Abstract
The E. coli mechanosensitive (MS) channel of small conductance (EcMscS) is the prototype of a diverse family of channels present in all domains of life. While EcMscS has been extensively studied, recent developments show that MscS may display some characteristics not widely conserved in this protein subfamily. With numerous members now electrophysiologically characterized, this subfamily of channels displays a breadth of ion selectivity with both anion and cation selective members. The selectivity of these channels may be relatively weak in comparison to voltage-gated channels but their selectivity mechanisms represent great novelty. Recent studies have identified unexpected residues important for selectivity in these homologs revealing different selectivity mechanisms than those employed by voltage gated K+, Na+, Ca2+ and Cl- channels whose selectivity filters are housed within their transmembrane pores. This commentary looks at what is currently known about MscS subfamily selectivity and begins to unravel the potential physiological relevance of these differences.
Keywords: Escherichia coli, selectivity, mechanosensitive, patch clamp, MscS-like
Introduction
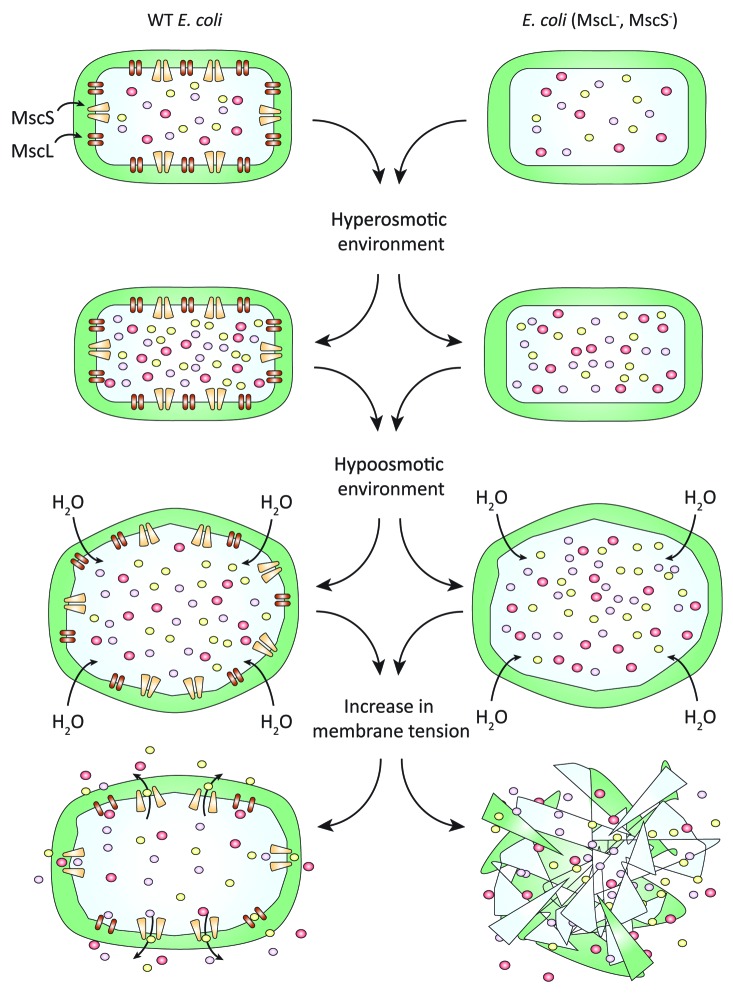
The E. coli mechanosensitive channel of small conductance (EcMscS) is often thought of as the archetypal member of the MscS channel subfamily. Characteristic stretch-activated activity of this family of channels was indeed the first report of bacterial mechanosensitive channels more than 25 y ago.1 This diverse family can be broadly subdivided into 2 groups; the smaller proteins such as EcMscS (ca. 250–500 aa) and the larger proteins such as MscK (ca. 500–1000+ aa).2-4 These channels, in the main, play an integral role in osmoprotection acting as emergency release valves in the face of large reductions in environmental osmolarity (Fig. 1).3,5 However, due to the seeming ‘redundancy’ of some paralogues, alternative, as yet undiscovered, physiological functions must be performed by these channels (see Malcolm et al., 2012 for review).6 Examples of electrophysiologically-characterized channels that potentially fulfill alternate or additional functions include MSC1 (Chlamydomonas reinhardtii), Msy1/2 (Saccharomyces pombe), and MSL10 (Arabidopsis thaliana).7-9 It is important to note that the seeming redundancy of some MscS-like channels may well be simply a function of their cellular expression levels and does not imply an inherent lack of mechanosensitivity or preclude roles in processes other than osmoprotection.10
Figure 1. Mechanosensitive channel function in E. coli. In response to a reduction in external osmolarity H2O floods into bacterial cells resulting in swelling and a corresponding rise in cellular turgor and membrane tension. This rise in membrane tension first gates MscS and then MscL (immediately below the lytic limit of the cell membrane) allowing the efflux of intracellular osmolytes thus relieving this pressure and preventing cellular lysis (left panel). In the absence of MS channels (MscS and MscL) this rise in membrane tension is left unchecked and results in cell lysis (right panel).
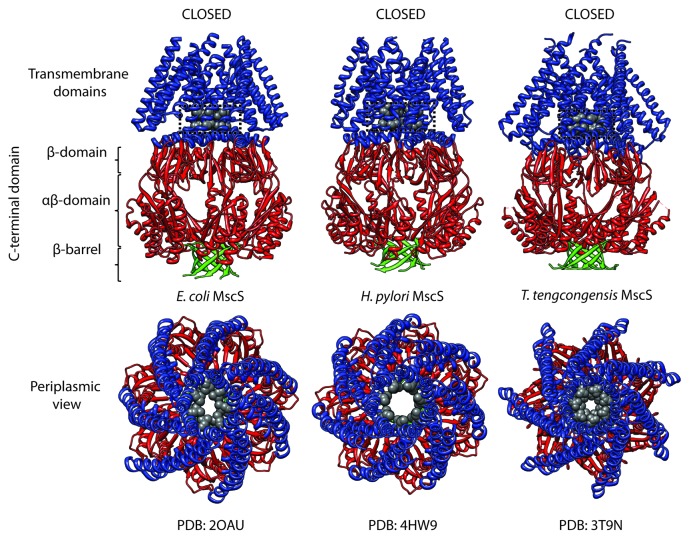
MscS family structure is characterized by multiple N-terminal transmembrane domains followed by a large water-filled C-terminal cytoplasmic vestibule.11,12 The number of N-terminal transmembrane helices varies greatly throughout the MscS family with channels like EcMscS and MscSP possessing 3 and larger proteins such as MscK likely possessing 11.13-15 The ubiquitous nature of the cytoplasmic domain is hinted at from hydropathy plots16-18 of MscS-like channels and is clearly illustrated in each of the 3 MscS homologs to have been crystallized thus far (Escherichia coli MscS, Helicobacter pylori MscS, and Thermoanerobacter tengcongensis MscS) (Fig. 2).19-21 In addition to this, all crystal structures we currently possess illustrate homoheptameric assembly. A notable feature of EcMscS is the seven lateral ~12 Å portals that perforate the cytoplasmic vestibule.11,12,22 Whether the vestibular perforations seen in E. coli MscS are a widely distributed structural characteristic of this family of channels is however unknown. This is further complicated by the fact that the C-terminal vestibule is an area in which large sequence insertions and/or deletions can be found in MscS homologs.2 As an example, these perforations seem to be greatly restricted and almost absent in an MscS-like homolog found in the anaerobic thermophile T. tengcongensis which leads to the possibility that larger conducting homologs possess these perforations, in various sizes, whereas they are largely constricted or absent in lower conducting homologs.20 In all likelihood, the presence of cytoplasmic perforations will impact greatly on both conductance and the location of the structural determinants of selectivity in MscS-like channels.
Figure 2. Structure of 3 crystallized MscS family members. Crystal structures of E. coli MscS (EcMscS), Helicobacter pylori MscS (HpMscS), and Thermoanaerobacter tengcongensis (TtMscS). Upper panel is a side view illustrating characteristic MscS family structure including a large water-filled C-terminal domain. The lower panel provides a periplasmic view of the respective channels with the residues colored in gray likely forming the vapor lock gates (EcMscS – L105 and L109, HpMscS – I94 and L98, TtMscS - L104, F108).
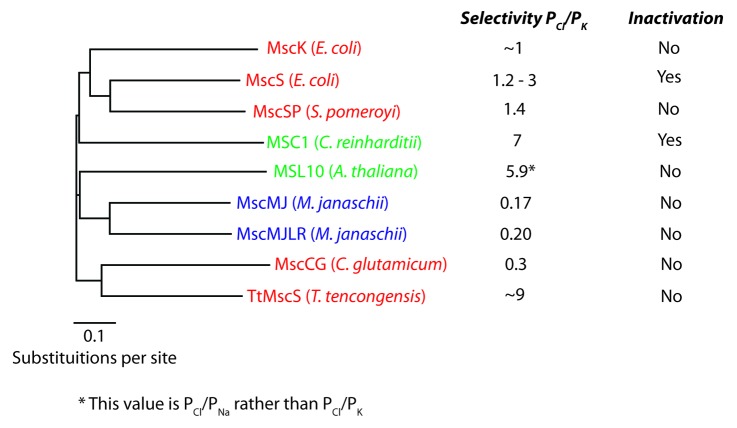
Many studies have focused on the role of the cytoplasmic vestibule in MscS channel function.23-25 In particular the possibility that the cytoplasmic vestibule is a dynamic structure which plays an active role in channel-gating.23,26,27 For example interactions between the amphiphilic TM3b helices and the roof of the cytoplasmic domain have been shown to be important in EcMscS adaptation/inactivation.26 Interestingly, inactivation in this channel family seems not to be wide spread with only 2 of the 9 currently characterized homologs exhibiting this attribute (Fig. 3.). Although, there is a recent report that suggests an MscS homolog in Vibrio cholerae may also exhibit inactivation.28 The suggestion that the cytoplasmic domain is involved in channel selectivity is not a new hypothesis29 and was strengthened greatly by the fact that mutations within the pore region of MscS do not affect its selectivity profile.30 In fact, looking at the putative pore-forming helices of all electrophysiologically-characterized MscS homologs shows they are devoid of any potential descriptors of ionic selectivity, being in the main hydrophobic.16 In addition, a molecular dynamics study also demonstrated the fact that the cytoplasmic domain may play an ion filtering role.25 Specific structural information about the cytoplasmic selectivity function of MscS-like channels has been provided by 2 recent publications. These have provided the first insights into the potential basis of ionic selectivity in MscS-like channels.16,20 By going beyond the current insight,31 this commentary aims to address what is currently known regarding MscS-like channel ionic selectivity with particular reference to the role of the C-terminal domain.
Figure 3. MscS subfamily phylogenetic tree displaying 9 electrophysiologically-characterized homologs. The reported anion-cation permeability ratios expressed as PCl/PK are illustrated along with whether these channels display inactivation. Bar represents 0.1 substitutions per site. (Green = archaea, Black = bacteria, Red = eukaryotes)
Reports of MscS selectivity date back to the very first description of mechanosensitive channels in bacteria and range between PCl/PK 1.2 - 3.30,32,33 Since that report 8 other members of this diverse family have been electrophysiologically characterized. Their selectivity ranges from a PCl/PK – 0.1 – 9 (Fig. 3). These anion-cation permeability ratios are, in the main, relatively modest especially when compared with chloride channels like the glycine receptor chloride channel (PCl/PNa = ~25)34 and cannot compare with the exquisite selectivity shown by other channels such as voltage-gated K+ channels (PCl = ~0).
In addition to these homologs, 4 other members of the MscS family from E. coli have been electrophysiologically characterized.17,35 These are the gene products of ybdG, ybiO, yjeP and ynaI. Initially thought to be a product of ybdG MscM activity is now thought to be related to the gene yjeP.17 This is due to the fact that MscM-like activity was still seen in a ΔybdG mutant and that MscM-like activity in spheroplasts overexpressing YbdG could only be identified via mutation (V229A).35 The selectivity is only known of an MscM-like channel identified by Berrier et al., 1996 (PCl/PK – 0.4)36 with the likely genetic origin of this activity being yjeP and not ybdG as previously suggested.10,17,31
Structural Determinants Of MscS-Like Channel Selectivity
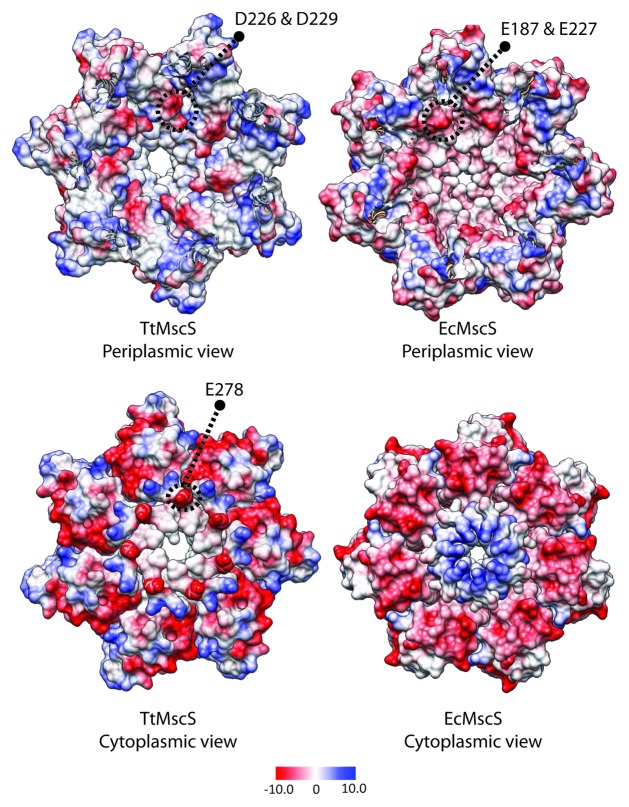
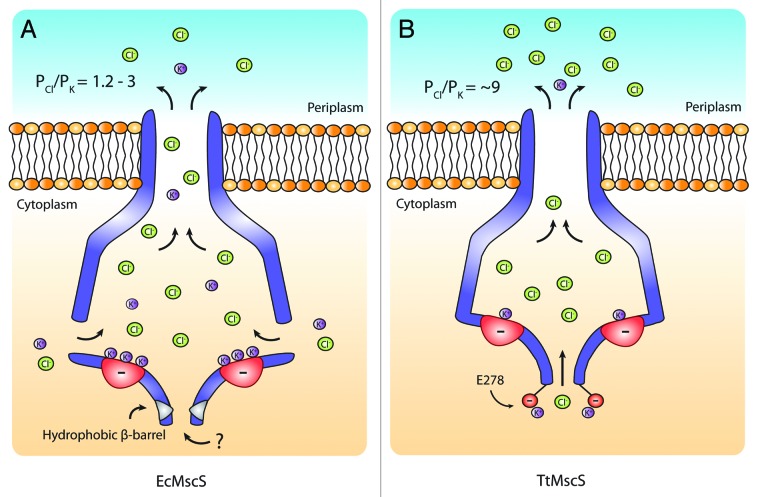
In E. coli MscS, 2 negatively charged residues (E187 and E227) situated in the cytoplasmic domain (Fig. 4, top right panel) in relatively close proximity to the lateral vestibular portals have been implicated in selectivity.16 This is proposed to occur via cation ‘trapping’ creating a more favorable path for anions (Fig. 5). This is supported by the larger transit times of cations through MscS compared with anions demonstrated in a molecular dynamic simulation study.25 In addition, Zhang et al., 201218 convincingly implicate a charged residue (E278) on the outside of the β-Barrel in the anion selectivity of an MscS homolog from T. tengcongensis. These authors suggest that this residue may also act by cation ‘trapping’ resulting in a more thermodynamically favorable path for the transit of anions (Fig. 4, bottom left panel, Fig. 5). This residue, however, is not well conserved throughout anion selective MscS homologs. In addition, neutralization of this charge (E278A) resulted in a mutant that still displayed anion selectivity. A possible explanation of such a result is that while E278 plays an integral role in the selectivity of TtMscS this is in addition to other charged residues within its cytoplasmic domain (Fig. 4, top left panel, Fig. 5).16 A region of high electronegativity is identifiable in the cytoplasmic domain of TtMscS (D226 and D229) akin to that seen in EcMscS (Fig. 4, top left and right panels). As a result the cytoplasmic domain in this MscS homolog may well ‘trap’ cations much in the same way as illustrated for EcMscS. Another interesting finding from this study relates to ion permeation via the β-barrel of E. coli MscS. A number of in silico studies utilizing molecular dynamics indicate that permeation via this narrow hydrophobic pore is unlikely.37 However, a chimera of the β-barrel region of E. coli MscS with the T. tengcongensis MscS protein creates a functional protein hinting that permeation may in fact be possible.20
Figure 4. Comparison between EcMscS and TtMscS cytoplasmic domains. Upper panel shows a Coulombic charge distribution of a transverse section as viewed from the periplasmic side of TtMscS (PDB: 3T9N; left side) and EcMscS (PDB: 2VV5; right side). Regions of electronegativity (denoted as red) can be identified centered around D226/D229 and E187/E227, respectively. The lower panel shows the underside of the cytoplasmic domain of TtMscS (right side) EcMscS (left side) highlighting the β-barrel residue implicated in anion selectivity in TtMscS (kcal/(mol·e) at 298 K).
Figure 5. Graphic illustration of the proposed selectivity mechanisms of E. coli MscS and T. tengcongensis MscS. (A) An electronegative region centered around E187 and E227 on the floor of the cytoplasmic domain ‘traps’ cations resulting in easier transit for anions. (B) A residue on the outside of the β-barrel ‘E278’ likely traps cations making an environment conducive to anion conduction. This is in addition to an electronegative region on the floor of the cytoplasmic domain that likely ‘traps’ permeating cations in a similar manner to EcMscS, which results in higher anion selectivity of TtMscS compared with EcMscS (Fig. 4).
These slightly different mechanisms may reveal an interesting feature of this family whereby selectivity mechanisms are unique to individual homologs. But a unifying fact is that the structural descriptors of ion selectivity are found in the cytoplasmic domain and not in the transmembrane pore region.
The MscS-like family contains a number of unique members one of the currently most intriguing being MscCG.38-40 This channel possesses an extra transmembrane domain after the large cytoplasmic vestibule and exhibits a slight cationic preference (PCl/PK – 0.3–0.8).39,41 This protein has been found to be responsible for glutamate efflux under certain conditions (i.e., penicillin or biotin treatment) and is utilized industrially for the production of glutamate.40,41 This raises the questions: (1) can the selectivity of this channel be modified to increase glutamate efflux, and (2) can the selectivity be modified to increase the efflux of other overproduced compounds? Intriguingly, the putative β-barrel structure of MscCG has a lysine at the equivalent position to M273 in EcMscS, which is a major constriction point in the β-barrel of EcMscS. It is possible that rearrangement of the cytoplasmic domain under certain conditions results in permeation via the β-barrel alone and this ring of lysines increases anion (glutamate) selectivity. A recent study indicates that a loop which forms part of the cytoplasmic cage between residues 221–232 containing 3 negatively charged residues (IAPEILGELDVH) is essential for glutamate efflux as ablation of this loop abolishes glutamate efflux.42 The exact role this loop plays in facilitating glutamate efflux is thus far unknown.
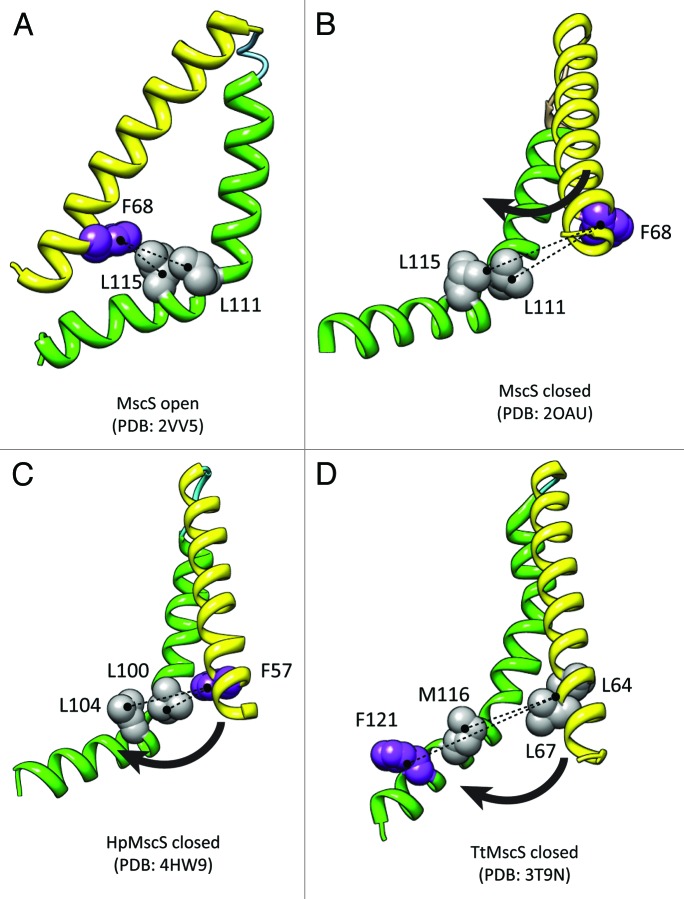
Another interesting development is the reporting of the crystal structure of the MscS homolog from Helicobacter pylori.43 The crystal structure illustrates a homoheptameric assembly of HpMscS thought to correspond to the channel closed state (Fig. 2). Many similar functional residues can be identified including a potentially conserved interaction with a phenylalanine (TM2) and two leucine residues (TM3) shown to be important in MscS mechanosensing43,44 (Fig. 6). This is in addition to hydrophobic residues which represent the channel gate (Figs. 2 and 6).45-47 This channel is yet to be electrophysiologically characterized but has conserved acidic residues in positions shown to be important for EcMscS anion selectivity, namely E187 and E227.
Figure 6. Conserved tension transmitting residues in EcMscS, HpMscS and TtMscS. (A) Open structure of EcMscS showing close association of F68 and L111/115. (B) Closed/inactivated structure of EcMscS showing dissociation of tension transmitting residues. (C) Illustration of similar position of a phenylalanine residue and leucine residues in closed structure of HpMscS. (D) In the closed structure of TtMscS the location of these residues may be switched. However, multiple alternative hydrophobic residues are present that may interact in a similar way to F68 and L111/115 in EcMscS.
MSL10, an anion selective (PCl/PNa – 5.9) MscS-like channel homolog expressed in the plant Arabidopsis thaliana shows little homology with EcMscS (< 5%) and displays vastly different gating kinetics.48 This makes structural comparisons from sequence alone difficult. There is at least no conservation of the residues proposed to be important for TtMscS and EcMscS selectivity, E278 and E187/E227 respectively. However, this does not preclude a similar selectivity mechanism in this homolog. The proposal has been made that the selectivity profile of this channel is intimately linked with its physiological function. An exciting paradigm is that this channel allows Cl- efflux potentially culminating in membrane depolarisation and the propagation of an electrical signal.48 Whereas such a proposition is attractive, assumptions about effects on membrane potential subsequent to channel gating are difficult when the ionic environment and resting membrane potential are to a large extent unknown.
The MscS-like channel MSC1 expressed in the chloroplasts of the green alga Chlamydomonas reinhardtii displays a similar selectivity profile to MSL10 (PCl/PK – 7).49 In addition, much like MSL10, there is no conservation of residues purported to be important for selectivity in TtMscS and EcMscS. Interestingly, this channel seems to be intimately involved in chloroplast organization, which is allegedly dependent on its anion preference. This channel has an N-terminal cleavable sequence, and failure to cleave this sequence results in an inability to incorporate in the E. coli membrane meaning this sequence would likely be a targeting sequence for the chloroplast membrane.
Biophysical Characterization Provides Functional Insight
All this structural information and in depth biophysical characterization of these channels is interesting, however, the question becomes are these differences in selectivity physiologically relevant. One attractive proposal for EcMscS is that rather than being an arbitrary biophysical characteristic, its selectivity is as important to its function as K+ selectivity is to K channels. By honing the selectivity of EcMscS and other MscS-like channels, whose main role is in osmoprotection, over many evolutionary years each organism has matched its MscS-like channel selectivity with the electrochemical gradient for efflux of major internal osmolytes to reduce the impact channel gating has on the resting membrane potential. As previously suggested for EcMscS, this ability to allow neutral efflux of positive and negatively charged solutes prevents any change in membrane potential conserving the ability of H+-driven ATPases to generate ATP.25 Any MscS-like channel whose major role is in osmoprotection would need to be able to balance anion and cation efflux in order to have as limited an effect on membrane potential as possible. Other MscS-like channels (MSC1 and MSL10) exhibit higher levels of selectivity that may have evolved over time and may indeed be indicative of their alternate functions within their host organism or may simply be a result of the different electrochemical environment that these respective channels find themselves in. In contrast, the lack of selectivity in MscL is indicative of its imperative role in osmolyte efflux. Its large pore diameter combined with a pressure threshold of activation lying directly below the lytic limit of the cell membrane makes this channel a perfect fit for its physiological role.
Conclusions
While initial inspection of the modest selectivity differences in the MscS family of channels seems unimportant it is in fact far more interesting. Selectivity studies from these channels have revealed numerous potential selectivity mechanisms within a single channel family. These reports compound the important function of the large cytoplasmic vestibulum and confirm its role in selectivity. In addition, far more than being just a biophysical characteristic, the selectivity of these channels is likely to have been refined over millions of years of evolution to provide channels that can balance osmolyte efflux with minimal effect on membrane potential. Knowledge regarding MscS-like channel selectivity may well be commercially and industrially important particularly in the case of amino acid production (glutamate) using channels such as MscCG.
Recent work suggests that the MscS family of channels, which contains anion and cation selective members, has a diverse set of structural motifs that dictate their selectivity. However, a unifying property is that the structural determinants of selectivity are not housed within the transmembrane region of these channels, as seen for all voltage gated Ca2+, K+, Na+, and Cl- channels.
Methods
Bioinformatics
Phylogenetic analysis was performed using Geneious software. Initial global alignment was carried using a Blosum matrix with the corresponding phylogenetic tree being produced using the Jukes-Cantor genetic distance model. Boot strap proportions are omitted for clarity.
Molecular modeling
The MscS crystal structures ([E. coli: PDB:2VV5 and 2OAU] [H. pylori: PDB:4HW9] [T. tengcongensis: PDB: 3T9N]) were viewed in Chimera UCSF 1.6 and used to generate Coulombic charge maps at pH 7.2. Coulombic charge maps were generated using standard histidine protonation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
C.D.C.: figure generation, manuscript generation. K.T.W.: manuscript generation. B.M.: manuscript generation.
Glossary
Abbreviations:
- MS
mechanosensitive
- TM
transmembrane domain
- MscS
mechanosensitive channel of small conductance
References
- 1.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2297–301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH., Jr. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [table of contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–7. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–30. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth IR, Blount P. The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol. 2012;194:4802–9. doi: 10.1128/JB.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcolm HR, Maurer JA. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem. 2012;13:2037–43. doi: 10.1002/cbic.201200410. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y, Fujiu K, Sokabe M, Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci U S A. 2007;104:5883–8. doi: 10.1073/pnas.0609996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci U S A. 2012;109:19015–20. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama Y, Yoshimura K, Iida H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat Commun. 2012;3:1020. doi: 10.1038/ncomms2014. [DOI] [PubMed] [Google Scholar]
- 10.Naismith JH, Booth IR. Bacterial mechanosensitive channels--MscS: evolution’s solution to creating sensitivity in function. Annu Rev Biophys. 2012;41:157–77. doi: 10.1146/annurev-biophys-101211-113227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–7. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–83. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Edwards MD, Jeong H, Roth J, Booth IR. Identification of mutations that alter the gating of the Escherichia coli mechanosensitive channel protein, MscK. Mol Microbiol. 2007;64:560–74. doi: 10.1111/j.1365-2958.2007.05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–30. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrov E, Palanivelu D, Constantine M, Rohde PR, Cox CD, Nomura T, Minor DL, Jr., Martinac B. Patch-clamp characterization of the MscS-like mechanosensitive channel from Silicibacter pomeroyi. Biophys J. 2013;104:1426–34. doi: 10.1016/j.bpj.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox CD, Nomura T, Ziegler CS, Campbell AK, Wann KT, Martinac B. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat Commun. 2013;4:2137. doi: 10.1038/ncomms3137. [DOI] [PubMed] [Google Scholar]
- 17.Edwards MD, Black S, Rasmussen T, Rasmussen A, Stokes NR, Stephen T-L, Miller S, Booth IR. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 2012;6:272–81. doi: 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–7. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai JY, Poon YS, Kaiser JT, Rees DC. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci. 2013;22:502–9. doi: 10.1002/pro.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Wang J, Feng Y, Ge J, Li W, Sun W, Iscla I, Yu J, Blount P, Li Y, et al. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc Natl Acad Sci U S A. 2012;109:18180–5. doi: 10.1073/pnas.1207977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–7. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 22.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Current Topics in Mechanosensitive Ion Channels. Current Topics in Mechanosensitive Ion Channels 2007;Part A. 58:1-24.
- 23.Nomura T, Sokabe M, Yoshimura K. Interaction between the cytoplasmic and transmembrane domains of the mechanosensitive channel MscS. Biophys J. 2008;94:1638–45. doi: 10.1529/biophysj.107.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koprowski P, Kubalski A. C termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J Biol Chem. 2003;278:11237–45. doi: 10.1074/jbc.M212073200. [DOI] [PubMed] [Google Scholar]
- 25.Gamini R, Sotomayor M, Chipot C, Schulten K. Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys J. 2011;101:80–9. doi: 10.1016/j.bpj.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic β domain as a new gating element. J Biol Chem. 2011;286:877–88. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machiyama H, Tatsumi H, Sokabe M. Structural changes in the cytoplasmic domain of the mechanosensitive channel MscS during opening. Biophys J. 2009;97:1048–57. doi: 10.1016/j.bpj.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe I, Elahi M, Huq A, Sukharev S. The mechanoelectrical response of the cytoplasmic membrane of Vibrio cholerae. J Gen Physiol. 2013;142:75–85. doi: 10.1085/jgp.201310985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotomayor M, van der Straaten TA, Ravaioli U, Schulten K. Electrostatic properties of the mechanosensitive channel of small conductance MscS. Biophys J. 2006;90:3496–510. doi: 10.1529/biophysj.105.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophys J. 2008;94:3003–13. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maksaev G, Haswell ES. Recent characterizations of MscS and its homologs provide insight into the basis of ion selectivity in mechanosensitive channels. Channels (Austin) 2013;7:215–20. doi: 10.4161/chan.24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2297–301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–8. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiharto S, Lewis TM, Moorhouse AJ, Schofield PR, Barry PH. Anion-cation permeability correlates with hydrated counterion size in glycine receptor channels. Biophys J. 2008;95:4698–715. doi: 10.1529/biophysj.107.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann U, Edwards MD, Rasmussen T, Bartlett W, van West P, Booth IR. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci U S A. 2010;107:12664–9. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol. 1996;151:175–87. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 37.Vora T, Corry B, Chung SH. Brownian dynamics investigation into the conductance state of the MscS channel crystal structure. Biochim Biophys Acta. 2006;1758:730–7. doi: 10.1016/j.bbamem.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K, Nakamura K, Kuroda T, Yabe I, Nakamatsu T, Kawasaki H. The protein encoded by NCgl1221 in Corynebacterium glutamicum functions as a mechanosensitive channel. Biosci Biotechnol Biochem. 2010;74:2546–9. doi: 10.1271/bbb.100636. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama Y, Yoshimura K, Iida H. Electrophysiological characterization of the mechanosensitive channel MscCG in Corynebacterium glutamicum. Biophys J. 2013;105:1366–75. doi: 10.1016/j.bpj.2013.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker M, Börngen K, Nomura T, Battle AR, Marin K, Martinac B, Krämer R. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828:1230–40. doi: 10.1016/j.bbamem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Börngen K, Battle AR, Möker N, Morbach S, Marin K, Martinac B, Krämer R. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta. 2010;1798:2141–9. doi: 10.1016/j.bbamem.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita C, Hashimoto K, Kumagai K, Maeda T, Takada A, Yabe I, Kawasaki H, Wachi M. L-Glutamate secretion by the N-terminal domain of the Corynebacterium glutamicum NCgl1221 mechanosensitive channel. Biosci Biotechnol Biochem. 2013;77:1008–13. doi: 10.1271/bbb.120988. [DOI] [PubMed] [Google Scholar]
- 43.Lai JY, Poon YS, Kaiser JT, Rees DC. Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci. 2013;22:502–9. doi: 10.1002/pro.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belyy V, Anishkin A, Kamaraju K, Liu N, Sukharev S. The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat Struct Mol Biol. 2010;17:451–8. doi: 10.1038/nsmb.1775. [DOI] [PubMed] [Google Scholar]
- 45.Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004;86:2883–95. doi: 10.1016/S0006-3495(04)74340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckstein O, Biggin PC, Sansom MSP. A hydrophobic gating mechanism for nanopores. J Phys Chem B. 2001;105:12902–5. doi: 10.1021/jp012233y. [DOI] [Google Scholar]
- 47.Beckstein O, Sansom MSP. Liquid-vapor oscillations of water in hydrophobic nanopores. Proc Natl Acad Sci U S A. 2003;100:7063–8. doi: 10.1073/pnas.1136844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci U S A. 2012;109:19015–20. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama Y, Fujiu K, Sokabe M, Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci U S A. 2007;104:5883–8. doi: 10.1073/pnas.0609996104. [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]