Abstract
Healing fractures resulting from osteoporosis or cancer remains a significant clinical challenge. In these populations, healing is often impaired not only due to age and disease, but also by other therapeutic interventions such as radiation, steroids, and chemotherapy. Despite substantial improvements in the treatment of osteoporosis over the few decades, osteoporotic fractures are still a major clinical challenge in the elderly population due to impaired healing. Similar fractures with impaired healing are also prevalent in cancer patients, especially those with tumor growing in bone. Treatment options for cancer patients are further complicated by the fact that bone anabolic therapies are contraindicated in patients with tumors. Therefore, many patients undergo surgery to repair the fracture, and bone grafts are often used to stabilize orthopaedic implants and provide a scaffold for ingrowth of new bone. Both synthetic and naturally occurring biomaterials have been investigated as bone grafts for repair of osteoporotic fractures, including calcium phosphate bone cements, resorbable polymers, and allograft or autograft bone. In order to re-establish normal bone repair, bone grafts have been augmented with anabolic agents, such as mesenchymal stem cells (MSC) or recombinant human bone morphogenetic protein-2 (rhBMP2). These developing approaches to bone grafting are anticipated to improve the clinical management of osteoporotic and cancer-induced fractures.
Keywords: Scaffold, bone graft, osteoporosis, fracture, cancer-induced bone disease
Introduction
Osteoporotic patients are typically treated with anabolic agents that stimulate bone formation (e.g., parathyroid hormone (PTH)) or anti-resorptive agents that inhibit bone resorption (e.g., bisphosphonates, calcitonin, raloxifene, and estrogen) to slow the progression of disease [1]. However, in many patients this loss of bone mass results in osteoporotic fractures, which account for approximately 1.5 million fractures in the US each year and are a significant cause of morbidity, mortality, and hospitalization. Treatment of osteoporotic fractures is challenging due to diminished capacity for fracture healing [2–4], and the reduced healing capacity of osteoporotic patients correlates with a much higher (~50%) failure rate of implant fixation compared to younger patients [5–7]. Since the bone is unlikely to heal on its own in osteoporotic fractures due to impaired healing, patients will frequently undergo surgical procedures to fix damaged bone using screws or fixation plates. Due to the high porosity and low strength of the osteoporotic cancellous bone, implants are often augmented with bone void fillers to improve outcomes. Restoration of normal bone repair through local delivery of biologics that enhance osteogenic differentiation has also been investigated to reduce the high complication rate associated with implant failure [2].
Cancer patients often develop bone metastatic diseases. Similar to osteoporotic patients, they are often treated with bisphosphonates, though typically given at higher doses. However, even with treatment, patients will eventually experience fractures, which are often slow to heal, significantly impeding their mobility and quality of life. While surgeries can improve quality of life, they are not a cure and are instead performed for palliative purposes. Bone surgeries performed on patients with traumatic bone injury are rarely effective in cancer patients, due to impaired healing from drug treatments as well as the tumor itself [8]. Patients with metastases to the proximal femur (one of the most common) typically undergo cemented endoprosthetic replacement/arthroplasty, which is similar to a hip replacement, while other long bone metastases are often repaired using locked intramedullary nails [8]. Lesions in the spine are typically treated using vertebroplasty, and other sites are often repaired using bone cements. Despite the improvements of treatment approaches over time, these surgeries are not without risk and patient’s survival after surgery is often short (whether from complications from surgery or from wide-spread disease) [9, 10].
Oral cancer comprises another disease often associated with rapid bone loss, resulting from both radiation treatments as well as tumor growth. To reduce morbidity associated with the disease, patients frequently require treatments consisting of tumor removal, reconstruction of the mandibular defect with vascularized bone from the fibula, and subsequent placement of dental implants [11]. Transplantation of the fibula flap introduces a significant source of patient morbidity, and is thus another limitation of the vascular bone graft approach. Importantly, current available therapies only address the need for bone regeneration without targeting the tumor, and thus therapeutic improvements are needed.
Regeneration of bone lost from osteoporosis or cancer presents the challenge of healing in patients with reduced repair mechanisms. These fractures frequently do not heal, may require multiple surgeries, and frequently re-fracture the same site. Recent reports from the German bone evaluation study (BEST) reported a 360-day re-fracture rate of 69% in osteoporosis patients treated with parathyroid hormone (PTH) and 85% in patients that do not receive medication [12]. Thus, there is a compelling need for improved bone grafts for healing osteoporotic fractures. In this review, we will highlight two recent strategies for significantly reducing the high complication rate resulting from implant failure and long-term immobilization: (1) osteoconductive bone grafts that provide mechanical stability and enhance osseointegration of the implant, and (2) osteoinductive bone grafts that enhance healing by re-establishing normal bone repair (i.e., coupling of the bone remodeling units) in osteoporotic patients (Figure 1).
Figure 1. Strategies for healing osteoporotic and cancer-induced bone disease (CIBD) fractures.
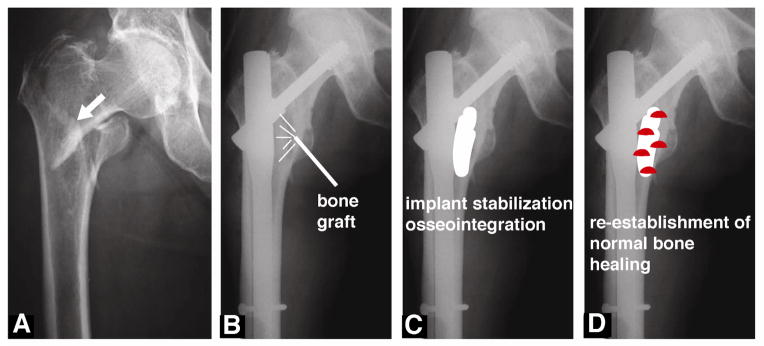
(A) Pre-operative radiograph of a hip fracture (arrow) in an 84 year-old female patient. (B) Postoperative radiograph showing fixation of the fracture with an intra-medullary hip lag screw coated with hydroxyapatite (HA). (C) Stabilization and osseointegration of implants using osteoconductive bone grafts. (D) Re-establishment of normal bone healing by local delivery of biologics (e.g., mesenchymal stem cells, rhBMP-2, or platelet-rich plasma (shown in red)) from bone grafts and scaffolds. Adapted from A Moroni et al. Can we improve fixation and outcomes? Use of bone substitutes. J Orthop Trauma 23:422–425, 2009 [29].
Biological Challenges of Healing Osteoporotic Bone
Patients with osteoporosis suffer a reduction in bone mineral density that can result from multiple pathological conditions and can lead to an increased risk of fracture. While bone mass is a major predictor of osteoporosis, other factors such as the material properties of bone can also affect the fracture risk [13]. A common observation associated with osteoporosis is that the bone deposition by osteoblasts cannot keep up with osteoclast-mediated bone resorption, ultimately resulting in a net loss of bone over time [14]. Furthermore, the healing potential of osteoporotic patients is impaired, in part due to reduced ability of mesenchymal stem cells (MSCs) to differentiate into osteoblasts and form new bone [2, 15]. MSCs from post-menopausal women exhibit a lower growth rate and deficient osteogenic potential compared to pre-menopausal women [16], and MSCs from osteoporotic patients synthesize less type I collagen [17]. The reduction in the number of MSCs with osteogenic potential during aging has been suggested to contribute to the age-related reduction in number of osteoblasts [18]. The use of intermittent PTH (or Forteo) stimulates osteoblast differentiation and is the only treatment that promotes healing and new bone formation in osteoporotic patients.
While patients with tumor-induced bone disease also experience an increase in osteoclast-mediated bone destruction that osteoblasts cannot repair, they often suffer more pronounced bone loss compared to osteoporotic patients due to the anti-cancer therapies [19]. Cancer patients are frequently treated with chemotherapeutic agents, radiation therapy, and/or steroids that can induce bone loss or necrosis, further complicating their ability to heal fractures or to heal from surgery [20]. Since PTH is contraindicated in cancer patients, there are no drugs used in cancer patients that stimulate new bone formation. While the anti-resorptive drugs (Denosumab and bisphosphonates) can successfully reduce bone destruction, they do not stimulate new bone and are associated with side-effects when given at high doses to cancer patients [21]. Better treatments are clearly needed for patients with osteoporosis or tumor-induced bone loss that enhance bone regeneration while reducing tumor growth.
Bone Grafts and Scaffolds for Healing Osteoporotic Fractures
The use of autogenous bone grafts has helped improve the impaired healing of patients with osteoporotic fractures. Autograft (bone harvested from the patient) or allograft (donor bone) bone is frequently used to enhance healing and fill space left by the fracture [22]. One study has reported that osteoporotic patients with acetabular fractures treated with total hip replacement supported by a fixation device and autografting of the acetabulum showed incorporation of the graft and good functional outcomes after 11 – 84 months [23]. In another study, treatment of osteoporotic humeral shaft non-unions with a vascularized fibular graft was found to achieve successful union in a small clinical study [24]. However, the bone harvesting surgical procedure is associated with additional morbidity, and the amount of autograft available is limited [25]. These limitations of autograft have generated considerable interest in synthetic scaffolds, which aim to reduce the high complication rate due to implant failure by addressing the need for stabilization of fixation devices and/or acceleration of fracture healing [2].
Typically, the primary failure mode for internal fixation devices is failure of the weak osteoporotic cancellous bone rather than the implant [26], which is consistent with observations that fractures in osteoporotic patients often present metaphyseal voids that are more extensive compared to younger patients [27]. Bone graft and bone substitutes are reported to be beneficial in maintaining metaphyseal reduction. Ideally, augmentation of osteoporotic fractures with osteoconductive bone grafts both maintains reduction of the fracture and also provides a scaffold for ingrowth of new bone near the interface between host bone and the fixation device. Settable calcium phosphate cements (CPCs) offer the advantages of good adhesion to bone, remodeling and consequent replacement with new bone, injectability [28], and reduced reliance on internal fixation devices [26], and are often used to fill voids caused by severe osteoporosis or comminution of the host bone [26]. Augmentation with CPCs has been reported to enhance the fixation stability of femoral neck and trochanteric fractures [29] as well fractures of the intertrochanteric crest [30]. While treatment of fragility fractures frequently focuses on the proximal femur, upper extremity fractures to the humerus and radius account for 33% of fractures in elderly patients [31]. Osteoporotic proximal humeral fractures are challenging to treat due to poor bone quality and unstable fixation [31]. Augmentation of proximal humeral fractures with Norian, an injectable hydroxyapatite (HA) cement, maintained reduction and promoted unions in all patients at 1 year follow-up [32]. Augmentation with CPCs has also been reported to maintain fixation of unstable distal radius fractures [27, 33, 34].
In order to improve the bioactivity or mechanical properties of the graft, calcium phosphate cements have been modified with other ions or polymers. Strontium (Sr)-substituted HA cements showed improved Sr and Ca release compared to stoichiometric HA granules [35], which is anticipated to enhance bone healing in vivo due to the anabolic and anti-catabolic properties of Sr [36]. Another study has reported that silicate-substituted calcium phosphate promoted osteogenic differentiation of MSCs [37]. Calcium phosphate/silk hybrid scaffolds have been fabricated as a composite bone graft for stimulating bone formation and reversing bone loss [38]. The hybrid scaffolds showed increased new bone formation and decreased bone resorption compared to the silk scaffold when implanted in the distal femoral epiphysis in ovariectomized rats.
While osteoconductive cements and scaffolds improve implant stability and provide a pathway for ingrowth of new bone, they do not address the impaired healing potential of osteoporotic bone. Thus, a number of approaches using osteoinductive scaffolds and grafts have been investigated to improve healing by stimulating osteoblast differentiation. Platelet-rich plasma (PRP) enhances healing of segmental femoral defects through expression of TGF-β1 and the osteoinductive factor bone morphogenetic protein-2 (BMP-2) [39]. In an osteoporotic model of ovariectomized mice, PRP enhanced healing by promoting new bone formation and suppressing adipogenesis within the bone marrow [40]. By providing a surface on which new bone can grow, local delivery of biologics (such as PRP or recombinant human BMP-2 (rhBMP-2)) from a scaffold is known to enhance bone formation [41, 42]. Local delivery of rhBMP-7 from poly(lactic glycolic) acid (PLGA) microspheres increased the mechanical strength of vertebral bodies in ovariectomized sheep [43]. In another study, sustained release of rhBMP-2 from gelatin microsphere/CPC composite scaffolds enhanced new bone formation compared to the CPC alone in osteoporotic goats [44]. Local delivery of MSCs from scaffolds has also been investigated as a strategy for healing osteoporotic fractures. Delivery of MSCs from PLGA/collagen Type I microspheres enhanced healing of trabecular bone defects in ovariectomized rats compared to MSCs alone [45]. However, healing of large cortical bone defects requires that the scaffold also deliver osteoinductive cues to induce differentiation of MSCs to osteoblasts. Delivery of an MSC sheet from osteoinductive calcined bovine bone increased new bone formation compared to individual MSCs in 8-mm calvarial defects in ovariectomized rats [46]. Other studies have shown that local delivery of MSCs transfected with BMP-2 from calcium phosphate scaffolds enhanced bone healing compared to untreated MSCs in cortical bone defects in the mandible [47] or femur [48] of osteoporotic rats. Mesoporous-glass/silk scaffolds seeded with MSCs transfected with both PDGF and BMP-2 have also been shown to increase new bone formation in segmental femoral defects in ovariectomized rats compared to BMP-2 alone [49].
Strategies for Healing Bone Damaged by Cancer-Induced Disease
Healing of fractures caused by cancer-induced bone disease (CIBD) presents additional challenges. Since expression of BMP receptors is up-regulated on cell membranes of certain cancers [50–52], local delivery of growth factors such as rhBMP-2 presents potential risks of stimulating tumor growth. In many cancer patients, management of pain is the primary concern (versus bone regeneration) due to the often limited life expectancy of the patient [53]. For example, malignant tumoral pathologies in the L5 vertebrae are typically stabilized using a titanium cage filled with poly(methyl methacrylate) (PMMA) bone cement, which effectively manages pain [54]. However, other studies have investigated the potential of vascularized autogenous bone grafts as a more regenerative approach compared to PMMA bone cement. Orthopaedic CIBD fractures have been successfully reconstructed using autogenous bone grafts. In one study, thirteen patients who underwent resection for a malignant pelvic lesion and were reconstructed with a total hip replacement augmented with an ipsilateral femoral autograft experienced a low (8%) probability of revision for mechanical failure after 2 years [55]. A recent case report has noted that the use of a free vascularized fibula graft resulted in a functional and pain-free hip for a patient with a large cavitary defect of the femoral head after resection of a chondroblastoma [56]. Additional studies report found that reconstruction of the distal radius with a free vascularized fibula graft after resection of a giant cell tumor resulted in good functional outcomes at 4 years [57].
Oral cancer patients present another challenge for healing CIBD fractures and bone damage. Bone destruction in the craniomaxillofacial (CMF) complex can result in dramatic changes in appearance, altered dentition, and reduced ability to speak. Thus, surgical intervention is required not only for palliative care but also to restore normal function. Oral cancer patients often require fixation or mandibulectomy (marginal or segmental) to remove tumor that has invaded the mandible or to repair treatment-induced bone destruction [11, 58]. The current one-stage procedure comprising tumor excision followed by immediate reconstruction of the excised mandible with vascularized bone has proven to be the most reliable and cost-effective approach for treatment of segmental mandible defects [11]. Since partial resections in patients with mandibular invasion may lead to recurrence [59], surgeons often are inclined to take large negative margins, which introduces cosmetic and functional defects [58, 59]. In order to preserve function, the clinical standard of care for reconstruction of large segmental mandibulectomies utilizes a vascularized free flap, in which a portion of the fibula is removed and grafted into the mandibular defect [11]. After grafting, patients frequently are treated with radiation monthly prior to placement of dental implants [60]. While success rates exceeding 90% have been reported for many types of mandibular surgeries, radiotherapy has been reported to lower success rates [60–63]. Thus, the radiation treatment intended to prevent recurrence in cancer patients can lead to complications such as osteoradionecrosis [64], resulting in failure of the graft and revision surgeries [63]. Despite these aggressive therapies of large surgical margins and radiation, recent studies have reported recurrence rates varying between 13 – 34% after mandibulectomy, which underscores the need for new approaches for reducing tumor recurrence while improving healing [58, 60, 61, 65, 66].
Recent studies have reported that nonvascular bone grafts (NVBGs), which are available in greater quantity and do not require an invasive harvesting procedure, can achieve successful outcomes for patients treated for marginal mandibulectomy or small segmental defects [67–69]. However, patients with large defects still have few options beyond the fibula free flap. While BMP-2 and other growth factors are used for healing CMF bone defects, the concern of stimulating tumor growth has prevented the use of these bone anabolic agents for oral cancer [70, 71]. Alternatively, the use of PRP has been effective for the treatment of refractory bisphosphonate-induced osteonecrosis of the jaw (BRONJ); however, it is unclear whether the increased concentration of growth factors will have negative effects on cancerous or pre-cancerous lesions [72].
Conclusions
Despite the introduction of new therapies for slowing the progression of disease in osteoporotic and CIBD patients, the loss of bone mass associated with these diseases results in pathologic fractures, which are difficult to treat due to impaired bone healing. New bone grafting strategies addressing the need for implant stabilization, bone ingrowth, and re-establishment of normal bone repair continue to be developed. Promising strategies include non-vascularized bone grafts and synthetic osteoconductive bone cements and scaffolds augmented with osteoinductive agents such as platelet rich plasma, rhBMP-2, and/or MSCs have shown promise in preclinical studies and clinical trials. Many groups continue to investigate improved strategies to enhance the mechanical properties of the graft and to stimulate improved healing. While similar approaches can be taken in cancer patients that suffer fractures, more research is needed to find drugs that can both stimulate healing while inhibiting tumor growth.
Acknowledgments
SA Guelcher has a consultancy with Medtronic and received research support from NIH/NCI and NIH/NIAMS. JA Sterling has received research support from the VA and NIH/NCI.
Footnotes
Human and Animal Rights and Informed Consent
All studies by the authors involving animal subjects were performed after approval by the appropriate institutional review boards.
Conflict of Interest
JA Sterling has received research support from the VA and NIH/NCI.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Rey-Rico A, Silva M, Couceiro J, Concheiro A, Alvarez-Lorenzo C. Osteogenic efficiency of in situ gelling poloxamine systems with and without bone morphogenetic protein-2. Eur Cell Mater. 2011;21:317–40. doi: 10.22203/ecm.v021a24. [DOI] [PubMed] [Google Scholar]
- 2.Egermann M, Schneider E, Evans CH, Baltzer AW. The potential of gene therapy for fracture healing in osteoporosis. Osteoporos Int. 2005;16 (Suppl 2):S120–8. doi: 10.1007/s00198-004-1817-9. [DOI] [PubMed] [Google Scholar]
- 3.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6. doi: 10.1016/s8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 4.Lill CA, Hesseln J, Schlegel U, Eckhardt C, Goldhahn J, Schneider E. Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J Orthop Res. 2003;21:836–42. doi: 10.1016/S0736-0266(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 5.Barrios C, Brostrom LA, Stark A, Walheim G. Healing complications after internal fixation of trochanteric hip fractures: the prognostic value of osteoporosis. J Orthop Trauma. 1993;7:438–42. doi: 10.1097/00005131-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cornell CN. Internal fracture fixation in patients with osteoporosis. J Am Acad Orthop Surg. 2003;11:109–19. doi: 10.5435/00124635-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Han CH, Park JI, Kim JY. Failure of intertrochanteric fracture fixation with a dynamic hip screw in relation to pre-operative fracture stability and osteoporosis. Int Orthop. 2001;25:360–2. doi: 10.1007/s002640100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboulafia AJ, Levine AM, Schmidt D, Aboulafia D. Surgical therapy of bone metastases. Seminars in oncology. 2007;34:206–14. doi: 10.1053/j.seminoncol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Utzschneider S, Wicherek E, Weber P, Schmidt G, Jansson V, Durr HR. Surgical treatment of bone metastases in patients with lung cancer. International orthopaedics. 2011;35:731–6. doi: 10.1007/s00264-010-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegener B, Schlemmer M, Stemmler J, Jansson V, Durr HR, Pietschmann MF. Analysis of orthopedic surgery of bone metastases in breast cancer patients. BMC musculoskeletal disorders. 2012;13:232. doi: 10.1186/1471-2474-13-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Hayden RE, Mullin DP, Patel AK. Reconstruction of the segmental mandibular defect: current state of the art. Current opinion in otolaryngology & head and neck surgery. 2012;20:231–6. doi: 10.1097/MOO.0b013e328355d0f3. This review paper summarizes the state of the art in clinical management of mandibular defects resulting from cancer-induced bone disease. [DOI] [PubMed] [Google Scholar]
- 12.Hadji P, Klein S, Haussler B, Kless T, Linder R, Rowinski-Jablokow M, et al. The bone evaluation study (BEST): patient care and persistence to treatment of osteoporosis in Germany. International journal of clinical pharmacology and therapeutics. 2013;51:868–72. doi: 10.5414/CP201931. [DOI] [PubMed] [Google Scholar]
- 13.Reid IR. Overview of Pathogenesis. In: Rosen C, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Ammes, Iowa, USA: John Wiley & Sons, Inc; 2013. pp. 357–60. [Google Scholar]
- 14.Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. International journal of medical sciences. 2008;5:263–72. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Benisch P, Schilling T, Klein-Hitpass L, Frey SP, Seefried L, Raaijmakers N, et al. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS ONE. 2012;7:e45142. doi: 10.1371/journal.pone.0045142. This study relates impaired healing in osteoporotic patients to altered gene expression in MSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–23. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez JP, Montecinos L, Rios S, Reyes P, Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557–65. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 19.Hadji P, Gnant M, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer treatment reviews. 2012;38:798–806. doi: 10.1016/j.ctrv.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer treatment reviews. 2010;36:615–20. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman RE. Adjuvant bone-targeted therapy to prevent metastasis: lessons from the AZURE study. Current opinion in supportive and palliative care. 2012;6:322–9. doi: 10.1097/SPC.0b013e32835689cd. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandra ML, David . Orthopedic Surgical Principles of Fracture Management. In: Rosen C, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Ames, iowa, USA: John Wiley & Sons; 2013. pp. 527–30. [Google Scholar]
- 23.Tidermark J, Blomfeldt R, Ponzer S, Soderqvist A, Tornkvist H. Primary total hip arthroplasty with a Burch-Schneider antiprotrusion cage and autologous bone grafting for acetabular fractures in elderly patients. J Orthop Trauma. 2003;17:193–7. doi: 10.1097/00005131-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Vidyadhara S, Vamsi K, Rao SK, Gnanadoss JJ, Pandian S. Use of intramedullary fibular strut graft: a novel adjunct to plating in the treatment of osteoporotic humeral shaft nonunion. Int Orthop. 2009;33:1009–14. doi: 10.1007/s00264-008-0596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996:300–9. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 26.Cornell CN, Lane JM, Poynton AR. Orthopedic management of vertebral and long bone fractures in patients with osteoporosis. Clinics in geriatric medicine. 2003;19:433–55. doi: 10.1016/s0749-0690(02)00076-9. [DOI] [PubMed] [Google Scholar]
- 27*.Zimmermann R, Gabl M, Lutz M, Angermann P, Gschwentner M, Pechlaner S. Injectable calcium phosphate bone cement Norian SRS for the treatment of intra-articular compression fractures of the distal radius in osteoporotic women. Arch Orthop Trauma Surg. 2003;123:22–7. doi: 10.1007/s00402-002-0458-8. This study highlights the use of injectable calcium phosphate bone cements for stabilization of osteoporotic fractures at sites with relatively low weight-bearing requirements. [DOI] [PubMed] [Google Scholar]
- 28.Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater. 2010;20:1–12. doi: 10.22203/ecm.v020a01. [DOI] [PubMed] [Google Scholar]
- 29*.Moroni A, Larsson S, Hoang Kim A, Gelsomini L, Giannoudis PV. Can we improve fixation and outcomes? Use of bone substitutes. J Orthop Trauma. 2009;23:422–5. doi: 10.1097/BOT.0b013e3181771426. This paper reviews the utility of synthetic bone grafts for improving fixation of osteoporotic fractures. [DOI] [PubMed] [Google Scholar]
- 30.Goodman SB, Bauer TW, Carter D, Casteleyn PP, Goldstein SA, Kyle RF, et al. Norian SRS cement augmentation in hip fracture treatment. Laboratory and initial clinical results. Clin Orthop Relat Res. 1998:42–50. [PubMed] [Google Scholar]
- 31.Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. Journal of shoulder and elbow surgery /American Shoulder and Elbow Surgeons [et al] 2012;21:1787–95. doi: 10.1016/j.jse.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Robinson CM, Page RS. Severely impacted valgus proximal humeral fractures. Results of operative treatment. J Bone Joint Surg Am. 2003;85-A:1647–55. doi: 10.2106/00004623-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Jupiter JB, Winters S, Sigman S, Lowe C, Pappas C, Ladd AL, et al. Repair of five distal radius fractures with an investigational cancellous bone cement: a preliminary report. J Orthop Trauma. 1997;11:110–6. doi: 10.1097/00005131-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ladd AL, Pliam NB. Use of bone-graft substitutes in distal radius fractures. J Am Acad Orthop Surg. 1999;7:279–90. doi: 10.5435/00124635-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 35**.Landi E, Tampieri A, Celotti G, Sprio S, Sandri M, Logroscino G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007;3:961–9. doi: 10.1016/j.actbio.2007.05.006. This study points to the use of calcium phosphate cements with enhanced bioactivity for both stabilization and restoration of normal bone healing in osteoporotic fractures. [DOI] [PubMed] [Google Scholar]
- 36**.Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O'Donnell MD, Hill RG, et al. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials. 2010;31:3949–56. doi: 10.1016/j.biomaterials.2010.01.121. This study points to the potential of bioactive bone cements for restoring normal osteoblast and osteoclast function. [DOI] [PubMed] [Google Scholar]
- 37.Cameron K, Travers P, Chander C, Buckland T, Campion C, Noble B. Directed osteogenic differentiation of human mesenchymal stem/precursor cells on silicate substituted calcium phosphate. J Biomed Mater Res A. 2013;101:13–22. doi: 10.1002/jbm.a.34261. [DOI] [PubMed] [Google Scholar]
- 38*.Cheng N, Dai J, Cheng X, Li S, Miron RJ, Wu T, et al. Porous CaP/silk composite scaffolds to repair femur defects in an osteoporotic model. J Mater Sci Mater Med. 2013;24:1963–75. doi: 10.1007/s10856-013-4945-y. This study points to composite scaffolds as a strategy for improving healing of osteoporotic fractures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simman R, Hoffmann A, Bohinc RJ, Peterson WC, Russ AJ. Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg. 2008;61:337–44. doi: 10.1097/SAP.0b013e318157a185. [DOI] [PubMed] [Google Scholar]
- 40*.Liu HY, Wu AT, Tsai CY, Chou KR, Zeng R, Wang MF, et al. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials. 2011;32:6773–80. doi: 10.1016/j.biomaterials.2011.05.080. This study highlights the potential of PRP for healing osteoporotic fractures. [DOI] [PubMed] [Google Scholar]
- 41.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32:5241–51. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue engineering Part A. 2011;17:1735–46. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 43.Phillips FM, Turner AS, Seim HB, 3rd, MacLeay J, Toth CA, Pierce AR, et al. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6:500–6. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Liu X, Liu X, Ge B. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin Orthop Relat Res. 2010;468:1978–85. doi: 10.1007/s11999-010-1321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Z, Zhu T, Li C, Shi X, Liu X, Yang X, et al. Improvement of intertrochanteric bone quality in osteoporotic female rats after injection of polylactic acid-polyglycolic acid copolymer/collagen type I microspheres combined with bone mesenchymal stem cells. Int Orthop. 2012;36:2163–71. doi: 10.1007/s00264-012-1543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu H, et al. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials. 2013;34:9998–10006. doi: 10.1016/j.biomaterials.2013.09.040. This study underscores the potential of dual delivery of MSCs and osteoinductive factors for restoration of normal bone healing and enhanced bone regeneration. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y, Tang W, Lin Y, Long J, Wang H, Liu L, et al. Combination of bone tissue engineering and BMP-2 gene transfection promotes bone healing in osteoporotic rats. Cell biology international. 2008;32:1150–7. doi: 10.1016/j.cellbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Yue B, Lu B, Dai KR, Zhang XL, Yu CF, Lou JR, et al. BMP2 gene therapy on the repair of bone defects of aged rats. Calcif Tissue Int. 2005;77:395–403. doi: 10.1007/s00223-005-0180-y. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Cheng N, Miron R, Shi B, Cheng X. Delivery of PDGF-B and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials. 2012;33:6698–708. doi: 10.1016/j.biomaterials.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–16. doi: 10.1016/s0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 51.Yoshikawa H, Rettig WJ, Takaoka K, Alderman E, Rup B, Rosen V, et al. Expression of bone morphogenetic proteins in human osteosarcoma. Immunohistochemical detection with monoclonal antibody. Cancer. 1994;73:85–91. doi: 10.1002/1097-0142(19940101)73:1<85::aid-cncr2820730116>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 52.Laitinen M, Jortikka L, Halttunen T. Measurement of total and local bone morphogenic protein concentration in bone tumors. Int Orthop. 1997;21:188. doi: 10.1007/s002640050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleary J, Ddungu H, Distelhorst SR, Ripamonti C, Rodin GM, Bushnaq MA, et al. Supportive and palliative care for metastatic breast cancer: resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast. 2013;22:616–27. doi: 10.1016/j.breast.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaner T, Oktenoglu T, Sasani M, Ozer AF. L5 vertebrectomy for the surgical treatment of tumoral and traumatic lesions of L5 vertebra. Orthopedic reviews. 2012;4:e10. doi: 10.4081/or.2012.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biau DJ, Thevenin F, Dumaine V, Babinet A, Tomeno B, Anract P. Ipsilateral femoral autograft reconstruction after resection of a pelvic tumor. J Bone Joint Surg Am. 2009;91:142–51. doi: 10.2106/JBJS.G.01061. [DOI] [PubMed] [Google Scholar]
- 56.Riedel B, Franklin C, Seal A, Stevanovic M. Free vascularized fibula graft to treat chondroblastoma of the hip. Orthopedics. 2012;35:e259–61. doi: 10.3928/01477447-20120123-20. [DOI] [PubMed] [Google Scholar]
- 57.Legname M, Barbary S, Dautel G. Distal radius reconstruction using a split vascularized fibula. Two cases following giant cell tumor resection. Orthopaedics & traumatology, surgery & research : OTSR. 2011;97:762–5. doi: 10.1016/j.otsr.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Rao LP, Shukla M, Sharma V, Pandey M. Mandibular conservation in oral cancer. Surg Oncol. 2012;21:109–18. doi: 10.1016/j.suronc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Kalavrezos ND, Gratz KW, Sailer HF, Stahel WA. Correlation of imaging and clinical features in the assessment of mandibular invasion of oral carcinomas. Int J Oral Maxillofac Surg. 1996;25:439–45. doi: 10.1016/s0901-5027(96)80079-8. [DOI] [PubMed] [Google Scholar]
- 60.Anne-Gaelle B, Samuel S, Julie B, Renaud L, Pierre B. Dental implant placement after mandibular reconstruction by microvascular free fibula flap: current knowledge and remaining questions. Oral oncology. 2011;47:1099–104. doi: 10.1016/j.oraloncology.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Smolka K, Kraehenbuehl M, Eggensperger N, Hallermann W, Thoren H, Iizuka T, et al. Fibula free flap reconstruction of the mandible in cancer patients: evaluation of a combined surgical and prosthodontic treatment concept. Oral oncology. 2008;44:571–81. doi: 10.1016/j.oraloncology.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Urken ML, Buchbinder D, Costantino PD, Sinha U, Okay D, Lawson W, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124:46–55. doi: 10.1001/archotol.124.1.46. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson AS, Zevallos J, Smith M, Lazarus CL, Husaini H, Okay D, et al. Quality of life after management of advanced osteoradionecrosis of the mandible. Int J Oral Maxillofac Surg. 2013;42:1121–8. doi: 10.1016/j.ijom.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 64.Alam DS, Nuara M, Christian J. Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngol Head Neck Surg. 2009;141:196–201. doi: 10.1016/j.otohns.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Munoz Guerra MF, Naval Gias L, Campo FR, Perez JS. Marginal and segmental mandibulectomy in patients with oral cancer: a statistical analysis of 106 cases. J oral Maxillofac Surg. 2003;61:1289–96. doi: 10.1016/s0278-2391(03)00730-4. [DOI] [PubMed] [Google Scholar]
- 66.Tei K, Totsuka Y, Iizuka T, Ohmori K. Marginal resection for carcinoma of the mandibular alveolus and gingiva where radiologically detected bone defects do not extend beyond the mandibular canal. J oral Maxillofac Surg. 2004;62:834–9. doi: 10.1016/j.joms.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Simon EN, Merkx MA, Kalyanyama BM, Shubi FM, Stoelinga PJ. Immediate reconstruction of the mandible after resection for aggressive odontogenic tumours: a cohort study. Int J Oral Maxillofac Surg. 2012 doi: 10.1016/j.ijom.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Gadre PK, Ramanojam S, Patankar A, Gadre KS. Nonvascularized bone grafting for mandibular reconstruction: myth or reality? J Craniofac Surg. 2011;22:1727–35. doi: 10.1097/SCS.0b013e31822e633b. [DOI] [PubMed] [Google Scholar]
- 69**.Matsuo A, Chiba H, Takahashi H, Toyoda J, Hasegawa O, Hojo S. Bone quality of mandibles reconstructed with particulate cellular bone and marrow, and platelet-rich plasma. J Craniomaxillofac Surg. 2011;39:628–32. doi: 10.1016/j.jcms.2011.01.003. This study shows that local delivery of MSCs and osteoinductive factors is an effective strategy for regeneration of mandibular bone defects. [DOI] [PubMed] [Google Scholar]
- 70.Kokorina NA, Lewis JS, Jr, Zakharkin SO, Krebsbach PH, Nussenbaum B. rhBMP-2 has adverse effects on human oral carcinoma cell lines in vivo. The Laryngoscope. 2012;122:95–102. doi: 10.1002/lary.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kokorina NA, Zakharkin SO, Krebsbach PH, Nussenbaum B. Treatment effects of rhBMP-2 on invasiveness of oral carcinoma cell lines. The Laryngoscope. 2011;121:1876–80. doi: 10.1002/lary.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immunity & ageing : I & A. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]


