Activation of AMPK and subsequent AMPK-sensitive regulation of this channel may contribute to the arrhythmia following cardiac ischemia.
AMPK reduces the activity of the epithelial Na+ channel ENaC57,98,99 and may therefore decrease Na+ transport in a variety of epithelia100 and nonepithelial tissues including endothelia.101 Decrease of Na+ entry lowers the work load of the respective epithelial cell and thus protects against energy depletion. As enhanced expression of endothelial ENaC is followed by endothelial stiffening with decreased endothelial NO release,101,102 reduction of ENaC activity by AMPK may at least in theory contribute to the stimulation of AMPK-sensitive endothelial NO release following ischemia.103
AMPK contributes to the regulation of the pore-forming subunits of Ca2+ release activated Ca2+ channels Orai1, Orai2, and Orai3 (Fig. 1), which are activated by the Ca2+-sensing subunits STIM1 and STIM2.104-106 Along those lines, the increase in the intracellular Ca2+ concentration ([Ca2+]i) following stimulation of the chemokine receptor CXCR4 with its ligand CXCL12 was more pronounced in dendritic cells (DCs) isolated from gene-targeted mice lacking functional AMPKα1 (ampk−/−) than in DCs isolated from wild type mice (ampk+/+).105 Inhibition of endosomal Ca2+ ATPase with thapsigargin in the absence of extracellular Ca2+ leads to a similar release of Ca2+ from the cytoplasmic stores, indicating that AMPK doses not significantly modify Ca2+ stores.105 The increase in cytosolic Ca2+ activity following readdition of extracellular Ca2+ is, however, more pronounced in ampk−/− DCs than in ampk+/+ DCs.105 AMPK further plays a role in the regulation of Orai1 in lymphocytes.107 The inhibition of Ca2+ channels by AMPK is part of a negative feedback,106 as an increase in [Ca2+]i is followed by activation of Ca2+/calmodulin-dependent kinase kinase-β (CaMKKβ) and CaMKKβ-dependent phosphorylation and thus activation of AMPK.7 Along those lines, AMPK phosphorylation following hypoxia is blunted by silencing of STIM1.7 Without this negative feeback, energy depletion would be expected to activate Orai1 due to impaired function of the endoplasmatic reticulum Ca2+ ATPase SERCA with subsequent depletion of intracellular Ca2+ stores and activation of Orai by STIM. AMPK-mediated inhibition of Ca2+ entry presumably contributes to the regulation of a wide variety of functions even without energy depletion, such as inhibition of cell proliferation,46,107 and of cell migration105 by AMPK. For instance, the chemokine CXCL12 enhances migration of immature ampk−/− DCs more potently than migration of immature ampk+/+ DCs.105 Notably, activation with bacterial lipopolysaccharides downregulates AMPK phosphorylation in DCs and thus stimulates migration to a similar extent in ampk−/− DCs and ampk+/+ DCs.105 AMPK further blunts DC activation.108 AMPK counteracts metabolic transition to aerobic glycolysis and cytokine release following triggering of Toll like receptors.108,109
AMPK downregulates the gap junctional protein connexin 26 (CX26) and may thus disrupt the connection between neighboring cells.55 Open gap junctions to intact neighboring cells support the survival of energy-depleted cells, as Na+ and K+ fluxes through gap junctions maintain ion gradients and the cell membrane potential difference across the cell membrane of the energy-depleted cells despite impaired Na+ extrusion and K+ uptake. The gap junctional fluxes impose, however, an additional burden to the Na+/K+ ATPase of the adjacent cell. Closure of the gap junctions thus jeopardizes the survival of the energy-depleted cell but by the same token protects the adjacent intact cells, which could otherwise be ripped to death by the energy-depleted neighboring cell.
AMPK-Regulated Carriers
AMPK regulates a wide variety of carriers. Most importantly, AMPK upregulates both Na+-independent (GLUT)33,34,110-113 and Na+-coupled (SGLT1)114,115 glucose carriers (Fig. 1). The carriers supply energy-depleted cells with glucose, which could be utilized for ATP generation by anaerobic glycolysis, i.e., without consumption of oxygen.116 Besides cells exposed to hypoxia, glycolysis is the preferential source of ATP in inflammatory immune cells and tumor cells.116 At first glance, it appears counterintuitive to employ secondary active Na+-coupled transport (SGLT1) for cellular glucose uptake into energy-depleted cells, as Na+ entering in parallel to glucose needs to be extruded by energy-consuming Na+/K+ ATPase. However, the amount of ATP generated from glycolytic degradation of glucose by far exceeds the amount of ATP required for extrusion of the cotransported Na+. In contrast to GLUT transporters, SGLT1 accomplishes cellular glucose uptake even at extracellular glucose concentrations lower than those prevailing in the cell. Thus, AMPK-stimulated SGLT1 activity could indeed contribute to energy repletion.
The utilization of glucose for glycolytic energy production requires an alkaline cytosolic pH, as the glycolytic enzymes are highly sensitive to cytosolic pH and are inhibited by cytosolic acidification.85 Cytosolic alkalinization may be caused by inhibition of K+ channels with subsequent depolarization of the cell membrane (Fig. 2). Beyond that AMPK stimulates H+ extrusion by the Na+/H+ exchanger117 and thus sets the stage for glucose utilization (Fig. 2). The lactate produced by anaerobic glycolysis could exit via the monocarboxylate transporters MCT1 and MCT4, which are both upregulated by AMPK.118 As lactate exit through MCT1 and MCT4 is paralleled by exit of H+,119 the carriers counteract cytosolic acidification.
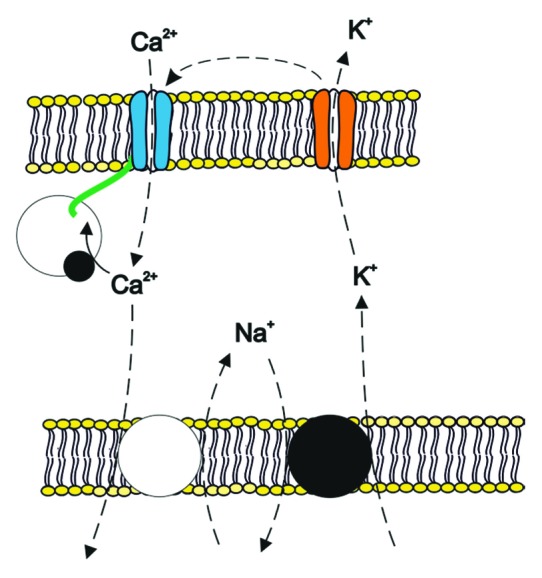
Figure 2. Tentative model illustrating AMPK-sensitive regulation of cytosolic Ca2+. AMPK inhibits Na+/K+ ATPase, K+ channels, Ca2+ release activated Ca2+ channel current (ICRAC, composed of pore forming Orai [blue] and its regulator STIM [green]) as well as Na+/Ca2+ exchanger activity (all dashed lines), which all indirectly or directly impact on cytosolic Ca2+ activity.
In contrast to its effect on SGLT1, AMPK downregulates a variety of Na+ coupled transporters, such as the Na+ coupled phosphate transporter NaPi-IIa,120 the Na+ coupled creatine transporter CreaT,121 the Na+ coupled myoinositol transporter SMIT, the NaCl coupled betaine,GABA transporter BGT122 as well as the Na+ coupled amino acid transporters EAAT3 and EAAT4.123 Inhibition of those transporters decreases the Na+ burden for the Na+/K+ ATPase and thus energy consumption. AMPK further downregulates the H+ driven peptide transporter PepT.124 Decreased activity of this carrier lowers the acid load of the cell.
AMPK inhibits Na+/Ca2+ exchangers105 (Fig. 2), which may limit Na+ entry but by the same token disrupts Ca2+ extrusion by this carrier.106 The purpose of this inhibition is presumably the avoidance of Ca2+ uptake into energy-depleted cells. Energy depletion compromizes Na+/K+ ATPase function due to lack of ATP and due to inhibition by AMPK. A decrease of Na+/K+ ATPase activity increases cytosolic Na+ activity and depolarizes the cell membrane which eventually leads to the reversal of the electrochemical gradients for the carrier. Without inhibition by AMPK the carrier would presumably contribute to cellular Ca2+ accumulation during energy depletion.
AMPK-Regulated Pumps
AMPK downregulates the vacuolar H+ ATPase125-127 and thus directly energy-consuming proton extrusion. Inhibition of the H+ ATPase in intercalated cells of the distal nephron impairs urinary acidification.
AMPK has been reported to inhibit the Na+/K+ ATPase and to contribute to its downregulation during hypoxia.128 AMPK-sensitive inhibition of Na+/K+ ATPase hence leads to downregulation of pulmonary transepithelial Na+ transport in hypoxia.129 As Na+/K+ ATPase is the most important ATP-consuming transport protein in the cell membrane, its downregulation has a profound effect on the energy balance of the cell. Inhibition of Na+/K+ ATPase is at least in some cells followed by inhibition of K+ channels with subsequent depolarization.83 As outlined above, the depolarization decreases the driving force for Na+ coupled transport and results in cytosolic alkalinization, which in turn favors glycolysis and lowers the requirement of primary or secondary active extrusion of H+.
In active skeletal muscle, however, AMPK is activated in parallel to Na+/K+ ATPase and contributes to the upregulation of the pump under this condition.130 It should be kept in mind that excitable cells would presumably not decrease energy consumption following depolarization (see above). Moreover, inhibition of Na+/K+ ATPase would be expected to increase the cytosolic Na+ concentration, which could, at least in theory, reverse the Na+/Ca2+ exchanger action thus resulting in Ca2+ uptake with subsequent energy-consuming muscle contraction. Clearly, inhibition of Na+/K+ ATPase serves to reduce energy expenditure but by the same token jeopardizes the second function of AMPK; i.e., the maintenance of low cytosolic Ca2+ activity.
Mechanisms Employed in AMPK-Sensitive Transport Regulation
AMPK may regulate transport proteins by direct phosphorylation,51 as shown for KCa1.1,82 Kv2.1,76 Kir6.2,79 K2P2.171 and K2P10.1,71 CFTR,93-95 as well as for H+ ATPase.125 AMPK stimulates Nedd4–2 (neuronal precursor cells expressed developmentally downregulated), an ubiquitin ligase labeling transport proteins for clearance from the cell membrane and subsequent degradation.57,58,98,99 For instance, Nedd4–2 mediates the downregulation of the epithelial Na+ channel ENaC,57,98,99 the inwardly rectifying K+ channel Kir2.1,54 and the voltage gated K+ channels Kv7.1.51,56,58 In theory, AMPK could similarly downregulate other Nedd4–2- sensitive transport proteins including the ion channels Nav1.5, Kv1.3, Kv1.5, Kv4.3, Kv7.2/3, CLCKa/barttin, Orai1, and ClC2,131-134 or the carriers SGLT1, NaPi-IIb, SN1, EAAT1, EAAT2, EAAT4.135-137
AMPK may enhance GLUT4 insertion into the cell membrane by phosphorylation and thus inhibition of TBC1D1, the Rab GTPase activating protein (GAP), which otherwise counteracts GLUT4 translocation into the plasma membrane.138 AMPK may upregulate carriers such as GLUT4 further by stimulating the phosphatidylinositol 3-phosphate 5-kinase PIKfyve, a kinase generating PtdIns(3,5)P2, which in turn mediates the trafficking of carrier-containing vesicles to the cell membrane.111 It is noteworthy that several further transport proteins are regulated by PIKfyve, such as the AMPA-type glutamate receptor GluA1,139 the K+ channels Kv11.1,140 Kir2.1, and Kir2.2,141,142 the Ca2+ channel TRPV6,143 the Cl- channel ClC2,144 the Na+, glucose cotransporter SGLT1,145 the creatine transporter CreaT146 as well as the amino acid transporters B0AT1,147 EAAT2148 EAAT3,149 and EAAT4.150 Whether or not AMPK-sensitivity of PIKfyve contributes to the regulation of those channels and carriers, remains, however, to be shown.
AMPK has been shown to modify KATP channel trafficking by inhibition of phosphatase and tensin homolog (PTEN) via glycogen synthase kinase 3β (GSK3β).90 Moreover, AMPK-sensitive KATP channel trafficking is impaired by stabilization of F-actin and stimulated by destabilization of F-actin.88 Whether those signaling mechanisms play a role in the regulation of other channels or carriers, is not known.
AMPK is further effective through downregulation of NF-κB (nuclear factor kappa B).151,152 As NF-κB stimulates the expression of Orai1,153,154 the inhibitory effect of AMPK on Orai1 may be partially due to downregulation of NF-κB. Channels upregulated by NF-κB further include the voltage-gated K+ channel Kv1.3155 and the epithelial Cl- channel CFTR.156 CFTR in turn downregulates the expression of the transcription factor.157 Channels downregulated by NF-κB include the epithelial Na+ channel ENaC.158 Among the carriers upregulated by NF-κB is the Na+/H+ exchanger NHE3,159 carriers downregulated by NF-κB include SGLT1.160 NF-κB presumably regulates the expression of multiple further transport proteins and/or several signaling cascades involved in transport regulation. For instance, NF-κB downregulates the expression of the serum and glucocorticoid inducible kinase SGK1,158 a powerful regulator of a wide variety of channels, carriers and Na+/K+ ATPase.137 Along those lines, NF-κB inhibits ENaC in the renal collecting duct by downregulating SGK1.158 However, the contribution of NF-κB or other transcription factors to AMPK-sensitive transport regulation has hitherto remained ill defined.
Conclusions
In conclusion, AMPK is a powerful regulator of a wide variety of channels, carriers and pumps. The kinase is at least partially effective by directly phosphorylating transport proteins, by stimulating Nedd4–2-sensitive transport protein degradation and by interference with NF-κB-sensitive transcription. AMPK-dependent regulation of transport proteins is an integral part of the cell survival strategy during energy depletion, Ca2+ overload and further threats of cell survival. Clearly, additional experimental effort is needed to fully understand the AMPK-dependent orchestration of transport under physiological and pathophysiological conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge the preparation of the manuscript by T. Loch, L. Subasic and Ali Soleimanpour. Research in the authors’ laboratory was supported by the Deutsche Forschungsgemeinschaft (Fo 695/1-1, GK 1302 and SFB 773) and the IZKF of the Medical Faculty of the University of Tübingen (Nachwuchsgruppe).
Glossary
Abbreviations:
- AMPK
AMP activated kinase
- B0AT
amino acid transporter
- BGT
NaCl coupled betaine,GABA transporter BGT
- BKCa
Big Ca2+ activated K+ channels
- CaMKKβ
Ca2+/calmodulin-dependent kinase kinase–beta
- CFTR
cystic fibrosis transmembrane transport regulator
- ClCKa/barttin
Cl- channels
- ClC2
Cl- channels
- CreaT
creatine transporter
- EAAT
Excitatory amino acid transporter
- ENaC
epithelial Na+ channel
- GAP
GTPase activating protein
- GLUT
glucose transporters
- GSK
glycogen synthase kinase
- hERG
human ether a gogo K+ channels
- KATP
ATP regulated K+ channels
- KCa
Ca2+ activated K+ channels
- KCNE1/KCNQ1
voltage gated K+ channels
- Kir
inwardly rectifying K+ channels
- Kv
voltage gated K+ channels
- LKB1
liver kinase B1
- MCT
monocarboxylate transporters
- NaPi-IIb
phosphate transporter
- Nav
voltage gated Na+ channels
- Nedd4.2
neuronal precursor cells expressed developmentally downregulated 4-2
- NF-κB
Nuclear factor kappa B
- OHC
outer hair cells
- Orai1
Ca2+ release activated Ca2+ channel
- PepT1
peptide transporter
- PIKfyve
phosphatidylinositol 3-phosphate 5-kinase
- PTEN
Phosphatase and tensin homologue (PTEN) via glycogen synthase
- ROMK
renal outer medullary K+ channels
- SGLT
Na+ coupled glucose transporter
- SMIT
Na+ coupled myoinositol transporter
- SN1
amino acid transporter
- STK11
serine/threonine kinase 11
- SOCE
store-operated Ca2+ entry
- TBC1D1
TBC domain Rab GTPase activating protein
- TASK
Tandem pore domain K+ channels
- TREK
Tandem pore domain K+ channels
References
- 1.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–4. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 4.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 5.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–47. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 6.Bair AM, Thippegowda PB, Freichel M, Cheng N, Ye RD, Vogel SM, Yu Y, Flockerzi V, Malik AB, Tiruppathi C. Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-kappaB activation in endothelial cells through AMP-activated protein kinase and protein kinase Cdelta. J Biol Chem. 2009;284:563–74. doi: 10.1074/jbc.M803984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–45. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Dey D, Bränström R, Forsberg L, Lu M, Zhang Q, Sjöholm A. BLX-1002, a novel thiazolidinedione with no PPAR affinity, stimulates AMP-activated protein kinase activity, raises cytosolic Ca2+, and enhances glucose-stimulated insulin secretion in a PI3K-dependent manner. Am J Physiol Cell Physiol. 2009;296:C346–54. doi: 10.1152/ajpcell.00444.2008. [DOI] [PubMed] [Google Scholar]
- 9.Wu WN, Wu PF, Zhou J, Guan XL, Zhang Z, Yang YJ, Long LH, Xie N, Chen JG, Wang F. Orexin-A Activates Hypothalamic AMPK Signaling Through a Ca2+-dependent Mechanism Involving Voltage-gated L-type Calcium Channel. Mol Pharmacol. 2013 doi: 10.1124/mol.113.086744. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 10.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 11.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32(Suppl 4):S7–12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Park JH, Park SH, Lim B, Baek WK, Suh SI, Lim JG, Ryu GR, Song DK. Protective role of glucagon-like peptide-1 against glucosamine-induced cytotoxicity in pancreatic beta cells. Cell Physiol Biochem. 2010;25:211–20. doi: 10.1159/000276555. [DOI] [PubMed] [Google Scholar]
- 14.Michell BJ, Stapleton D, Mitchelhill KI, House CM, Katsis F, Witters LA, Kemp BE. Isoform-specific purification and substrate specificity of the 5′-AMP-activated protein kinase. J Biol Chem. 1996;271:28445–50. doi: 10.1074/jbc.271.45.28445. [DOI] [PubMed] [Google Scholar]
- 15.McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front Biosci. 2008;13:3022–33. doi: 10.2741/2907. [DOI] [PubMed] [Google Scholar]
- 16.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–22. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 17.Carling D. The role of the AMP-activated protein kinase in the regulation of energy homeostasis. Novartis Found Symp. 2007;286:72–81, discussion 81-5, 162-3, 196-203. doi: 10.1002/9780470985571.ch7. [DOI] [PubMed] [Google Scholar]
- 18.Guan F, Yu B, Qi GX, Hu J, Zeng DY, Luo J. Chemical hypoxia-induced glucose transporter-4 translocation in neonatal rat cardiomyocytes. Arch Med Res. 2008;39:52–60. doi: 10.1016/j.arcmed.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Horie T, Ono K, Nagao K, Nishi H, Kinoshita M, Kawamura T, Wada H, Shimatsu A, Kita T, Hasegawa K. Oxidative stress induces GLUT4 translocation by activation of PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J Cell Physiol. 2008;215:733–42. doi: 10.1002/jcp.21353. [DOI] [PubMed] [Google Scholar]
- 20.Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab. 2007;293:E286–92. doi: 10.1152/ajpendo.00693.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–82. doi: 10.1016/j.bbrc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Kohan AB, Talukdar I, Walsh CM, Salati LM. A role for AMPK in the inhibition of glucose-6-phosphate dehydrogenase by polyunsaturated fatty acids. Biochem Biophys Res Commun. 2009;388:117–21. doi: 10.1016/j.bbrc.2009.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lage R, Vázquez MJ, Varela L, Saha AK, Vidal-Puig A, Nogueiras R, Diéguez C, López M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010;24:2670–9. doi: 10.1096/fj.09-150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei B, Matsuo K, Labinskyy V, Sharma N, Chandler MP, Ahn A, Hintze TH, Stanley WC, Recchia FA. Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo. Proc Natl Acad Sci U S A. 2005;102:6966–71. doi: 10.1073/pnas.0500768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Hu X, Selvakumar P, Russell RR, 3rd, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–41. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Gauthier MS, Sun L, Ruderman N, Lodish H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 2010;24:4229–39. doi: 10.1096/fj.10-159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, Glatz JF. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448:1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- 28.MacLean PS, Zheng D, Jones JP, Olson AL, Dohm GL. Exercise-induced transcription of the muscle glucose transporter (GLUT 4) gene. Biochem Biophys Res Commun. 2002;292:409–14. doi: 10.1006/bbrc.2002.6654. [DOI] [PubMed] [Google Scholar]
- 29.Natsuizaka M, Ozasa M, Darmanin S, Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W, Koide H, et al. Synergistic up-regulation of Hexokinase-2, glucose transporters and angiogenic factors in pancreatic cancer cells by glucose deprivation and hypoxia. Exp Cell Res. 2007;313:3337–48. doi: 10.1016/j.yexcr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol (1985) 2000;88:1072–5. doi: 10.1152/jappl.2000.88.3.1072. [DOI] [PubMed] [Google Scholar]
- 31.Osawa Y, Seki E, Kodama Y, Suetsugu A, Miura K, Adachi M, Ito H, Shiratori Y, Banno Y, Olefsky JM, et al. Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression. FASEB J. 2011;25:1133–44. doi: 10.1096/fj.10-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota S, Horigome K, Ishii T, Nakai M, Hayashi K, Kawamura T, Kishino A, Taiji M, Kimura T. Metformin suppresses glucose-6-phosphatase expression by a complex I inhibition and AMPK activation-independent mechanism. Biochem Biophys Res Commun. 2009;388:311–6. doi: 10.1016/j.bbrc.2009.07.164. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Scheffler TL, Gunawan AM, Shi H, Zeng C, Hannon KM, Grant AL, Gerrard DE. Chronic elevated calcium blocks AMPK-induced GLUT-4 expression in skeletal muscle. Am J Physiol Cell Physiol. 2009;296:C106–15. doi: 10.1152/ajpcell.00114.2008. [DOI] [PubMed] [Google Scholar]
- 34.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–91. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol (1985) 2000;88:2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 36.Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol (1985) 2001;91:1073–83. doi: 10.1152/jappl.2001.91.3.1073. [DOI] [PubMed] [Google Scholar]
- 37.Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun. 2010;398:178–83. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 38.Nagendran J, Waller TJ, Dyck JR. AMPK signalling and the control of substrate use in the heart. Mol Cell Endocrinol. 2013;366:180–93. doi: 10.1016/j.mce.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Lantier L, Mounier R, Leclerc J, Pende M, Foretz M, Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 2010;24:3555–61. doi: 10.1096/fj.10-155994. [DOI] [PubMed] [Google Scholar]
- 40.Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB J. 2009;23:2264–73. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- 41.Summermatter S, Mainieri D, Russell AP, Seydoux J, Montani JP, Buchala A, Solinas G, Dulloo AG. Thrifty metabolism that favors fat storage after caloric restriction: a role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J. 2008;22:774–85. doi: 10.1096/fj.07-8972com. [DOI] [PubMed] [Google Scholar]
- 42.Caretti A, Bianciardi P, Sala G, Terruzzi C, Lucchina F, Samaja M. Supplementation of creatine and ribose prevents apoptosis in ischemic cardiomyocytes. Cell Physiol Biochem. 2010;26:831–8. doi: 10.1159/000323992. [DOI] [PubMed] [Google Scholar]
- 43.Föller M, Sopjani M, Koka S, Gu S, Mahmud H, Wang K, Floride E, Schleicher E, Schulz E, Münzel T, et al. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 2009;23:1072–80. doi: 10.1096/fj.08-121772. [DOI] [PubMed] [Google Scholar]
- 44.Lang F, Gulbins E, Lang PA, Zappulla D, Föller M. Ceramide in suicidal death of erythrocytes. Cell Physiol Biochem. 2010;26:21–8. doi: 10.1159/000315102. [DOI] [PubMed] [Google Scholar]
- 45.Yang D, Yaguchi T, Nakano T, Nishizaki T. Adenosine activates AMPK to phosphorylate Bcl-XL responsible for mitochondrial damage and DIABLO release in HuH-7 cells. Cell Physiol Biochem. 2011;27:71–8. doi: 10.1159/000325207. [DOI] [PubMed] [Google Scholar]
- 46.Theodoropoulou S, Kolovou PE, Morizane Y, Kayama M, Nicolaou F, Miller JW, Gragoudas E, Ksander BR, Vavvas DG. Retinoblastoma cells are inhibited by aminoimidazole carboxamide ribonucleotide (AICAR) partially through activation of AMP-dependent kinase. FASEB J. 2010;24:2620–30. doi: 10.1096/fj.09-152546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem. 2011;27:681–90. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]
- 48.Bae HB, Zmijewski JW, Deshane JS, Tadie JM, Chaplin DD, Takashima S, Abraham E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011;25:4358–68. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-β peptide degradation. FASEB J. 2011;25:219–31. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen MN, Rasmussen HB. AMPK: A regulator of ion channels. Commun Integr Biol. 2012;5:480–4. doi: 10.4161/cib.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pastor-Soler NM, Hallows KR. AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens. 2012;21:523–33. doi: 10.1097/MNH.0b013e3283562390. [DOI] [PubMed] [Google Scholar]
- 53.Albert AP, Woollhead AM, Mace OJ, Baines DL. AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2008;295:L837–48. doi: 10.1152/ajplung.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alesutan I, Munoz C, Sopjani M, Dërmaku-Sopjani M, Michael D, Fraser S, Kemp BE, Seebohm G, Föller M, Lang F. Inhibition of Kir2.1 (KCNJ2) by the AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;408:505–10. doi: 10.1016/j.bbrc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Alesutan I, Sopjani M, Munoz C, Fraser S, Kemp BE, Föller M, Lang F. Inhibition of connexin 26 by the AMP-activated protein kinase. J Membr Biol. 2011;240:151–8. doi: 10.1007/s00232-011-9353-y. [DOI] [PubMed] [Google Scholar]
- 56.Alesutan I, Föller M, Sopjani M, Dërmaku-Sopjani M, Zelenak C, Fröhlich H, Velic A, Fraser S, Kemp BE, Seebohm G, et al. Inhibition of the heterotetrameric K+ channel KCNQ1/KCNE1 by the AMP-activated protein kinase. Mol Membr Biol. 2011;28:79–89. doi: 10.3109/09687688.2010.520037. [DOI] [PubMed] [Google Scholar]
- 57.Almaça J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K. AMPK controls epithelial Na(+) channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch. 2009;458:713–21. doi: 10.1007/s00424-009-0660-4. [DOI] [PubMed] [Google Scholar]
- 58.Alzamora R, Gong F, Rondanino C, Lee JK, Smolak C, Pastor-Soler NM, Hallows KR. AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299:F1308–19. doi: 10.1152/ajprenal.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen MN, Krzystanek K, Jespersen T, Olesen SP, Rasmussen HB. AMP-activated protein kinase downregulates Kv7.1 cell surface expression. Traffic. 2012;13:143–56. doi: 10.1111/j.1600-0854.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 60.Dallas ML, Scragg JL, Peers C. Inhibition of L-type Ca(2+) channels by carbon monoxide. Adv Exp Med Biol. 2009;648:89–95. doi: 10.1007/978-90-481-2259-2_10. [DOI] [PubMed] [Google Scholar]
- 61.Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, et al. Ion channel regulation by AMPK: the route of hypoxia-response coupling in thecarotid body and pulmonary artery. Ann N Y Acad Sci. 2009;1177:89–100. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- 62.Hirschler-Laszkiewicz I, Tong Q, Waybill K, Conrad K, Keefer K, Zhang W, Chen SJ, Cheung JY, Miller BA. The transient receptor potential (TRP) channel TRPC3 TRP domain and AMP-activated protein kinase binding site are required for TRPC3 activation by erythropoietin. J Biol Chem. 2011;286:30636–46. doi: 10.1074/jbc.M111.238360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mace OJ, Woollhead AM, Baines DL. AICAR activates AMPK and alters PIP2 association with the epithelial sodium channel ENaC to inhibit Na+ transport in H441 lung epithelial cells. J Physiol. 2008;586:4541–57. doi: 10.1113/jphysiol.2008.158253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Myerburg MM, King JD, Jr., Oyster NM, Fitch AC, Magill A, Baty CJ, Watkins SC, Kolls JK, Pilewski JM, Hallows KR. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–84. doi: 10.1165/2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sidani S, Kopic S, Socrates T, Kirchhoff P, Föller M, Murek M, Capasso A, Geibel JP. AMP-activated protein kinase: a physiological off switch for murine gastric acid secretion. Pflugers Arch. 2009;459:39–46. doi: 10.1007/s00424-009-0698-3. [DOI] [PubMed] [Google Scholar]
- 66.Tang C, To WK, Meng F, Wang Y, Gu Y. A role for receptor-operated Ca2+ entry in human pulmonary artery smooth muscle cells in response to hypoxia. Physiol Res. 2010;59:909–18. doi: 10.33549/physiolres.931875. [DOI] [PubMed] [Google Scholar]
- 67.Turrell HE, Rodrigo GC, Norman RI, Dickens M, Standen NB. Phenylephrine preconditioning involves modulation of cardiac sarcolemmal K(ATP) current by PKC delta, AMPK and p38 MAPK. J Mol Cell Cardiol. 2011;51:370–80. doi: 10.1016/j.yjmcc.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Kopic S, Corradini S, Sidani S, Murek M, Vardanyan A, Föller M, Ritter M, Geibel JP. Ethanol inhibits gastric acid secretion in rats through increased AMP-kinase activity. Cell Physiol Biochem. 2010;25:195–202. doi: 10.1159/000276553. [DOI] [PubMed] [Google Scholar]
- 69.Dallas ML, Scragg JL, Wyatt CN, Ross F, Hardie DG, Evans AM, Peers C. Modulation of O(2) sensitive K (+) channels by AMP-activated protein kinase. Adv Exp Med Biol. 2009;648:57–63. doi: 10.1007/978-90-481-2259-2_6. [DOI] [PubMed] [Google Scholar]
- 70.Ross FA, Rafferty JN, Dallas ML, Ogunbayo O, Ikematsu N, McClafferty H, Tian L, Widmer H, Rowe IC, Wyatt CN, et al. Selective expression in carotid body type I cells of a single splice variant of the large conductance calcium- and voltage-activated potassium channel confers regulation by AMP-activated protein kinase. J Biol Chem. 2011;286:11929–36. doi: 10.1074/jbc.M110.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kréneisz O, Benoit JP, Bayliss DA, Mulkey DK. AMP-activated protein kinase inhibits TREK channels. J Physiol. 2009;587:5819–30. doi: 10.1113/jphysiol.2009.180372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida H, Bao L, Kefaloyianni E, Taskin E, Okorie U, Hong M, Dhar-Chowdhury P, Kaneko M, Coetzee WA. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J Mol Cell Cardiol. 2012;52:410–8. doi: 10.1016/j.yjmcc.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein H, Garneau L, Trinh NT, Privé A, Dionne F, Goupil E, Thuringer D, Parent L, Brochiero E, Sauvé R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am J Physiol Cell Physiol. 2009;296:C285–95. doi: 10.1152/ajpcell.00418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mia S, Munoz C, Pakladok T, Siraskar G, Voelkl J, Alesutan I, Lang F. Downregulation of Kv1.5 K channels by the AMP-activated protein kinase. Cell Physiol Biochem. 2012;30:1039–50. doi: 10.1159/000341480. [DOI] [PubMed] [Google Scholar]
- 75.Almilaji A, Munoz C, Elvira B, Fajol A, Pakladok T, Honisch S, Shumilina E, Lang F, Föller M. AMP-activated protein kinase regulates hERG potassium channel. Pflugers Arch. 2013;465:1573–82. doi: 10.1007/s00424-013-1299-8. [DOI] [PubMed] [Google Scholar]
- 76.Ikematsu N, Dallas ML, Ross FA, Lewis RW, Rafferty JN, David JA, Suman R, Peers C, Hardie DG, Evans AM. Phosphorylation of the voltage-gated potassium channel Kv2.1 by AMP-activated protein kinase regulates membrane excitability. Proc Natl Acad Sci U S A. 2011;108:18132–7. doi: 10.1073/pnas.1106201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siraskar B, Huang DY, Pakladok T, Siraskar G, Sopjani M, Alesutan I, Kucherenko Y, Almilaji A, Devanathan V, Shumilina E, et al. Downregulation of the renal outer medullary K(+) channel ROMK by the AMP-activated protein kinase. Pflugers Arch. 2013;465:233–45. doi: 10.1007/s00424-012-1180-1. [DOI] [PubMed] [Google Scholar]
- 78.Wang CZ, Wang Y, Di A, Magnuson MA, Ye H, Roe MW, Nelson DJ, Bell GI, Philipson LH. 5-amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem Biophys Res Commun. 2005;330:1073–9. doi: 10.1016/j.bbrc.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 79.Chang TJ, Chen WP, Yang C, Lu PH, Liang YC, Su MJ, Lee SC, Chuang LM. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazone-induced closure of the K(ATP) channel and insulin secretion in rats. Diabetologia. 2009;52:1112–21. doi: 10.1007/s00125-009-1337-4. [DOI] [PubMed] [Google Scholar]
- 80.Lim A, Park SH, Sohn JW, Jeon JH, Park JH, Song DK, Lee SH, Ho WK. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic beta-cells. Diabetes. 2009;58:2813–9. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Föller M, Jaumann M, Dettling J, Saxena A, Pakladok T, Munoz C, Ruth P, Sopjani M, Seebohm G, Rüttiger L, et al. AMP-activated protein kinase in BK-channel regulation and protection against hearing loss following acoustic overstimulation. FASEB J. 2012;26:4243–53. doi: 10.1096/fj.12-208132. [DOI] [PubMed] [Google Scholar]
- 82.Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007;282:8092–8. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang F, Rehwald W. Potassium channels in renal epithelial transport regulation. Physiol Rev. 1992;72:1–32. doi: 10.1152/physrev.1992.72.1.1. [DOI] [PubMed] [Google Scholar]
- 84.Lang F, Messner G, Rehwald W. Electrophysiology of sodium-coupled transport in proximal renal tubules. Am J Physiol. 1986;250:F953–62. doi: 10.1152/ajprenal.1986.250.6.F953. [DOI] [PubMed] [Google Scholar]
- 85.Boiteux A, Hess B. Design of glycolysis. Philos Trans R Soc Lond B Biol Sci. 1981;293:5–22. doi: 10.1098/rstb.1981.0056. [DOI] [PubMed] [Google Scholar]
- 86.Schmid E, Bhandaru M, Nurbaeva MK, Yang W, Szteyn K, Russo A, Leibrock C, Tyan L, Pearce D, Shumilina E, et al. SGK3 regulates Ca(2+) entry and migration of dendritic cells. Cell Physiol Biochem. 2012;30:1423–35. doi: 10.1159/000343330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turner RW, Anderson D, Zamponi GW. Signaling complexes of voltage-gated calcium channels. Channels (Austin) 2011;5:440–8. doi: 10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen PC, Kryukova YN, Shyng SL. Leptin Regulates KATP Channel Trafficking in Pancreatic beta-cells by a Signaling Mechanism Involving AMPK and PKA. J Biol Chem. 2013 Forthcoming doi: 10.1074/jbc.M113.516880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng R, Nie A, Jian F, Liu Y, Tang H, Zhang J, Zhang Y, Shao L, Li F, Zhou L, et al. Acute exposure of beta-cells to troglitazone decreases insulin hypersecretion via activating AMPK. Biochim Biophys Acta. 2014;1840:577–85. doi: 10.1016/j.bbagen.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 90.Park SH, Ho WK, Jeon JH. AMPK regulates K(ATP) channel trafficking via PTEN inhibition in leptin-treated pancreatic β-cells. Biochem Biophys Res Commun. 2013;440:539–44. doi: 10.1016/j.bbrc.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 91.Evans AM, Peers C, Wyatt CN, Kumar P, Hardie DG. Ion channel regulation by the LKB1-AMPK signalling pathway: the key to carotid body activation by hypoxia and metabolic homeostasis at the whole body level. Adv Exp Med Biol. 2012;758:81–90. doi: 10.1007/978-94-007-4584-1_11. [DOI] [PubMed] [Google Scholar]
- 92.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–55. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105:1711–21. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.King JD, Jr., Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol. 2009;297:C94–101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kongsuphol P, Cassidy D, Hieke B, Treharne KJ, Schreiber R, Mehta A, Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J Biol Chem. 2009;284:5645–53. doi: 10.1074/jbc.M806780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kunzelmann K, Mehta A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+ FEBS J. 2013;280:4417–29. doi: 10.1111/febs.12457. [DOI] [PubMed] [Google Scholar]
- 97.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–5. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 98.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2006;281:26159–69. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- 99.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005;280:17608–16. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- 100.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–96. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 101.Drüppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstädt H, Oberleithner H, Kliche K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–9. doi: 10.1096/fj.13-228312. [DOI] [PubMed] [Google Scholar]
- 102.Lang F. Stiff endothelial cell syndrome in vascular inflammation and mineralocorticoid excess. Hypertension. 2011;57:146–7. doi: 10.1161/HYPERTENSIONAHA.110.164558. [DOI] [PubMed] [Google Scholar]
- 103.Ahn YJ, Kim H, Lim H, Lee M, Kang Y, Moon S, Kim HS, Kim HH. AMP-activated protein kinase: implications on ischemic diseases. BMB reports. 2012;45:489–95. doi: 10.5483/bmbrep.2012.45.9.169. [DOI] [PubMed] [Google Scholar]
- 104.Eylenstein A, Gehring EM, Heise N, Shumilina E, Schmidt S, Szteyn K, Münzer P, Nurbaeva MK, Eichenmüller M, Tyan L, et al. Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1) FASEB J. 2011;25:2012–21. doi: 10.1096/fj.10-178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nurbaeva MK, Schmid E, Szteyn K, Yang W, Viollet B, Shumilina E, Lang F. Enhanced Ca²⁺ entry and Na+/Ca²⁺ exchanger activity in dendritic cells from AMP-activated protein kinase-deficient mice. FASEB J. 2012;26:3049–58. doi: 10.1096/fj.12-204024. [DOI] [PubMed] [Google Scholar]
- 106.Lang F, Eylenstein A, Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell Calcium. 2012;52:347–54. doi: 10.1016/j.ceca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Bhavsar SK, Schmidt S, Bobbala D, Nurbaeva MK, Hosseinzadeh Z, Merches K, Fajol A, Wilmes J, Lang F. AMPKα1-sensitivity of Orai1 and Ca(2+) entry in T - lymphocytes. Cell Physiol Biochem. 2013;32:687–98. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 108.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–74. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Lai YC, Hill EV, Tyteca D, Carpentier S, Ingvaldsen A, Vertommen D, Lantier L, Foretz M, Dequiedt F, et al. Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem J. 2013;455:195–206. doi: 10.1042/BJ20130644. [DOI] [PubMed] [Google Scholar]
- 112.Friedrichsen M, Mortensen B, Pehmøller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013;366:204–14. doi: 10.1016/j.mce.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Oliveira RL, Ueno M, de Souza CT, Pereira-da-Silva M, Gasparetti AL, Bezzera RM, Alberici LC, Vercesi AE, Saad MJ, Velloso LA. Cold-induced PGC-1alpha expression modulates muscle glucose uptake through an insulin receptor/Akt-independent, AMPK-dependent pathway. Am J Physiol Endocrinol Metab. 2004;287:E686–95. doi: 10.1152/ajpendo.00103.2004. [DOI] [PubMed] [Google Scholar]
- 114.Sopjani M, Bhavsar SK, Fraser S, Kemp BE, Föller M, Lang F. Regulation of Na+-coupled glucose carrier SGLT1 by AMP-activated protein kinase. Mol Membr Biol. 2010;27:137–44. doi: 10.3109/09687681003616870. [DOI] [PubMed] [Google Scholar]
- 115.Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res. 2004;12:862–70. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- 116.Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35:965–73. doi: 10.1002/bies.201300084. [DOI] [PubMed] [Google Scholar]
- 117.Rotte A, Pasham V, Eichenmüller M, Bhandaru M, Föller M, Lang F. Upregulation of Na+/H+ exchanger by the AMP-activated protein kinase. Biochem Biophys Res Commun. 2010;398:677–82. doi: 10.1016/j.bbrc.2010.06.135. [DOI] [PubMed] [Google Scholar]
- 118.Takimoto M, Takeyama M, Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metabolism. 2013;62:1633–40. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 119.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008;10:311–21. doi: 10.1208/s12248-008-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dërmaku-Sopjani M, Almilaji A, Pakladok T, Munoz C, Hosseinzadeh Z, Blecua M, Sopjani M, Lang F, Lang F. Down-Regulation of the Na-Coupled Phosphate Transporter NaPi-IIa by AMP-Activated Protein Kinase. Kidney Blood Press Res. 2013;37:547–56. doi: 10.1159/000355735. [DOI] [PubMed] [Google Scholar]
- 121.Li H, Thali RF, Smolak C, Gong F, Alzamora R, Wallimann T, Scholz R, Pastor-Soler NM, Neumann D, Hallows KR. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am J Physiol Renal Physiol. 2010;299:F167–77. doi: 10.1152/ajprenal.00162.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Munoz C, Sopjani M, Dërmaku-Sopjani M, Almilaji A, Föller M, Lang F. Downregulation of the osmolyte transporters SMIT and BGT1 by AMP-activated protein kinase. Biochem Biophys Res Commun. 2012;422:358–62. doi: 10.1016/j.bbrc.2012.04.092. [DOI] [PubMed] [Google Scholar]
- 123.Sopjani M, Alesutan I, Dërmaku-Sopjani M, Fraser S, Kemp BE, Föller M, Lang F. Down-regulation of Na+-coupled glutamate transporter EAAT3 and EAAT4 by AMP-activated protein kinase. J Neurochem. 2010;113:1426–35. doi: 10.1111/j.1471-4159.2010.06678.x. [DOI] [PubMed] [Google Scholar]
- 124.Pieri M, Christian HC, Wilkins RJ, Boyd CA, Meredith D. The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. Am J Physiol Gastrointest Liver Physiol. 2010;299:G136–43. doi: 10.1152/ajpgi.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alzamora R, Al-Bataineh MM, Liu W, Gong F, Li H, Thali RF, Joho-Auchli Y, Brunisholz RA, Satlin LM, Neumann D, et al. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. Am J Physiol Renal Physiol. 2013;305:F943–56. doi: 10.1152/ajprenal.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol. 2010;298:F1162–9. doi: 10.1152/ajprenal.00645.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol. 2009;296:C672–81. doi: 10.1152/ajpcell.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol. 2011;31:3546–56. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan CD, Smolenski RT, Harhun MI, Patel HK, Ahmed SG, Wanisch K, Yáñez-Muñoz RJ, Baines DL. AMP-activated protein kinase (AMPK)-dependent and -independent pathways regulate hypoxic inhibition of transepithelial Na+ transport across human airway epithelial cells. Br J Pharmacol. 2012;167:368–82. doi: 10.1111/j.1476-5381.2012.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Benziane B, Björnholm M, Pirkmajer S, Austin RL, Kotova O, Viollet B, Zierath JR, Chibalin AV. Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. J Biol Chem. 2012;287:23451–63. doi: 10.1074/jbc.M111.331926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflugers Arch. 2011;461:1–21. doi: 10.1007/s00424-010-0893-2. [DOI] [PubMed] [Google Scholar]
- 132.Embark HM, Böhmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, Capasso G, Waldegger P, Seyberth HW, Waldegger S, et al. Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4-2 and the serum- and glucocorticoid-dependent kinases. Kidney Int. 2004;66:1918–25. doi: 10.1111/j.1523-1755.2004.00966.x. [DOI] [PubMed] [Google Scholar]
- 133.Palmada M, Dieter M, Boehmer C, Waldegger S, Lang F. Serum and glucocorticoid inducible kinases functionally regulate ClC-2 channels. Biochem Biophys Res Commun. 2004;321:1001–6. doi: 10.1016/j.bbrc.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 134.Baltaev R, Strutz-Seebohm N, Korniychuk G, Myssina S, Lang F, Seebohm G. Regulation of cardiac shal-related potassium channel Kv 4.3 by serum- and glucocorticoid-inducible kinase isoforms in Xenopus oocytes. Pflugers Arch. 2005;450:26–33. doi: 10.1007/s00424-004-1369-z. [DOI] [PubMed] [Google Scholar]
- 135.Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97:911–21. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- 136.Rajamanickam J, Palmada M, Lang F, Boehmer C. EAAT4 phosphorylation at the SGK1 consensus site is required for transport modulation by the kinase. J Neurochem. 2007;102:858–66. doi: 10.1111/j.1471-4159.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 137.Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 138.Pehmøller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–75. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seebohm G, Neumann S, Theiss C, Novkovic T, Hill EV, Tavaré JM, Lang F, Hollmann M, Manahan-Vaughan D, Strutz-Seebohm N. Identification of a novel signaling pathway and its relevance for GluA1 recycling. PLoS One. 2012;7:e33889. doi: 10.1371/journal.pone.0033889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pakladok T, Almilaji A, Munoz C, Alesutan I, Lang F. PIKfyve sensitivity of hERG channels. Cell Physiol Biochem. 2013;31:785–94. doi: 10.1159/000350096. [DOI] [PubMed] [Google Scholar]
- 141.Munoz C, Almilaji A, Setiawan I, Föller M, Lang F. Up-regulation of the inwardly rectifying K⁺ channel Kir2.1 (KCNJ2) by protein kinase B (PKB/Akt) and PIKfyve. J Membr Biol. 2013;246:189–97. doi: 10.1007/s00232-012-9520-9. [DOI] [PubMed] [Google Scholar]
- 142.Seebohm G, Strutz-Seebohm N, Ursu ON, Preisig-Müller R, Zuzarte M, Hill EV, Kienitz MC, Bendahhou S, Fauler M, Tapken D, et al. Altered stress stimulation of inward rectifier potassium channels in Andersen-Tawil syndrome. FASEB J. 2012;26:513–22. doi: 10.1096/fj.11-189126. [DOI] [PubMed] [Google Scholar]
- 143.Sopjani M, Kunert A, Czarkowski K, Klaus F, Laufer J, Föller M, Lang F. Regulation of the Ca(2+) channel TRPV6 by the kinases SGK1, PKB/Akt, and PIKfyve. J Membr Biol. 2010;233:35–41. doi: 10.1007/s00232-009-9222-0. [DOI] [PubMed] [Google Scholar]
- 144.Klaus F, Laufer J, Czarkowski K, Strutz-Seebohm N, Seebohm G, Lang F. PIKfyve-dependent regulation of the Cl- channel ClC-2. Biochem Biophys Res Commun. 2009;381:407–11. doi: 10.1016/j.bbrc.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 145.Shojaiefard M, Strutz-Seebohm N, Tavaré JM, Seebohm G, Lang F. Regulation of the Na(+), glucose cotransporter by PIKfyve and the serum and glucocorticoid inducible kinase SGK1. Biochem Biophys Res Commun. 2007;359:843–7. doi: 10.1016/j.bbrc.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 146.Strutz-Seebohm N, Shojaiefard M, Christie D, Tavare J, Seebohm G, Lang F. PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cell Physiol Biochem. 2007;20:729–34. doi: 10.1159/000110433. [DOI] [PubMed] [Google Scholar]
- 147.Bogatikov E, Munoz C, Pakladok T, Alesutan I, Shojaiefard M, Seebohm G, Föller M, Palmada M, Böhmer C, Bröer S, et al. Up-regulation of amino acid transporter SLC6A19 activity and surface protein abundance by PKB/Akt and PIKfyve. Cell Physiol Biochem. 2012;30:1538–46. doi: 10.1159/000343341. [DOI] [PubMed] [Google Scholar]
- 148.Gehring EM, Zurn A, Klaus F, Laufer J, Sopjani M, Lindner R, Strutz-Seebohm N, Tavaré JM, Boehmer C, Palmada M, et al. Regulation of the glutamate transporter EAAT2 by PIKfyve. Cell Physiol Biochem. 2009;24:361–8. doi: 10.1159/000257428. [DOI] [PubMed] [Google Scholar]
- 149.Klaus F, Gehring EM, Zürn A, Laufer J, Lindner R, Strutz-Seebohm N, Tavaré JM, Rothstein JD, Boehmer C, Palmada M, et al. Regulation of the Na(+)-coupled glutamate transporter EAAT3 by PIKfyve. Neurochem Int. 2009;54:372–7. doi: 10.1016/j.neuint.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 150.Alesutan IS, Ureche ON, Laufer J, Klaus F, Zürn A, Lindner R, Strutz-Seebohm N, Tavaré JM, Boehmer C, Palmada M, et al. Regulation of the glutamate transporter EAAT4 by PIKfyve. Cell Physiol Biochem. 2010;25:187–94. doi: 10.1159/000276569. [DOI] [PubMed] [Google Scholar]
- 151.Bess E, Fisslthaler B, Frömel T, Fleming I. Nitric oxide-induced activation of the AMP-activated protein kinase α2 subunit attenuates IκB kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6:e20848. doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han Q, Zhang X, Xue R, Yang H, Zhou Y, Kong X, Zhao P, Li J, Yang J, Zhu Y, et al. AMPK potentiates hypertonicity-induced apoptosis by suppressing NFκB/COX-2 in medullary interstitial cells. J Am Soc Nephrol. 2011;22:1897–911. doi: 10.1681/ASN.2010080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borst O, Schmidt EM, Münzer P, Schönberger T, Towhid ST, Elvers M, Leibrock C, Schmid E, Eylenstein A, Kuhl D, et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood. 2012;119:251–61. doi: 10.1182/blood-2011-06-359976. [DOI] [PubMed] [Google Scholar]
- 154.Eylenstein A, Schmidt S, Gu S, Yang W, Schmid E, Schmidt EM, Alesutan I, Szteyn K, Regel I, Shumilina E, et al. Transcription factor NF-κB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J Biol Chem. 2012;287:2719–30. doi: 10.1074/jbc.M111.275925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Visentin S, Renzi M, Levi G. Altered outward-rectifying K(+) current reveals microglial activation induced by HIV-1 Tat protein. Glia. 2001;33:181–90. doi: 10.1002/1098-1136(200103)33:3<181::AID-GLIA1017>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 156.Song Y, Wang Q, Huang W, Xiao L, Shen L, Xu W. NF kappaB expression increases and CFTR and MUC1 expression decreases in the endometrium of infertile patients with hydrosalpinx: a comparative study. Reprod Biol Endocrinol. 2012;10:86. doi: 10.1186/1477-7827-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hunter MJ, Treharne KJ, Winter AK, Cassidy DM, Land S, Mehta A. Expression of wild-type CFTR suppresses NF-kappaB-driven inflammatory signalling. PLoS One. 2010;5:e11598. doi: 10.1371/journal.pone.0011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de Seigneux S, Leroy V, Ghzili H, Rousselot M, Nielsen S, Rossier BC, Martin PY, Féraille E. NF-kappaB inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem. 2008;283:25671–81. doi: 10.1074/jbc.M803812200. [DOI] [PubMed] [Google Scholar]
- 159.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2012;303:F1617–28. doi: 10.1152/ajprenal.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Viñuales C, Gascón S, Barranquero C, Osada J, Rodríguez-Yoldi MJ. Inhibitory effect of IL-1β on galactose intestinal absorption in rabbits. Cell Physiol Biochem. 2012;30:173–86. doi: 10.1159/000339056. [DOI] [PubMed] [Google Scholar]