Abstract
Background
Honey is widely used in folk medicine to treat cough, fever, and inflammation. In this study, the effect of aerosolised honey on airway tissues in a rabbit model of ovalbumin (OVA)-induced asthma was investigated. The ability of honey to act either as a rescuing agent in alleviating asthma-related symptoms or as a preventive agent to preclude the occurrence of asthma was also assessed.
Methods
Forty New Zealand white rabbits were sensitized twice with mixture of OVA and aluminium hydroxide on days 1 and 14. Honey treatments were given from day 23 to day 25 at two different doses (25% (v/v) and 50% (v/v) of honey diluted in sterile phosphate buffer saline. In the aerosolised honey as a rescue agent group, animals were euthanized on day 28; for the preventive group, animals were further exposed to aerosolised OVA for 3 days starting from day 28 and euthanized on day 31. The effects of honey on inflammatory cell response, airway inflammation, and goblet cell hyperplasia were assessed for each animal.
Results
Histopathological analyses revealed that aerosolised honey resulted in structural changes of the epithelium, mucosa, and submucosal regions of the airway that caused by the induction with OVA. Treatment with aerosolised honey has reduced the number of airway inflammatory cells present in bronchoalveolar lavage fluid and inhibited the goblet cell hyperplasia.
Conclusion
In this study, aerosolised honey was used to effectively treat and manage asthma in rabbits, and it could prove to be a promising treatment for asthma in humans. Future studies with a larger sample size and studies at the gene expression level are needed to better understand the mechanisms by which aerosolised honey reduces asthma symptoms.
Background
Asthma is a chronic inflammatory airway disease that affects around 300 million people worldwide. According to a survey conducted in 2006, the prevalence of asthma among Malaysian adults was about 4.5%, and the rate was increasing, especially among children [1]. Asthma accounted for 0.4% of all deaths worldwide, and the number is increasing annually. In fact, this disease has become a significant cause of morbidity and mortality in developed countries [2].
Asthmatic patients usually experience recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, especially at night and early in the morning [3]. The disease is characterised by variable degrees of chronic inflammation and airway remodelling. Airway remodelling refers to alteration of the structure of the airway, which includes epithelium, mucosa, and submucosa, leading to thickening of the airway wall [4]. Other important features are goblet cell hyperplasia and metaplasia, increased smooth muscle mass, bronchial gland enlargement, angiogenesis, and alteration of the extracellular matrix components [5,6]. Both goblet cell metaplasia and bronchial gland enlargement lead to airway mucus hypersecretion, which contributes to airway obstruction [6].
Asthma is thought to occur due to over-expression of Th2 cytokines such as interleukin (IL)-4, IL-5, and IL-13 [3,7]. Over-expression of IL-4 leads to eosinophilia and mucus metaplasia as well as subepithelial fibrosis [7], whereas IL-5 over-expression is thought to result in an increased number of eosinophils in the airway, which is the hallmark of asthma [3]. IL-13 over-expression causes subepithelial fibrosis, mucus metaplasia, and induced infiltration of eosinophils and macrophages into the airways [6,8]. Over-expression of these Th2 interleukin molecules results in airway hyperresponsiveness (AHR) [7].
Drug therapy has become the most common treatment to manage asthma. Examples include short acting β-agonists, inhaled corticosteroids, and long acting β-agonists. These drugs alleviate asthmatic attacks by relaxing the airway smooth muscle. However, asthma attacks and exacerbations can still occur, as the treatment does not alter the underlying pathology [9]. Unfortunately, prolonged use of drug therapy can cause local and systemic side effects, including oral candidiasis, dysphonia, growth failure, accelerated loss of bone mass, elevated intraocular pressure, and mild tachyphylaxis [10]. These problems highlight the need to find an alternative treatment for asthma with fewer side effects, and the use of natural products is a promising approach.
Honey has been widely used as a remedy for treating ailments such as cough, fever, infections, and inflammation. It is reportedly has antioxidant, anti-immunomodulatory, and antibacterial effects [11,12]. Although honey is usually taken orally, inhalation is a more suitable way to administer honey to treat asthma; it would ensure that the maximum amount of honey is deposited in the airways to treat the disease. Maksoud and Rahman [13] showed that honey nebulisation was effective in treating upper acute asthma in paediatric patients. However, there have been no comprehensive studies examining the effect of nebulised honey (i.e., aerosolised) on pathophysiological changes of the airway tissues in association with asthma-related features such as on goblet cell hyperplasia, mucus over-production, and inflammatory cell responses.
The traditional oral administration of honey routes it towards the digestive system, therefore delaying its efficacy in treating patients with an acute asthma attack. Thus, the goal of the present study was to investigate the effect of aerosolised honey on histopathological changes of the airway in a rabbit model of ovalbumin (OVA)-induced chronic lung disease, which mimics the human condition of asthma. Experiments were conducted to determine whether aerosolised honey can act as a rescue agent by reducing asthma-related symptoms and/or as a preventive agent by preventing the occurrence of asthma.
Methods
Ethics
This study was conducted in strict accordance with university guidelines. The protocol was approved by the Animal Ethics Committee of Universiti Sains Malaysia (USM/Animal Ethics Approval/2012/(77)(379)).
Animals
Forty rabbits (breed: New Zealand white, Oryctolagus cuniculus; sex: male = 37, female = 3; weight: 2.40 ± 0.56 [mean, standard deviation, SD]) were used in this study. The rabbits were housed in an air-conditioned room, kept on a 12 hour light/dark cycle, and had access to clean food and water.Animals were divided into eight groups of five rabbits each. Control groups were treated as follows: group 1, naïve (normal); group 2, injected intraperitoneally (i.p.) twice with OVA at days 1 and 14; group 3, i.p. injection twice with OVA at the same time points, followed by aerosolised OVA for three consecutive days at day 28 to day 30; group 4, treated twice with i.p. phosphate buffered saline (PBS) at days 1 and 14 followed by aerosolised PBS for three consecutive days at day 28 to day 30 (the negative control). Asthma was induced in rabbits in the other groups twice via i.p. injection of OVA at days 1 and 14, and they then were treated as follows: groups 5 and 6, honey at 25% (v/v) and 50% (v/v), respectively, treatment with aerosolised-honey for five consecutive days from day 23 to day 27, followed by euthanization, to test for rescue effects; groups 7 and 8, treatment with aerosolised honey at the same concentrations and time period, followed by aerosolised OVA for three consecutive days from day 28 to day 30, before euthanized on the following day to test for preventative effects – Figure 1.
Figure 1.
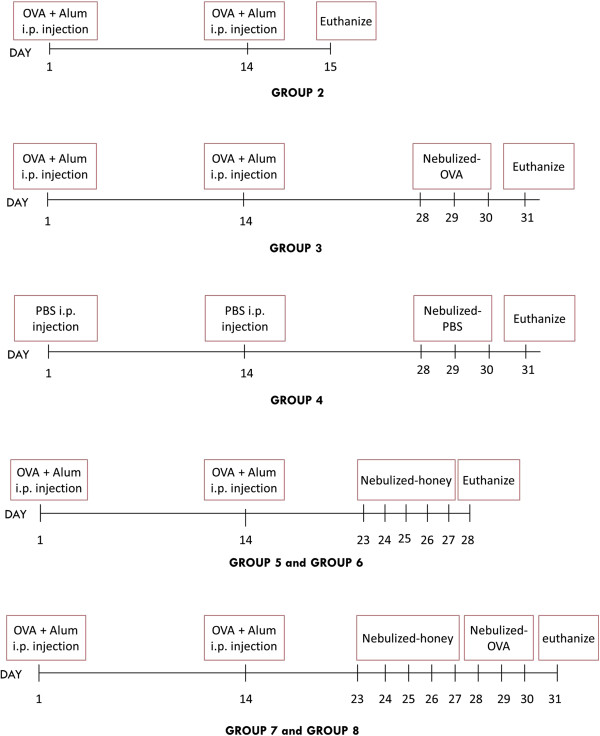
Procedural timeline. Schematic diagrams showing the procedures and timelines for OVA induction of airway inflammation in the rabbit model and for treatment with aerosolised honey.
Experimental protocol
Experiments were followed protocols adopted from El. Gazzar et al. [14]. Induction of allergic airway inflammation was performed by i.p. sensitization and airway challenge through nasal inhalation (Figure 1). Animals in group 2 were sensitized (i.p.) on days 1 and 14 with 0.1 mg of OVA (Sigma A5503; Sigma-Aldrich, St. Louis, MO, USA) and 10 mg of aluminium hydroxide (alum) (donated by the EMAN Testing and Research Laboratory, School of Pharmaceutical Sciences, Universiti Sains Malaysia) in 2 ml of PBS. Twenty-four hours after the second sensitization, animals in group 2 were sacrificed. Animals in the aerosolised OVA group (group 3) were further challenged with aerosolised OVA (10 mg/ml) for 3 consecutive days starting from day 28 until day 30. Aerosolised OVA was generated using an ultrasonic nebuliser (MabisMistTM, Mabis Healthcare Inc, USA). Rabbits in the negative control group (group 4) were treated with both i.p PBS and aerosolised PBS. Rabbits in groups 3 and 4 were euthanized at day 31 using intravenous injection of sodium pentobarbital (1 ml/kg body weight) (Dolethal, Lure Cedex, France).
For the honey treatment groups, asthma was first induced via i.p. OVA in rabbits in groups 5, 6, 7, and 8. They then were treated for 20 min with 5 ml of aerosolised Tualang honey (Federal Agricultural Marketing Authority (FAMA) of Malaysia). Honey was diluted with PBS to either 25% (v/v) or 50% (v/v). Animals in groups 5, 6, 7 and 8 were treated with 25% (v/v) and 50% (v/v) honey. Treatment was given daily starting from day 23 until day 27. Rabbits in groups 5 and 6 (models of honey as rescue agent) were euthanized on day 28, whereas rabbits in groups 7 and 8 (honey as preventive agent) were subsequently challenged with aerosolised OVA (10 mg/ml) starting from day 28 until day 30 and euthanized at day 31. Figure 1 outlines of the study procedures and time points for each group.
Collection of bronchoalveolar lavage (BAL) fluid
Twenty four hours after the final treatment, rabbits were anaesthetised and lungs were lavaged three times by instillation and withdrawal of 3 ml of sterile PBS through an endotracheal tube. BAL fluid from each animal was placed in sterile plastic tubes, cooled in ice, and centrifuged (1200 × g) at room temperature for 5 min. The cell pellets were resuspended in 200 μl of sterile PBS. Total cell counts were determined using a Neubauer hemocytometer (Marienfeld, Germany). A 10 μl aliquot of the cell suspension was mixed with 10 μl of Trypan blue (Gibco-Life Technologies, NY, USA) solution to allow counting of nucleated cells. For differential cell counts, 100 μl of the cell suspension were applied to a slide by cytospinning at 500 rpm for 5 min followed by staining with Wright-Giemsa solution (Sigma-Aldrich).
Histological and morphometric analyses
The right lung of each rabbit was fixed in 10% (v/v) formalin overnight and processed according to standard histological protocols. Tissues ultimately were embedded in paraffin (Paraplast® Plus, Leica Biosystems, Ayer Rajah, Singapore) and cut into 3 μm sections with a rotary microtome (Accu-Cut®, SRM™ 200, Sakura®, CA, USA). The sections were stained with haematoxylin and eosin (H&E) (Sigma-Aldrich) to examine the histology of airways and cellular infiltration into peribronchial connective tissues [15] and with Alcian Blue (AB; Merck, Darmstadt, Germany) and Periodic Acid Schiff (PAS; Sigma-Aldrich) to detect mucus-secreting goblet cells.
The slides stained with H&E (n = 5 for each group) were analysed for airway tissue structure and morphometric features such as thickness of epithelium, mucosal, and submucosal regions. For these measurements, photomicrographs were taken using a DP72 Olympus digital camera (Tokyo, Japan) attached to an Olympus BX53 microscope. Measurements were taken from five different points on larger airways for each slide.
To measure the effect of honey on mucus production, the numbers of mucus-secreting goblet cells were counted. The goblet cells were examined in five different fields in three large lung sections from each animal (n = 5 for each group). Results were calculated as the number of positively stained goblet cells within 100 epithelial cells. All slides were blinded prior to morphometric analysis.
Statistical analyses
Our preliminary results showed that there was no statistically significant difference in terms of the degree of inflammatory responses and airway epithelial structures in both naïve (group 1) and PBS-treated (group 4) groups (data not shown); therefore, these two groups were combined and served as the control animal group. The i.p. OVA (group 2) and i.p. + aerosolised OVA (group 3) groups were combined and were later known as the OVA-induced injury group.
For statistical analyses, results were compared among groups to determine the following: (1) the effect of aerosolised honey on airway structures and the number of mucus-secreting cells; (2) the effect of aerosolised honey as rescue and preventive agents; (3) the effect of treatment with 25% (v/v) and 50% (v/v) honey; and (4) the effect of treatment with 25% (v/v) and 50% (v/v) honey for both rescue and preventive groups.
Comparisons between groups were made using independent t-tests, and the data are presented as means (SD). A p value of < 0.05 was considered to be significant, whereas p < 0.001 was considered to be highly significant. Statistical analysis was performed using SPSS Statistics software version 20 (IBM, NY, USA).
Results
Aerosolised honey reduced the inflammatory cell response
Figure 2 shows the representative microscopic images of cytospun BAL fluid stained with Wright-Giemsa, and Table 1 summarises the histopathological examination of the effect of aerosolised honey on inflammatory cell response. Exposure to OVA led to the infiltration of inflammatory cells into the airway, such as eosinophils, mononuclear cells (lymphocytes and plasma cells), neutrophils, and macrophages (Figure 2b). OVA-induced asthmatic rabbits were characterised by dense peribronchial inflammatory cell infiltration, especially eosinophils (which are the hallmark of asthma). The accumulation of eosinophils in the BAL fluid was reduced by treatment with aerosolised honey (Figure 2c). However, further exposure to aerosolised OVA in the honey preventive groups led to an increase in infiltration of inflammatory cells (Figure 2d). Based on the finding as shown in the Table 1, the honey treatment in the rescue group shows less numbers of inflammatory cells present as compared to honey treatment in the preventive group, with 50% of honey gave a better effect. Nevertheless, this result shows that treatment with aerosolised honey can reduce the inflammatory cell response.
Figure 2.
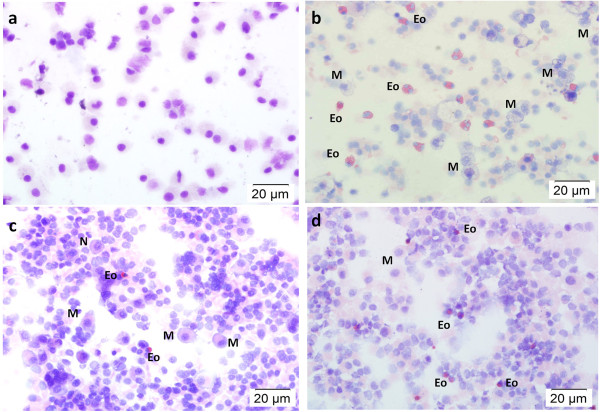
Effect of aerosolised honey on inflammatory cell response. The panels show Wright-Giemsa stained BAL fluid from the control group (a), injury group (b), and 50% honey-treated group (rescue, c; preventive, d). Rabbits in the aerosolised OVA group (group 3 – OVA-induced injury) resulted in greater infiltration of neutrophils (N), eosinophils (Eo), and macrophages (M), whereas the number of infiltrated inflammatory cells was low (control animal group). Treatment with aerosolised honey reduced the infiltration of these inflammatory cells. However, further exposure to OVA following treatment elicited the second phase of the inflammatory reaction.
Table 1.
Summary of the effect of aerosolised honey on inflammatory cell response
| Group |
Inflammatiory cells |
|||
|---|---|---|---|---|
| Eosinophils | Mononuclear | Neutrophils | Macrophage | |
| Control |
- |
- |
- |
- |
| OVA-induced injury |
+++ |
+++ |
++ |
+++ |
| 25% Honey rescue |
++ |
+++ |
+ |
++ |
| 25% Honey preventive |
+ |
+ |
- |
+ |
| 50% Honey rescue |
- |
- |
- |
+ |
| 50% Honey preventive | + | + | - | ++ |
Indicator: -(normal), +(mild), ++(moderate), +++(dense).
Aerosolised honey had a significant effect on airway structures
OVA treatment led to the infiltration of inflammatory cells into the peribronchial region of the lungs (Table 1). The histological appearance of the airway structures in the injury group differed compared to the morphology of the airway in the control group. Figure 3 shows representative microscopic images of tissue sections from different groups stained with H&E. The structure of the epithelial layer was normal and peribronchial cell infiltration was absent in lung sections from the control animal group (Figure 3a). OVA sensitization and repeated OVA challenge caused thickening of the airway wall, including the epithelium, mucosa, submucosa, and smooth muscle. It was also associated with epithelial folding and epithelial cell shedding, elongation of cellular epithelial nuclei, and goblet cell hyperplasia, which causes accumulation of mucus in the lumen of the bronchioles (Figure 3b). Compared to the OVA-induced injury group, aerosolised honey at both the 25% and 50% doses in the rescue groups reduced the infiltration of inflammatory cells in the peribronchial region (Figure 3c, e). In the preventive groups with both doses of honey, epithelial thickening was increased compared to that of the control group (Figures 3d, f).
Figure 3.
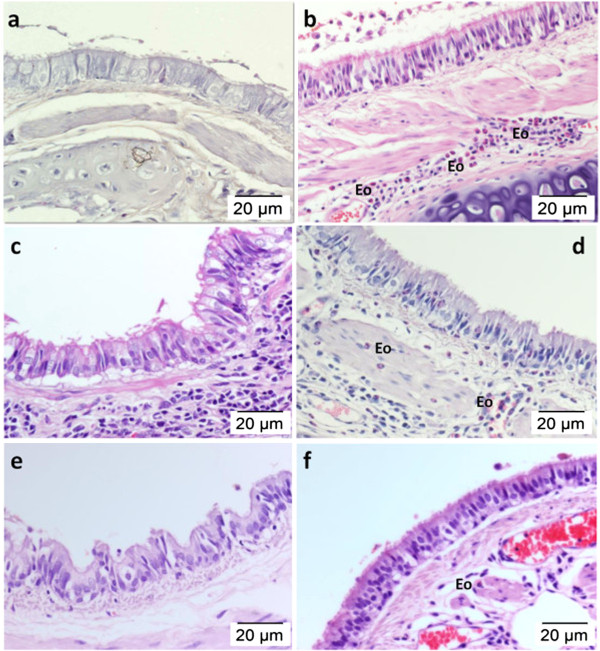
Effect of aerosolised honey on airway structures in the OVA-induced asthma rabbit model. The panels show H&E stained lung tissues from the control group (a), OVA-induced injury group (b), 25% honey-treated group (rescue, c; preventive, d), and 50% honey-treated group (rescue, e; preventive, f). Exposure of the lungs to OVA led to epithelial hypertrophy and peribronchial inflammatory cell infiltration (b). Treatment with aerosolised honey for three consecutive days following OVA sensitization and before the OVA challenge (honey rescue groups) markedly attenuated airway inflammation (c and e). However, the subsequent OVA challenge (honey preventive groups) caused epithelial hypertrophy as well as peribronchial cell infiltration to occur (d andf).
Figure 4 shows (1) OVA induced the development of epithelial hyperplasia and thickening of mucosal and submucosal regions and (2) the effect of treatment with 25% and 50% aerosolised honey on airway structures following OVA treatment. Treatment with aerosolised honey at both concentrations in both the rescue and preventive experiments restored the airway structures following induction with OVA (Table 2). In all four treatment groups, honey treatment was significantly reduced the thickness of the airway epithelial and mucosal regions (p < 0.05) following asthma induction with OVA. However, inhalation of honey did not significantly restore the submucosal region (p > 0.05).
Figure 4.
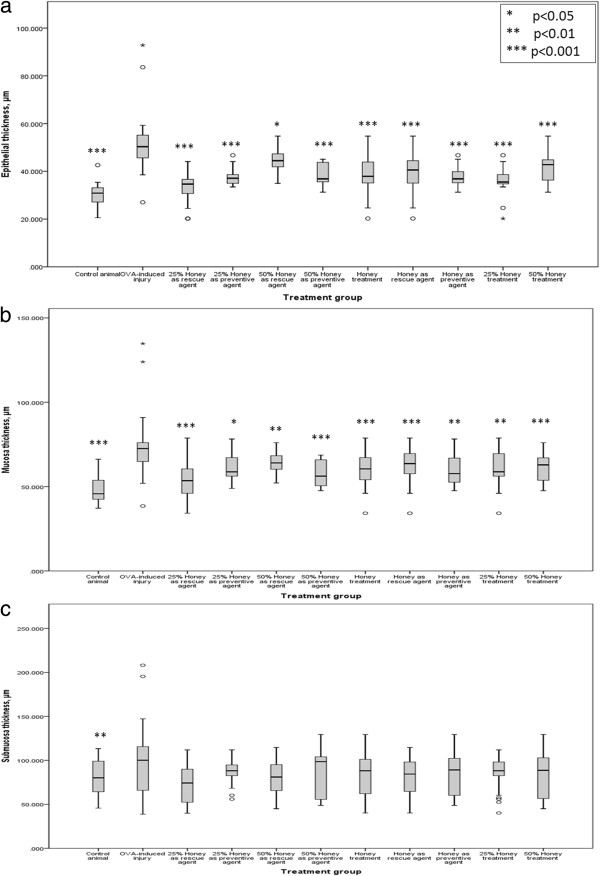
Effect of aerosolised honey treatment on airway structure. The three parameters measured were (a) epithelial thickness, (b) mucosa thickness, and (c) submucosa thickness. Statistical analyses were performed to compare: 1) the control animal group with the OVA-induced injury group and 2) the OVA-induced injury group with different treatment groups. p < 0.05 was considered to be statistically significant.
Table 2.
Statistical summary of the effect of aerosolised honey on airway structure
| Treatment Group | Lung Structure | p-value |
|---|---|---|
| Control Animal vs OVA-induced Injury*** |
Epithelial Thickness |
0.000 |
| Control Animal vs OVA-induced Injury*** |
Mucosal Thickness |
0.000 |
| Control Animal vs OVA-induced Injury** |
Sub Mucosal Thickness |
0.003 |
| OVA-induced Injury vs 25% Honey as Rescue Agent*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs 25% Honey as Rescue Agent* |
Mucosal Thickness |
0.018 |
| OVA-induced Injury vs 25% Honey as Rescue Agent# |
Sub Mucosal Thickness |
0.219 |
| OVA-induced Injury vs 50% Honey as Rescue Agent* |
Epithelial Thickness |
0.036 |
| OVA-induced Injury vs 50% Honey as Rescue Agent** |
Mucosal Thickness |
0.009 |
| OVA-induced Injury vs 50% Honey as Rescue Agent# |
Sub Mucosal Thickness |
0.155 |
| OVA-induced Injury vs 25% Honey as Preventive Agent*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs 25% Honey as Preventive Agent** |
Mucosal Thickness |
0.006 |
| OVA-induced Injury vs 25% Honey as Preventive Agent# |
Sub Mucosal Thickness |
0.326 |
| OVA-induced Injury vs 50% Honey as Preventive Agent*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs 50% Honey as Preventive Agent*** |
Mucosal Thickness |
0.000 |
| OVA-induced Injury vs 50% Honey as Preventive Agent# |
Sub Mucosal Thickness |
0.211 |
| OVA-induced Injury vs Honey as Rescue Agent*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs Honey as Rescue Agent** |
Mucosal Thickness |
0.002 |
| OVA-induced Injury vs Honey as Rescue Agent# |
Sub Mucosal Thickness |
0.104 |
| OVA-induced Injury vs Honey as Preventive Agent*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs Honey as Preventive Agent*** |
Mucosal Thickness |
0.000 |
| OVA-induced Injury vs Honey as Preventive Agent# |
Sub Mucosal Thickness |
0.168 |
| OVA-induced Injury vs Honey Treatment*** |
Epithelial Thickness |
0.000 |
| OVA-induced Injury vs Honey Treatment*** |
Mucosal Thickness |
0.000 |
| OVA-induced Injury vs Honey Treatment# | Sub Mucosal Thickness | 0.081 |
*p < 0.05.
**p < 0.01.
***p < 0.001.
#p > 0.05.
Effect of aerosolised honey on goblet cells
Figure 5 shows representative microscopic images of lung sections stained with AB-PAS. Figure 6 shows the results of morphometric analysis of the effect of OVA induction on goblet cell hyperplasia as compared to the control and aerosolisation of 25% and 50% honey on goblet cell numbers in different treatment groups. Following OVA sensitization and challenge, AB-PAS positive goblet cells were significantly more abundant in the OVA-induced injury group compared to the control group (Figure 6; Table 3) (p < 0.001). Following honey inhalation, the numbers of goblet cells were significantly reduced in all treatment groups (p < 0.05) (Figure 6; Table 3).
Figure 5.
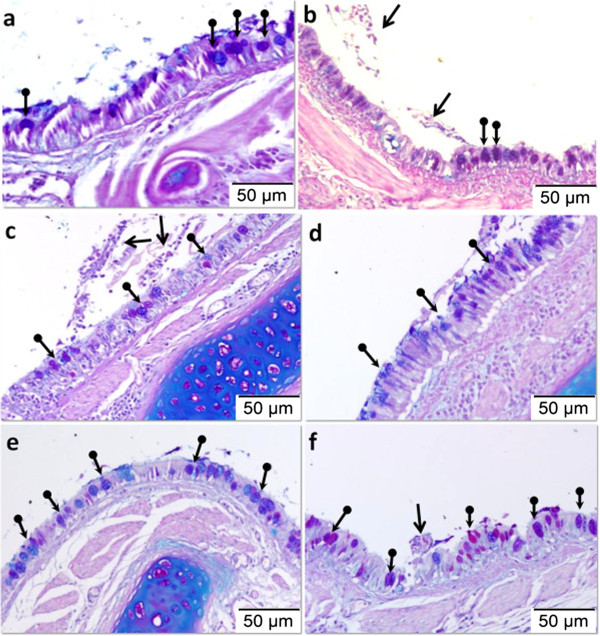
Effect of aerosolised honey treatment on goblet cell hyperplasia. The panels show AB-PAS stained lung tissues from the control group (a), injury group (b), 25% honey-treated group (rescue, c; preventive, d), and 50% honey-treated group (rescue, e; preventive, f). Arrows in the figure indicate the accumulation of mucus in the airway lumen, and round-ended arrows indicate representative images of goblet cells.
Figure 6.
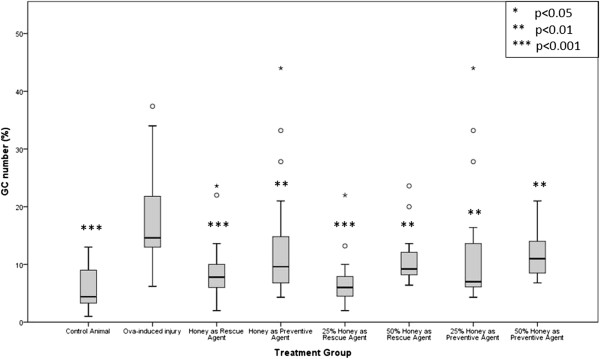
Effect of aerosolised honey treatment on goblet cell numbers. Results of morphometric analysis of the effect of OVA induction on goblet cell numbers as compared to control animals and the effect of aerosolisation of 25% and 50% honey in different treatment groups on goblet cell numbers following OVA-induced injury. p < 0.05 is considered to be statistically significant.
Table 3.
Statistical summary of the effect of aerosolised honey on goblet cell numbers
| Treatment Group | p -value |
|---|---|
| OVA-induced Injury vs Control Animal |
0.000 |
| OVA-induced Injury vs 25% Honey as Rescue Agent |
0.000 |
| OVA-induced Injury vs 25% Honey as Preventive Agent |
0.008 |
| OVA-induced Injury vs 50% Honey as Rescue Agent |
0.001 |
| OVA-induced Injury vs 50% Honey as Preventive Agent | 0.006 |
Discussion
The aim of the present study was to investigate the effect of aerosolised honey as an agent to alleviate the asthma-related histopathological changes that occur in the rabbit airway following OVA-induced injury. The effects of aerosolised honey on inflammatory cell response, histopathological changes of lung tissues, and goblet cell hyperplasia were assessed. Although many studies have used mice as a model, we used the rabbit as our model system to imitate human chronic lung disease because it is phylogenetically closer to humans and offers a better understanding of lung structure than smaller animal models [16].
After asthma was induced by OVA, honey was administered in the form of aerosol particles through a nebuliser. This technique was selected because it ensures maximum deposition of honey on the surfaces of the airway. The aerosolised honey particles generated by a nebuliser are between 1 to 5 μm in size [17]. Adjei et al. [18] reported that particles must be less than 3 μm in order to be deposited in the pulmonary zone of the lungs. Thus, aerosol particles generated by a nebuliser are small enough to reach the lungs and provide the optimal effect on the subjects. In comparison to smaller animals, findings from other study showed that delivery of aerosol via nasal inhalation led to deposition of more than 80% of the aerosol particles in the nasal region [19], thus hindering the effectiveness of the treatment.
The pathogenesis of asthma begins with alteration in the structure of the airway components, such as thickening of the epithelial and subepithelial regions [5], epithelial folding and desquamation [4,6], goblet cell metaplasia, enlargement of blood vessels [5], increased infiltration of inflammatory cells, and excessive mucus secretion into the airways [20]. The results of our study showed that the OVA sensitization and airway challenge led to thickening of the epithelial layer, mucosa, and submucosa of the lung tissues, indicating that OVA treatment effectively established the model for airway remodelling in rabbits.
In this study, aerosolised honey was used to investigate the capability of honey to 1) alleviate the effects of asthma (i.e., a rescue treatment) and 2) assess the potential for honey to prevent the occurrence of asthma (i.e., a preventive agent). In general, aerosolised honey treatment (both 25% and 50% for both rescue and preventative treatments) resulted in a significant decrease in the thickening of the epithelial and mucosal regions. However, the submucosal region was not affected by the honey treatment. Thickening of the mucosal regions depends on thickening of the epithelial layer, whereas the submucosal region of the rabbit lung contains no (or less) smooth muscle and submucosal glands – are the main contributors for submucosal thickening. In summary, both the 25% and 50% honey treatments significantly alleviated airway inflammation following induction of injury with OVA, and this was true for both the rescue and preventive experiments. This finding suggests that honey may prove useful in alleviating asthma-associated histopathological changes as well as preventing the development of asthma upon subsequent induction with an allergen.
In the present study, some of the lung tissue samples for all groups showed signs of epithelial desquamation. This feature is often considered to be a pathological feature of asthma based on observation of post-mortem specimens and endobronchial biopsies from asthmatic patients [7,21]. However, epithelial shedding has also been reported from bronchial biopsy specimens from healthy nonasthmatic subjects [22]. Hence, the presence of epithelial desquamation may be related to the sampling technique used [7], and it may have occurred during collection of the BAL fluid samples in this study. Infiltration of inflammatory cells into the peribronchial region is thought to be a pivotal process in the pathogenesis of asthma. In response to injury, Th2 cells are activated and produce a large amount of IL-4, IL-5, and IL-13. These cytokines are responsible for recruiting inflammatory cells such as eosinophils and macrophages into the bronchial region. Thus, eosinophilic inflammation is the pathological hallmark of asthma, as the cells act as effectors in allergic airway disease by releasing cytotoxic granule proteins [15,23].
OVA-induced asthma results in chronic airway inflammation and characteristically is associated with infiltration of lymphocytes, eosinophils, macrophages, and neutrophils into the peribronchial region [24]. In our study, treatment with aerosolised honey decreased the number of inflammatory cells, especially eosinophils, in the airway region (Figure 2c; Table 1). Treatment with 25% honey in the rescue group was more effective at suppressing the infiltration of eosinophils than treatment with 25% honey in the preventive group. This suggests that treatment with honey at 25% concentration effectively reduced the inflammatory cell response. In addition, inhalation of honey prevented the occurrence of asthma in the model system. The number of infiltrating cells in the 50% dosage group was lower than that of the 25% dosage group in the asthma prevention experiment.
The anti-inflammatory effect of honey in both in vivo and in vitro systems has been described previously. However, most of these studies focused on the role of honey in wound healing. Bashkaran et al. [25] assessed the anti-inflammatory and antioxidant properties of Tualang honey in treating alkaline injury to the eyes of rabbits and reported that honey contained an anti-inflammatory component with an effectiveness comparable to that of conventional treatment. In vitro studies showed that honey types such as Gelam[25], Manuka[26], and buckwheat[27] have anti-inflammatory components. Manuka honey was reported to increase the expression of the pro-inflammatory cytokine TNF-α as well as anti-inflammatory cytokines such as IL-10, IL-1ra, and growth factors PDG and TGF-β [26]. Kassim et al. [28] suggested that the anti-inflammatory properties of honey may be attributable to its phenolic compounds.
Goblet cells are responsible for secreting mucus on the surface of the airway. Airway mucus protects the epithelial surfaces from injury and facilitates the removal of bacterial, cellular, and particulate debris from the lung [29]. In the case of asthma, goblet cells become hyperplastic, and a high proportion of goblet cells and submucosal gland enlargement lead to mucus hypersecretion and contribute significantly to airflow limitation [6,29]. As shown in Table 3 and Figure 6, treatment with aerosolised honey (regardless of dosage and its mechanism of action) significantly inhibited (p < 0.05) goblet cell hyperplasia and over-production of mucus following OVA induction. Thus, the results indicate that aerosolised honey may have an inhibitory effect on the development of asthma. The ability of aerosolised honey to eliminate goblet cell hyperplasia and mucus over-production suggest that honey treatment has great potential in the management of patients with asthma, both in terms of prevention of disease and rescuing patients from symptoms.
Conclusion
Herein we demonstrated that treatment with honey at both concentrations tested effectively inhibited OVA-induced airway inflammation by alleviating asthma-related histopathological changes in the airway and also prevented the occurrence of asthma. Inhalation of honey was also found to effectively eliminate mucus-secreting goblet cell hyperplasia. Future studies of these effects at the gene expression level are needed to better understand the mechanisms by which aerosolised honey reduces asthma symptoms.
Financial disclosure
This study was supported by the E-Science Fund Grant Scheme from the Ministry of Science, Technology and Innovation (MOSTI), Malaysia (305/CIPPT/613224).
Competing interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Authors’ contributions
Conceived and designed the experiment: NAK, SAS, BY. Performed the experiment: NAK. Analyzed the data: NAK, GK, BY. Wrote the first draft of the manuscript: NAK, BY. Contributed to the writing of the manuscript: NAK, GK, BY. Read and approved with manuscript results and conclusions: NAK, SAS, GK, BY.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Nurfatin Asyikhin Kamaruzaman, Email: fatin_asyikhin@live.com.
Siti Amrah Sulaiman, Email: sbamrah@kb.usm.my.
Gurjeet Kaur, Email: gurjeet@usm.my.
Badrul Yahaya, Email: badrul@amdi.usm.edu.my.
Acknowledgements
Special thanks go to the staff of the Pre-Clinical Research Laboratory of AMDI, USM and to Dr. Nor Azlina Khalil, Dr. Sawibah Yahaya, Mrs. Adilah Abdul Khalil, and Mrs. Rodiah Mohd Radzi for their guidance in the animal study. The authors also would like to thank Mr. Zali Kassim for his technical assistance with the histological work, and the Research Committee of AMDI for proofread the manuscript.
References
- Usha Devi B, Hairul Izwan AR, Munjeet KPS, Rosidah CP. An audit of bronchial asthma control at a primary public health care facility. Malaysian J Pub Health Med. 2011;11:22–26. [Google Scholar]
- Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- Balaha MF, Tanaka H, Yamashita H, Rahman MN, Inagaki N. Oral Nigella sativa oil ameliorates ovalbumin-induced bronchial asthma in mice. Int Immunopharmacol. 2012;14:224–231. doi: 10.1016/j.intimp.2012.06.023. doi:10.1016/j.intimp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med. 2000;6:15–20. doi: 10.1097/00063198-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Infalammation and remodeling in the adult and child with asthma. Pediatr Pulmonol Suppl. 2001;21:3–16. [PubMed] [Google Scholar]
- Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol. 2007;120:997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012;2012:1–12. doi: 10.1155/2012/316049. Doi: 10.1155/2012/316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Yang WM. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 2011;136:230–235. doi: 10.1016/j.jep.2011.04.048. doi:10.1016/j.jep.2011.04.048. [DOI] [PubMed] [Google Scholar]
- Fanta CH. Drug therapy: Asthma. N Engl J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- Khalil MI, Sulaiman SA, Boukraa L. Antioxidant properties of honey and its role in preventing health disorder. Open Neutraceuticals J. 2010;3:6–16. doi: 10.2174/18763960010030100006. [DOI] [Google Scholar]
- Tan HT, Rahman RA, Gan SH, Halim AS, Hassan SA, Sulaiman SA, Kirnpal-Kaur B. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement Altern Med. 2009;9:1–8. doi: 10.1186/1472-6882-9-1. doi:10.1186/1472-6882-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud AM, Rahman AM. Bee honey nebulisation as a non traditional treatment of acute bronchial asthma in infants and children. First North–south Conference and Workshop on Pharmacogenetics (Beating the Gene: From the Bench to the Bedside) 2006. p. 115.
- El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6:1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Lee JS, Yun CY, Kim JH, Kim JS, Kim DH, Kim IS. Inhibitory effects of Duchesnea chrysantha extract on ovalbumin-induced lung inflammation in a mouse model of asthma. J Ethnopharmacol. 2008;118:102–107. doi: 10.1016/j.jep.2008.03.012. doi:10.1016/j.jep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Kamaruzaman NA, Kardia E, Kamaldin NA, Latahir AZ, Yahaya B. The rabbit as a model for studying lung disease and stem cell therapy. Biomed Res Int. 2013;2013:1–12. doi: 10.1155/2013/691830. doi:10.1155/2013/691830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabis Healthcare Inc. MabisMist Ultrasonic Nebuliser Guidebook. 2012. [cited 2013 20th August] available from: http://www.mabisdmi.com/assets/downloadsupport/documents/40-270-000_english_instructions.pdf.
- Adjei AL, Gupta PK, Qiu Y. In: Inhalation Aerosols Physical and Biological Basis for Therapy. Hickey AJ, editor. New York: Informa Healthcare; 2007. Bioavailability and pharmacokinetics of inhaled drugs. [Google Scholar]
- Szelenyi I. Animal models of bronchial asthma. Inflamm Res. 2000;49:639–654. doi: 10.1007/s000110050642. [DOI] [PubMed] [Google Scholar]
- Busse WW, Rosenwasser LJ. Mechanisms of asthma. J Allergy Clin Immunol. 2003;111:S799–S804. doi: 10.1067/mai.2003.158. [DOI] [PubMed] [Google Scholar]
- Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med. 2001;164:S46–S51. doi: 10.1164/ajrccm.164.supplement_2.2106066. [DOI] [PubMed] [Google Scholar]
- Ordonez C, Ferrando R. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Jang HY, Ahn KS, Park MJ, Kwon OK, Lee HK, Oh SR. Skullcapflavone II inhibits ovalbumin-induced airway inflammation in a mouse model of asthma. Int Immunopharmacol. 2012;12:666–674. doi: 10.1016/j.intimp.2012.01.010. doi:10.1016/j.intimp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Bashkaran K, Zunaina E, Bakiah S, Sulaiman SA, Sirajudeen K, Naik V. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: Experimental animal study. BMC Complement Altern Med. 2011;11:1–11. doi: 10.1186/1472-6882-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean A. Investigating the Anti-inflammatory Activity of Honey. University of Waikato: Doctoral Dissertation; 2012. [Google Scholar]
- van den Berg AJ, van den Worm E, Van Ufford HC, Halkes SB, Hoekstra MJ, Beukelman CJ. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17:176–178. doi: 10.12968/jowc.2008.17.4.28839. [DOI] [PubMed] [Google Scholar]
- Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res. 2010;30:650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007;56:331–340. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]


