Abstract
We recently reported gating currents recorded from hERG channels expressed in mammalian TSA cells and assessed the kinetics at different voltages. We detected 2 distinct components of charge movement with the bulk of the charge being carried by a slower component. Here we compare our findings in TSA cells with recordings made from oocytes using the Cut Open Vaseline Gap clamp (COVG) and go on to directly compare activation of gating charge and ionic currents at 0 and +60 mV. The data show that gating charge saturates and moves more rapidly than ionic current activates suggesting a transition downstream from the movement of the bulk of gating charge is rate limiting for channel opening.
Keywords: hERG, gating currents, activation, kinetics, potassium channel
Introduction
hERG (Kv11.1) channels display a unique gating phenotype which is characterized by the very slow activation of channels on depolarization followed by a rapid inactivation and subsequent recovery from inactivation on repolarization.1,2 These atypical gating kinetics seen in hERG pose the important question of what specific structural or voltage sensing elements result in the slow gating. Recently we addressed this problem by recording hERG voltage sensor kinetics from mammalian cells through 2 methodologies; a direct measurement of gating currents using solutions depleted of conducting ions, and a study of the covalent rates of modification of a cysteine residue exposed only as voltage sensors are activated.3
The general architecture and gating mechanisms of the Kv1 and hERG channels are similar4,5 so it seems likely that different energetic loads upon the VSD or on the coupling mechanism might be responsible for the slow gating of hERG channels. Prior investigations into hERG voltage sensing have utilized 2 main techniques to characterize the kinetics of rearrangements of the VSD of hERG: Voltage clamp fluorometry (VCF) and direct measurement of gating currents. The first VCF study using fluorescent molecules attached to introduced cysteine residues at the top of the S4 segment reported both slow and fast environmental changes where the slow changes correlated with channel activation and deactivation, leading to the conclusion that VSD rearrangements were slow and coupled closely with pore opening.6 Direct measurements of gating currents were then made using a COVG recording system and showed that there were two components of charge movement in hERG gating, a rapid component with time constant < 1 ms and a slower component carrying the bulk of the charge.7 The Q-V was left-shifted to the G-V in this study, which led to the conclusion that the majority of gating charge moved prior to the channel opening and was thus not rate limiting in opening, however, the kinetics of the charge movement were not assessed. Subsequent VCF studies that removed the native cysteine residues in hERG found a fast fluorescent report from the top of S4 that had a left shifted voltage dependence from the G-V that was interpreted to track VSD movements and a slower fluorescent component that tracked pore rearrangements resulting from the channel opening.8 It is clear that a more detailed analysis of the kinetics of gating charge movement in hERG will aid in the understanding of the sequence of voltage dependent events in gating and their correlation with fluorescent signals.
Our recent study approached this problem by recording gating current kinetics to investigate whether the slow gating is due to intrinsically slow movement of the voltage sensor, or a slower rate limiting step coupling the voltage sensor to the pore gate. The study revealed 2 charge systems with different kinetics that exhibited some dissimilarities to the gating currents first reported from COVG by Piper et al. To investigate the basis for these differences and further our studies of the kinetics of hERG gating we have utilized the COVG recording system9 to record WT hERG gating currents using potassium-free solutions to compare the different methodologies.
Results
Prior to our recent paper in which we reported hERG gating currents from mammalian TSA cells,3 the only reported gating currents from hERG channels were recorded using the COVG technique and focused on measurements of total charge movement over 300 ms depolarizing steps.7,13 In our study, we used a variety of protocols to try to dissect components of charge movement that occurred in the mammalian expression system including shorter pulses and extending duration protocols to assess the kinetics of charge movement.
COVG oocyte recordings reveal a slow component of ON gating currents
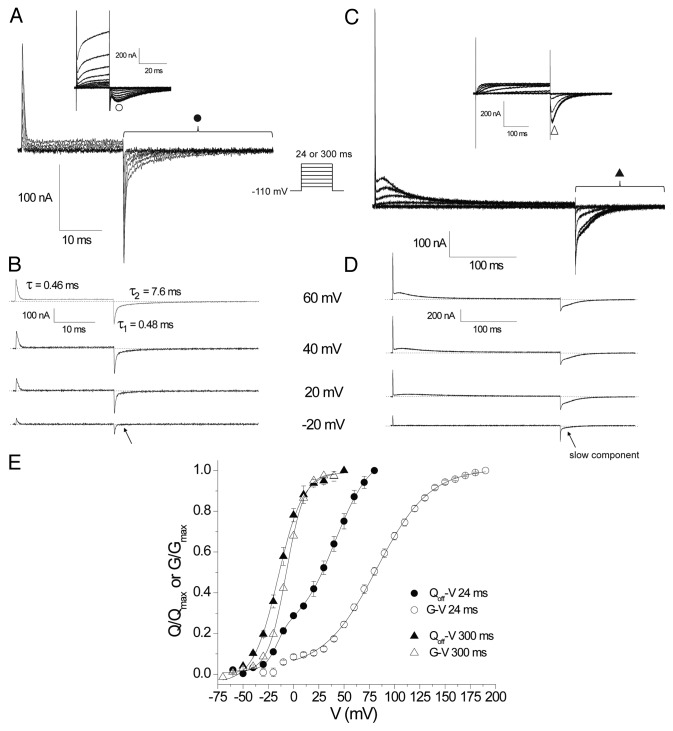
Using the COVG clamp we recorded hERG gating currents using a 24 ms protocol as shown in Figure 1A and B. We observed a fast component that moved at negative potentials with a fast return upon repolarization to -110 mV as illustrated by the fast decaying ON gating currents (IgON) and OFF gating currents (IgOFF) displayed in the isolated -20 mV trace. During stronger depolarizations a slower component in IgOFF emerged and the current was best fit by a two component exponential with time constants of ~0.5 and 8 ms. It is difficult to accurately resolve charge by integrating slow gating transitions that produce small IgON. Therefore, it is common to assess the amount of charge moved in a given conditioning pulse by integrating the IgOFF upon repolarization as OFF gating currents are typically faster as voltage sensors return from activated positions. The Qoff measurement was therefore used to plot the Qoff-V shown in Figure 1E (●). The data was best fit with a double component Boltzmann equation with parameters V0.5a = -14.2 ± 0.2 mV, ka = 9.4 ± 1.7 mV, Aa = 0.31 ± 0.04, V0.5b = 41.2 ± 1.9 mV, kb = 13.1 ± 1.1 mV (n = 3), where Aa is the amplitude of the first component fit which represents the faster charge system that appears before the emergence of the slower charge system, and at more negative potentials.
Figure 1. Comparison of WT hERG gating and ionic currents over 24 ms and 300 ms depolarizing pulses. (A) Representative traces of gating currents recorded in response to depolarizing steps of 24 ms from a HP of -110 mV. Traces from depolarizations between -60 and +60 are shown in 20 mV increments. Inset are representative traces of ionic currents recorded using the same protocol illustrating traces from depolarizations between -90 and +190 in 20 mV increments. (B) Isolated traces of gating currents demonstrate a fast IgON component of charge movement at all voltages and the emergence of a slower component of charge movement positive to 20 mV which is clear in the biexponential fit of the IgOFF currents at +60 mV. (C) Representative gating (from -60 to +60 mV) and ionic (inset, from -60 to +80 mV) current traces from a family of 300 ms depolarizing pulses. (D) Isolated gating current traces illustrating the development of a slow IgON component which develops concurrently with a slowing in the IgOFF currents. (E) Isochronal peak ionic tail current GV relationships for 24 ms (○) and 300 ms (∆) depolarizations and QOFF-V relationships from integration of IgOFF currents for 24 ms (●) and 300 ms (▲) depolarizations. Data points were fit with a single Boltzmann function of the form where y/ymax is the normalized response; either G/Gmax or Q/Qmax, V0.5 the half activation potential and k the slope factor or a double Boltzmann function where A is the amplitude of the fit component.
Data in Figure 1E illustrates that the 24ms Qoff-V was left-shifted compared with the isochronal tail current G-V (representative traces shown in Fig. 1A inset) which is consistent with the charge moving prior to pore opening and ionic current activation. The G-V also displayed a smaller more hyperpolarized component to activation similar in voltage dependence to the gating charge movement which could represent the contamination of the measurement of the peak ionic tail current by the gating currents. To isolate the ionic current activation the G-V was plot (○) and fit with a single Boltzmann between 0 and 190 mV (V0.5 = 83.1 ± 3.3 mV, k = 25.3 ± 0.4 mV, n = 5). It is important to note that these measurements of Qoff-V and G-V after 24 ms pulses are not at equilibrium at each voltage. The slow activation gating of hERG channels requires that long duration depolarizing pulses are used to find the true equilibrium measurement of gating. Measuring charge movement and ionic activation over pulse durations that have not reached a steady-state (equilibrium) leads to a right-shift of the V0.5 and a shallower slope. The G-V and Q-V based on a 24 ms pulse is therefore expected to be significantly right shifted compared with equilibrium measurements. Despite this, the left shifted position of the Q-V compared with the G-V in these isochronal measurements suggests that the kinetics of gating charge movement are hyperpolarized to, and faster than, channel opening over this short 24 ms pulse.
To further resolve the slower component of the charge movement we applied the same protocol as Piper et al. using 300 ms depolarizing steps. The traces shown in Figure 1C show the fast IgON as a sharp transient current at the start of the pulse followed by a slow rising and decaying component of charge movement. Figure 1D displays isolated traces to highlight the development of the slow IgON and IgOFF with increasing depolarizations. At -20 mV the slow IgON is not clearly resolved but is most likely present as the IgOFF at -20 mV displays a slow component not present after 24 ms at -20 mV (indicated by arrows in Fig. 1B and D). Upon depolarizations > -20 mV the slow IgON becomes clearly visible over 300 ms and displays a voltage dependent increase in rate of rise and decay, typical of gating currents. The appearance of the slow component is also reflected by the slowing in the return of charge in the IgOFF coincident with the appearance of the slow IgON. A plot of the Qoff-V was fit with parameters of V0.5 = -14.2 ± 0.2 mV, k = 9.4 ± 1.7 mV (n = 3), and showed a saturation of Qoff at around +20 mV (Fig. 1E), which suggests that after 300 ms at +20 mV the majority of the slow charge system has moved into its final, presumably fully activated position. The isochronal G-V exhibited a slightly right shifted voltage dependence where V0.5 = -6.9 ± 0.6 mV, k = 9.4 ± 0.4 mV (n = 5).
Kinetics of charge movement and pore opening
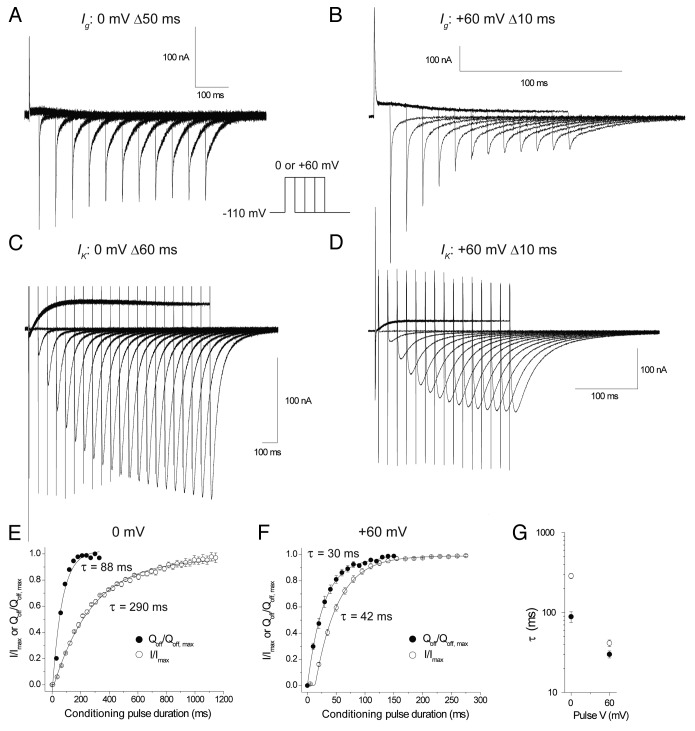
The slower component of the IgON waveform over 300 ms was not easily fit with simple exponential functions. To clarify the kinetic relationship between channel pore opening and gating charge movement we measured Qoff and ionic current activation after conditioning depolarizations to 0 and +60 mV for increasing durations. Our previous study also used this protocol as it effectively measures the rate of IgON movement in a more robust way than directly measuring IgON integrals over long periods.
Data in Figure 2 shows overlain traces of gating currents in response to conditioning pulses of increasing duration at 0 mV (Fig. 2A) and +60 mV (Fig. 2B) and ionic currents at 0 mV (Fig. 2C) and +60 mV (Fig. 2D). Figure 2E and F show the normalized Qoff and peak ionic tail currents plotted against pulse duration at 0 mV and +60 mV. The development of Qoff was fit with a single exponential function at 0 mV (Fig. 2E, ●) (τ = 88.8 ± 13.7 ms [n = 5]) and at +60 mV (Fig. 2F, ●) (τ = 30.1 ± 3 ms [n = 5]). It should be noted that the time course of the development of the fast IgON is not captured in these protocols as the minimum duration pulse applied was greater than the duration of the fast IgON. Ionic currents exhibited further latency to opening which results from the transitioning of multiple closed states before opening,14 even after movement of the VSD’s to their activated position. To account for this latency the data were fit with an exponential function with a delay (τ0) at 0 mV (Fig. 2E, ○) (τ = 287 ± 23 ms, τ0 = 12 ± 4 ms [n = 4]) and +60 mV (Fig. 2F, ○) (τ = 42 ± 4 ms, τ0 = 12 ± 0.5 ms [n = 4]). The voltage dependence of the time constants is compared in Figure 2G which, in combination with the plotted data, shows clearly that gating current saturates and thus moves more quickly than channels activate at both 0 mV and +60 mV, suggesting that the bulk of gating charge moves before the channel pore opens.
Figure 2. Kinetics of hERG charge movement and ionic activation at 0 mV and +60 mV. Representative trace of gating currents evoked by steps to (A) 0 mV extending in 50 ms increments or (B) +60 mV in 10 ms increments. (C) Representative trace of ionic currents evoked by steps to 0 mV extending in 60 ms increments or (D) +60 mV in 10 ms increments. Plot of the normalized Qoff (●) and normalized peak ionic tail currents (○) after increasing durations of a 0 mV (E) or +60 mV (F) conditioning step. Charge movement was fit with a single exponential () and ionic currents with a delayed exponential where y is the normalized response, t is the duration of the conditioning pulse, A the amplitude, τ the time constant and H the unit step function where t0 is the delay before onset of the exponential rise. (G) Time constants for gating charge movement and ionic current activation at 0 and +60 mV.
Discussion
The objectives of this study were to (1) Compare the gating currents of hERG channels expressed in oocytes using COVG with our previous results from channels expressed in TSA cells recorded using patch clamping and (2) Investigate the kinetic relationship of the slow charge system to ionic activation.
Two charge systems of hERG gating
Our previous study3 showed that during a series of 24 ms pulses a fast ON gating (IgON) and OFF gating (IgOFF) current was elicited that was joined by a slower component that carried the bulk of the charge (~70%) at more depolarized potentials. These kinetically distinct systems were labeled Q1 (fast Ig) and Q2 (slow Ig) in keeping with the finding of dual charge systems in other Kv channels such as Shaker and Kv1.5 channels.15,16 In the COVG experiments presented in Figure 1 we used a 24 ms protocol and replicated the findings from our previous study in which we observed 2 systems of charge that correlate to the fast Q1 and slower Q2 systems. These 2 systems showed similar voltage dependence in the Qoff-V as represented by the double Boltzmann fit in Figure 1E. The additional finding using this technique was the emergence of a prominent slower IgON component at voltages > 0 mV that did not decay over the 24 ms pulse, in contrast to our prior study where a persistent slow IgON current was not observed over 24 ms. This suggests that in the oocyte this slow component of the gating current is more easily resolved. Comparing the COVG Qoff-V parameters (V0.5a = -14.2 ± 0.2 mV, ka = 9.4 ± 1.7 mV, V0.5b = 41.2 ± 1.9 mV, kb = 13.1 ± 1.1) with those we reported in the mammalian cell system for the 24 ms protocol (V0.5a = -24 mV, ka = 8.5 mV, V0.5b = 26 mV, kb = 12 mV) reveals that the V0.5b from the COVG recordings is more depolarized which would result from the greater resolution of the slower component that moves at more depolarized potentials.
The duration of depolarising pulses is critical for extracting equilibrium measurements of channel gating and the highly depolarized G-V from 24 ms (Fig. 1E, V0.5~80 mV) indicates that the measurements after 24 ms were not at equilibrium. As COVG recordings are extremely stable over time, the recording of much longer duration depolarizing pulses is possible. This allowed measurement of the slow charge movement closer to equilibrium. We employed a 300 ms protocol similar to Piper et al. which revealed the full rise and decay of the slow IgON system at depolarized potentials (Fig. 1C&D). The Qoff-V (V0.5 = -14 mV) for this protocol was only slightly left shifted compared with the isochronal G-V (V0.5 = -7 mV) with activation saturating around the same voltage as the gating charge. The narrowing of the gap between the G-V and Q-V after 300 ms is a result of more of the slow gating system reaching equilibrium and suggests that the slower gating charge system is more closely associated with pore opening than the smaller and earlier emerging fast charge system. Our Qoff-V0.5 is somewhat more depolarized than that reported by Piper et al. (-25 mV) for unknown reasons but is still consistent with those findings.
The bulk of charge movement precedes channel opening
Equilibrium measurements reflect the relative free energy differences between states but do not give any information about the kinetics of the transitions. We wanted to know if the movement of the slow charge system was kinetically distinct from channel opening. We measured ionic activation and the development of Qoff at 0 mV and +60 mV and found that in both cases the movement of the gating charge preceded the opening of the channels (Fig. 2). Gating current moved ~3 fold faster than ionic current activation at 0 mV but only ~1.5 fold more quickly at +60 mV potentially reflecting the approach to the saturation of ionic current activation kinetics that is seen in hERG at depolarized potentials which reflects the presence of a voltage independent or very weakly voltage dependent step in the activation pathway.14 In our previous study in TSA cells we used a similar protocol to assess rate of charge movement and found that at 0 mV the larger Q2 system moved with a time constant of ~36 ms and at +60 mV had a time constant of ~4 ms. The main disparity in the data lies between the time constants reported for charge movement at +60 mV where the COVG charge movement is 10-fold slower. It is possible that because the slow IgON is so small it was not clearly resolvable and that mammalian cells did not withstand depolarizing pulses of sufficient duration to resolve the slower movement in the Qoff. It is also important to note that the ionic conditions for these recordings was of different composition; whereas NMDG+ was typically used as the major cation in the TSA cell study, we used TEA+ based solutions in the COVG. External TEA+ has previously been shown to have effects on hERG inactivation17 and more generally the choice of ions have been shown to have effects on charge immobilization and IgOFF kinetics.18,19 Further studies will uncover the origin of these differences.
Taken together, the data presented here and in our previous study establishes that there are two charge systems operating in the hERG channel, a fast and small charge carrying Q1 component and a large slow Q2 component that carries the bulk of the charge. The Q1 component is unlikely to be associated with channel opening or inactivation as the kinetics are very fast compared with opening (τ~0.5 ms, Fig. 1B) and a channel mutation that removes inactivation was shown to have no effect on this component.7 The fast gating charge has therefore been hypothesized to represent an early transition of the VSD between closed states of the channel.7 The structural basis of this rearrangement is not yet clear but may involve a small movement or reorientation of S4 or other regions of the channel that are yet to be identified. The larger and slower bulk charge movement Q2 is more closely related to channel opening, as demonstrated by the proximity of the Q-V and G-V (Fig. 1E), and represents significant movement of the S4 segment3 which could set up the VSD in a position that primes the pore for the opening transition. Further experiments will be required to establish the precise structural basis for these charge movements and whether Q1 and Q2 are different movements of the same structural moiety, or represent movements of separate structural entities with different voltage dependencies and charge.
In conclusion, our study has shown that despite the slow onset of the bulk of hERG charge movement, it is not rate limiting for pore gating, as time constants for gating charge movement are still orders of magnitude faster than ionic current activation. This strongly suggests the critical involvement of further transitions downstream from VSD rearrangements that electromechanically couple the gating charge movement to the pore gate in hERG channels.
Methods
Oocyte preparation and injection
Stage IV and V oocytes were isolated and injected as previously described.3 Typically, 10–14 ng of cRNA was injected in 50 nL volume. Oocytes were incubated in ND96 ringers solution at 20 °C for 3–4 d before recording.
COVG recording
A Dagan CA-1B COVG clamp system was used (Minneapolis). For ionic current recordings external solution was (in mM): 96 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, and 5 HEPES, pH 7.4. Internal solution contained: 120 K-Glutamate, 10 HEPES, pH 7.0. For gating current recordings external solution consisted of: 120 Tetraethlyammonium-hydroxide (TEA-OH), 120 Methanesulfonic acid (MES), 10 HEPES, 1 Ca-MES, pH 7.4. Internal solution: 120 TEA-OH, 120 MES, 10 HEPES, pH 7.0. All chemicals from Sigma. Oocytes were permeabilized with 0.3% saponin internal solution. The membrane was clamped at 0 or -10 mV for 20–30 min after permeabilization to deplete K+ ions from the oocyte and both internal and external solutions contained 20 μM terfenadine to block any residual hERG ionic current. Terfenadine is an open channel blocker of hERG that occupies the inner pore cavity of the channel to obstruct ion flow and can be trapped in the closed pore.10-12This open channel blocking mechanism renders terfenadine unlikely to affect rearrangements in the structurally distinct voltage sensing. The voltage electrode had resistance ~0.5 MΩ and was filled with 3M KCl for ionic or CsCl for gating measurements. Clamp was controlled with pClamp 10.1 (Molecular Devices). Data was analog low pass filtered at 5 KHz and digitized at 25–50 KHz. Holding potential was -110 mV. For gating currents an online P/-6 or P/-8 leak subtraction protocol was applied.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Goodchild SJ held postdoctoral fellowship awards from the Heart and Stroke Foundation of Canada and the Mitacs Elevate program over the course of this study.
References
- 1.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–5. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Dou Y, Goodchild SJ, Es-Salah-Lamoureux Z, Fedida D. Components of gating charge movement and S4 voltage-sensor exposure during activation of hERG channels. J Gen Physiol. 2013;141:431–43. doi: 10.1085/jgp.201210942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng YM, Claydon TW. Voltage-dependent gating of HERG potassium channels. Front Pharmacol. 2012;3:83. doi: 10.3389/fphar.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 6.Smith PL, Yellen G. Fast and slow voltage sensor movements in HERG potassium channels. J Gen Physiol. 2002;119:275–93. doi: 10.1085/jgp.20028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piper DR, Varghese A, Sanguinetti MC, Tristani-Firouzi M. Gating currents associated with intramembrane charge displacement in HERG potassium channels. Proc Natl Acad Sci U S A. 2003;100:10534–9. doi: 10.1073/pnas.1832721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Es-Salah-Lamoureux Z, Fougere R, Xiong PY, Robertson GA, Fedida D. Fluorescence-tracking of activation gating in human ERG channels reveals rapid S4 movement and slow pore opening. PLoS One. 2010;5:e10876. doi: 10.1371/journal.pone.0010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefani E, Bezanilla F. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 1998;293:300–18. doi: 10.1016/S0076-6879(98)93020-8. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya K, Niwa R, Morishima M, Honjo H, Sanguinetti MC. Molecular determinants of hERG channel block by terfenadine and cisapride. J Pharmacol Sci. 2008;108:301–7. doi: 10.1254/jphs.08102FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci U S A. 2000;97:12329–33. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stork D, Timin EN, Berjukow S, Huber C, Hohaus A, Auer M, Hering S. State dependent dissociation of HERG channel inhibitors. Br J Pharmacol. 2007;151:1368–76. doi: 10.1038/sj.bjp.0707356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper DR, Hinz WA, Tallurri CK, Sanguinetti MC, Tristani-Firouzi M. Regional specificity of human ether-a’-go-go-related gene channel activation and inactivation gating. J Biol Chem. 2005;280:7206–17. doi: 10.1074/jbc.M411042200. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol. 1997;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezanilla F, Perozo E, Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994;66:1011–21. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesketh JC, Fedida D. Sequential gating in the human heart K(+) channel Kv1.5 incorporates Q(1) and Q(2) charge components. Am J Physiol. 1999;277:H1956–66. doi: 10.1152/ajpheart.1999.277.5.H1956. [DOI] [PubMed] [Google Scholar]
- 17.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–6. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 18.Goodchild SJ, Fedida D. Contributions of intracellular ions to kv channel voltage sensor dynamics. Front Pharmacol. 2012;3:114. doi: 10.3389/fphar.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodchild SJ, Xu H, Es-Salah-Lamoureux Z, Ahern CA, Fedida D. Basis for allosteric open-state stabilization of voltage-gated potassium channels by intracellular cations. J Gen Physiol. 2012;140:495–511. doi: 10.1085/jgp.201210823. [DOI] [PMC free article] [PubMed] [Google Scholar]