Abstract
Mathematical models have been central to understanding the interaction between neural control and breathing. Models of the entire respiratory system – which comprises the lungs and the neural circuitry that controls their ventilation - have been derived using simplifying assumptions to compartmentalise each component of the system and to define the interactions between components. These full system models often rely – through necessity - on empirically derived relationships or parameters, in addition to physiological values. In parallel with the development of whole respiratory system models are mathematical models that focus on furthering a detailed understanding of the neural control network, or of the several functions that contribute to gas exchange within the lung. These models are biophysically based, and rely on physiological parameters. They include single-unit models for a breathing lung or neural circuit, through to spatially-distributed models of ventilation and perfusion, or multi-circuit models for neural control. The challenge is to bring together these more recent advances in models of neural control with models of lung function, into a full simulation for the respiratory system that builds upon the more detailed models but remains computationally tractable. This requires first understanding the mathematical models that have been developed for the respiratory system at different levels, and which could be used to study how physiological levels of O2 and CO2 in the blood are maintained.
[Every living cell in the human body (as well as in other mammals and animals) needs oxygen (O2) to survive. O2, combined with the breakdown products of carbohydrate, fat or protein, releases the energy required for the function of cells. This process results in production of carbon dioxide (CO2) which needs to be removed from the cells1. Effective delivery of O2 and removal of CO2 from all of the cells in large organisms is achieved by coordination between a blood circulatory system that transports the required amount of O2-rich blood to the tissue cells and returns CO2 to the heart and lungs; and an external breathing system (the lungs) that transports air to and from a vast gas exchange surface. Together the two transport systems bring external air into close proximity with blood in the lung, hence enabling gas exchange2. The rate at which air and blood are delivered to the gas exchange surface is critical for effective gas exchange: hence, to ensure adequate and matched rates of delivery of both air and blood to the gas exchange surface, the body has evolved respiratory control systems that operate both centrally and locally to adapt the minute ventilation (the total amount of new air that moves into the lungs each minute) and the cardiac output (the volume of blood being pumped by the heart) over long and short periods of time.
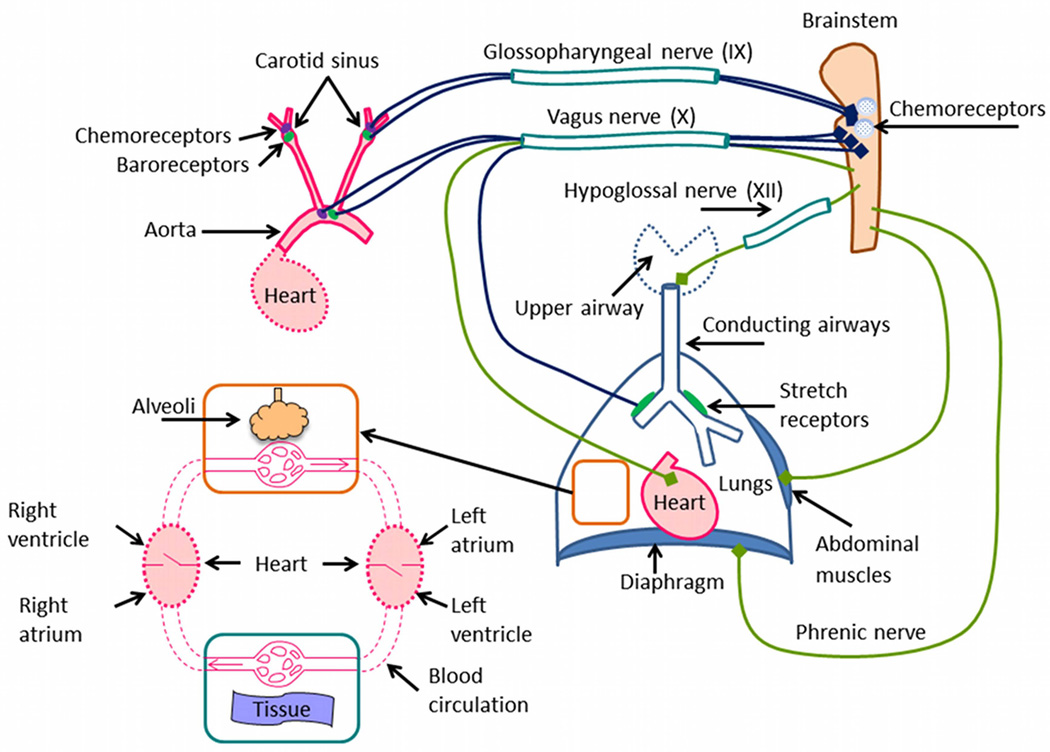
Figure 1 shows the components of the cardio-respiratory system that are known to be essential for the effective exchange of respiratory gases with the environment and the body tissues. Central control of lung movement and heart rate is initiated from a complex interacting neural network, located in the brainstem. The neural network receives signals from various parts of the body including from chemoreceptors within the brainstem3, and in response it generates the signals that affect lung movement and heart rate. The lung movement is mainly affected by the diaphragm, which is excited during inspiration by a signal from the phrenic nerve, and which recoils during expiration. Expiration is usually passive in mammals, however, when forced expiration occurs, it is mainly affected by the abdominal muscles2. The resistance to flow in the upper airway and movements of the tongue are affected by signals from the hypoglossal nerve. The vagus nerve is an important bundle of nerves that carries signals to and from the brainstem. Signals from chemoreceptors and baroreceptors in the aorta as well as signals from stretch receptors in the lungs are transferred via the vagus nerve to the brainstem, and signals that act to slow the heart rate are transferred by the vagus nerve to the heart. Chemoreceptors and baroreceptors are also located in the carotid sinus and their signals are transferred to the brainstem via the glossopharyngeal nerve. The lung itself is a complex organ that comprises of airways and blood vessels that are ‘suspended’ within a delicate network of gas exchange tissue4. The airways, pulmonary arteries, and pulmonary veins converge at the alveoli (microscopic air-filled sacs, with walls comprised of capillaries) where respiratory gas exchange occurs.
Figure 1.
A schematic description of the respiratory system and other organs with which it interacts.
The cardio-respiratory system has been studied extensively both experimentally and theoretically using mathematical models, although many elements of the system have been studied in isolation (for example, the heart is often studied separately from the lungs, and the neural system is studied in isolation from the rest of the body). Isolated studies are possible mathematically by providing adequate boundary conditions for each element. However, understanding what the correct boundary conditions are under different physiological conditions in health and disease requires studying the system as a whole, in particular since the interactions between the different components of the system are difficult to predict. Understanding how isolated studies contribute to the integrated behaviour of the cardio-respiratory system is important for achieving the ultimate goal of improving human health. Here we review mathematical models that have been developed for the respiratory system at different levels, focusing on the need to maintain physiological levels of O2 and CO2 in the blood and ignoring other functions of the respiratory system such as speaking, coughing, laughing and sniffing2. We also focus on response times over a period of minutes and do not look at the transitions from acute to chronic conditions that occur over time periods of days and years. Our review is not intended to be comprehensive and we do not mention all of the models in the field: more comprehensive literature reviews have been published in the past5–8 to which we refer the interested reader. Instead, we focus in more detail on a number of models and use them to highlight the different approaches and trends in the field.
MODELS OF THE ENTIRE RESPIRATORY SYSTEM
“The essence of physiology is regulation”. F.S. Grodins et al.9
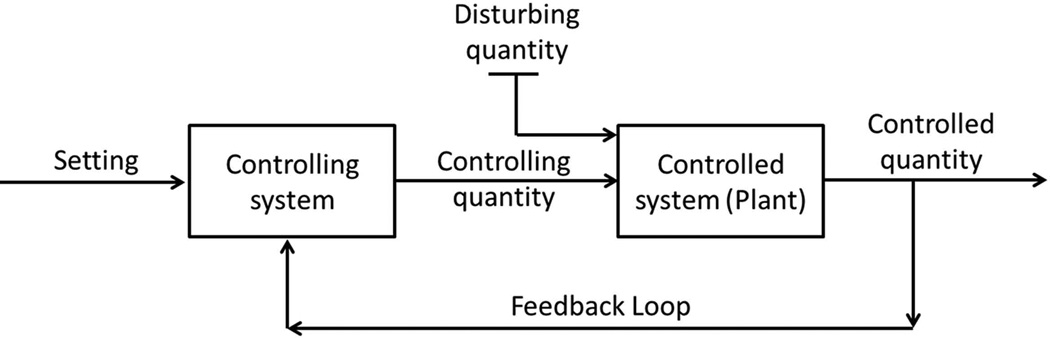
The idea that lung ventilation is regulated so that alveolar partial pressure of CO2 remains constant was expressed as early as 190510, however, it was after World War II and the consequent rapid development of control-system theory that the first quantitative respiratory model appeared11. The earliest models, led by Gray12, described an empirical relationship between minute ventilation and steady state measurements of arterial pH, partial pressure of CO2 and/or partial pressure of O26. The first dynamic model of the respiratory system was developed by Grodins et al. (1954)9. Although it is the later more elaborate model by Grodins et al. (1967)13 that became famous, the 1954 model provided the basic principles on which many models of the entire respiratory system are based. Figure 2, adapted from the 1954 publication9, describes the general features of a feedback regulator. The most important idea behind Figure 2 is that there is some quantity (the “controlled quantity”) that is kept as close as possible to some set value. This is achieved by feeding the value of the controlled quantity into the controlling system, which in turn changes some controlling quantity that affects the controlled system. As a result the controlled quantity varies such that it is kept close to the set value even if there are some disturbances to the system. Note that by algebraic manipulations the “setting value” can be “hidden” among other parameters of the controlled system and may not appear explicitly in the model equations. However, having a “setting value” in Figure 2 helps emphasize that the control system has a purpose - in the case of the respiratory system it is to maintain adequate levels of arterial partial pressures of O2 and CO22.
Figure 2.
General features of a feedback regulator (adapted from Grodins et al. (1954)9, with permission, American Physiological Society).
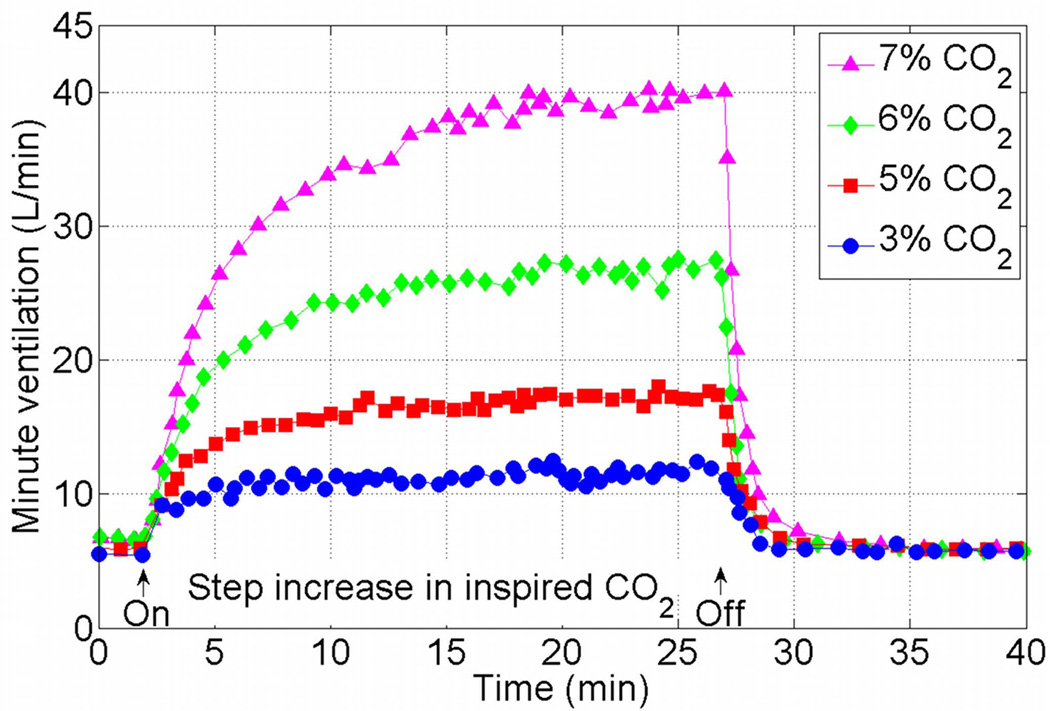
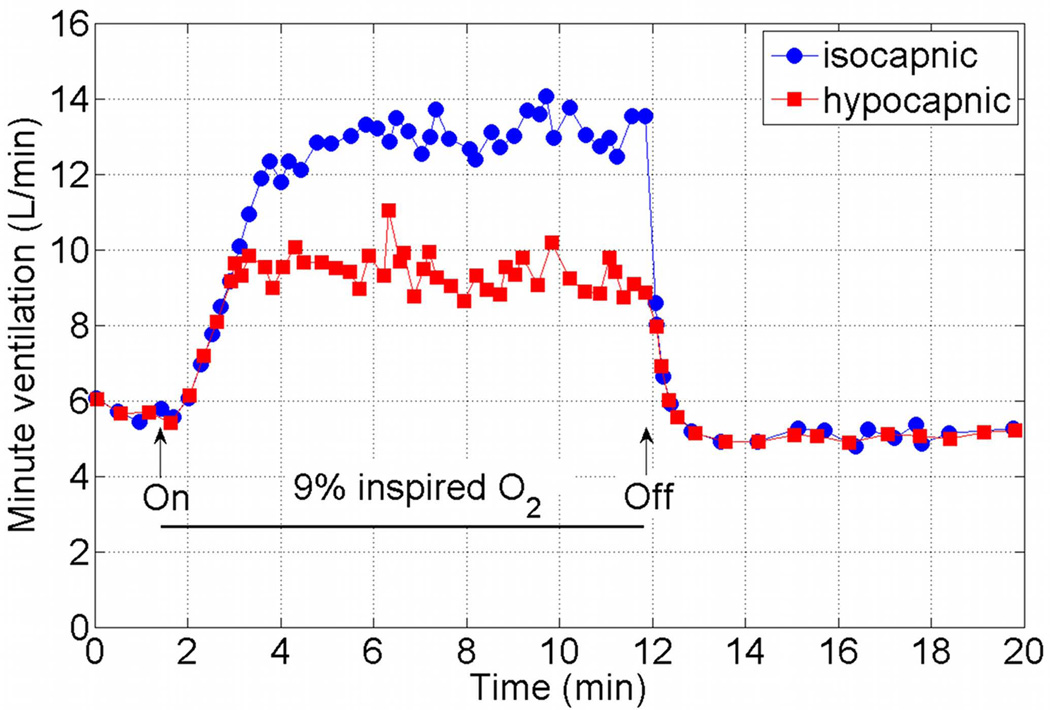
Models of the entire respiratory system include the general features seen in Figure 2 and differ in the details of each component, including the number of controlled and controlling quantities. The models also differ in the experiments that they have been designed for or shown to mimic. Mathematical models should of course be tested against a variety of physiological conditions, however, when reviewing mathematical models in this section we will focus on the model responses to hypoxia (decrease in inspired O2) and hypercapnia (increase in inspired CO2). These responses are used routinely to test the control function of the respiratory system and were mimicked by many mathematical models. A typical minute ventilation response to hypercapnia measured in humans is shown in Figure 3. The data was extracted from Figure 10 of Reynolds et al. (1972)14 using the ENGAUGE software15. Note that the minute ventilation increases with increased inspired CO2, that it takes a longer time to reach steady state when the inspired CO2 increases, and that the “on” step response is slower than the “off” step response. Also note that the data is an average of the responses of 14 human subjects when 7% CO2 is inhaled, and the average of 10 subjects in all the other cases. We do not know why a different number of subjects was chosen in one case and how the subjects were chosen from a pool of 15 subjects. These limitations of the experimental data will be discussed further later in this review. Figure 4 shows the mean response of minute ventilation in 10 human subjects to 9% inspired O2. The data was extracted from Figure 8 of Reynolds et al. (1973)16 using the ENGAUGE software15. The upper curve shows the results when the alveolar CO2 concentration was kept constant. In the lower curve the alveolar CO2 concentration was not controlled and has changed as a result of the increase in minute ventilation.
Figure 3.
Mean response of minute ventilation to 7, 6, 5 and 3% inspired CO2 in 10 human subjects except for 7% where the mean response is in 14 human subjects. The data was extracted from Figure 10 of Reynolds et al. (1972)14 (figure used with permission, American Physiological Society) using the ENGAUGE software available from http://sourceforge.net15.
Figure 4.
Mean response of minute ventilation in 10 human subjects to 9% inspired O2. The upper curve shows the results when the alveolar CO2 concentration was controlled. The data was extracted from Figure 8 of Reynolds & Milhorn (1973)16 (figure used with permission, American Physiological Society) using the ENGAUGE software available from http://sourceforge.net15.
Below we discuss three models in detail. 1) The Grodins model which served as a basis and inspiration to other models in the field. 2) The Khoo/PNEUMA model that represents a large-scale computational effort to model the cardio-respiratory system and couples together several sub-models that have been developed by various groups; and 3) the Ben-Tal and Smith model which represents the first attempts to couple recent knowledge gained on neural dynamics with lung mechanics and gas exchange.
The Grodins model
As mentioned previously, the first dynamic model of the respiratory system was developed by Grodins et al. (1954)9, but it is the 1967 model13 that people regard as the “Grodins model” and which we will describe here (for a review of the 1954 model see Khoo & Yamashiro6). The controlled system in the Grodins model13 consists of 12 ordinary differential equations (ODEs) that describe the rate of change of CO2, O2 and N2 concentrations (or volume fractions) and partial pressures in the lungs, brain and tissue. The lung is modeled as a rigid container with uniform content and zero dead space ventilated by a continuous unidirectional stream of air and perfused by a unidirectional flow of blood. The brain compartment includes two reservoirs, one through which blood flows and one containing cerebrospinal fluid. The tissue compartment represents O2 consumption and CO2 production in the entire body. Time delays in the model represent the blood transport time between the different compartments. Grodins et al.13 studied three versions of their model (each taking into account different levels of complexity). In the second and third versions of the model, the controlled quantities were the brain H+ as well as arterial H+ and partial pressure of O2 at the peripheral chemoreceptors (recall that pH is a function of log[H+] and that [H+] can be expressed as a function of CO2). The controlling system consisted of an empirical function (based on experimental data) whose entries were the controlled quantities and whose output was the minute ventilation (the controlling quantity).
In response to a step increase of 5% inhaled CO2 there was a fast increase in minute ventilation followed by a slower increase, but the model did not reach steady state after 30 min of simulation (although steady state of about 20 L/min has been reached after 49 min). The return of minute ventilation back to normal once the CO2 stimulation was removed was slower than the experimental observation. Similarly, the response to a 30 min step decrease of 10% O2 with a subsequent return to air (21% O2) was also simulated by Grodins et al.13. The model produced ventilation overshoot during the on transient and a prolonged interval of periodic breathing after the stimulation was turned off, both of which are inconsistent with experiments.
Despite the obvious limitations of the Grodins model it has served as a basis for many other models and has inspired other studies in the field (see, for example, references17–23), perhaps because it could accept a variety of stimulations and physiological conditions and because it could produce periodic breathing (although this has not been shown in the original paper).
The Khoo/PNEUMA model
Perhaps the most detailed (non-commercial) model to date of the cardio-respiratory system has been developed over the past 30 years by Prof. Michael Khoo and his collaborators. A comprehensive description of the model can be found in Cheng et al.24 and an implementation of the model using SIMULINK®/MATLAB® (The Mathworks, Natick, MA) is available as a software package named “PNEUMA” (see http://bmsr.usc.edu/software/pneuma/).
The model, which has over 250 parameters and variables, is described as a collection of modules each with a set of inputs and outputs that are sometimes related via differential equations and sometimes via empirical functions (this block diagram representation is the working environment for SIMULINK®). At the highest level of organization, the model is divided into four interconnected main units: respiratory system, cardiovascular system, central neural control and sleep mechanism, but we will focus our attention on the respiratory system unit (which we will regard as the controlled system) and the modules within the central neural control unit that affect the respiratory system directly (we will regard these as the controlling system). The controlled quantity in the Khoo/PNEUMA model (within the context described above) is a chemoreflex-related ventilator drive (called Dchem) which is the sum of central and peripheral chemoreflex components (as well as a sleep-wake state index). The controlling quantities are total ventilator drive (called DT or DTotal) and the breathing rhythm (which is a square signal giving 1 during inspiration and 0 during expiration with equal periods for inspiration and expiration over a single breath). The respiratory system module includes upper airway and inspiratory muscle mechanics, and gas exchange in the lungs, body tissues and brain region. The central and peripheral chemoreceptors are also included under “Gas Exchange and Transport”. The central neural control unit contains a “Ventilatory Drive” module that combines the chemical drive, Dchem, with other neural signals (such as the sleep/wake state) and produces the total ventilator drive.
The Khoo/PNEUMA model has been used to study a variety of responses including the appearance of periodic breathing under different physiological conditions. Several of these simulations are shown in Cheng et al.24 and one of them shows the transient response to isocapnic hypoxia (the transient response to hypercapnia is not shown). The breathing frequency and the lung volume (called tidal volume in the paper but note that this differs from the physiological definition, see for example Guyton & Hall1) do not increase as much as seen experimentally when inspired O2 is reduced from 21% to 10% (the volume amplitude increases from 0.6 L to 0.7 L and the breathing frequency increases from 12.5 to 12.8 breaths/min; this is roughly an increase of 1.5 L/min, far smaller than the increase in minute ventilation shown in Figure 4). Some of the parameters used in the Khoo/PNEUMA model are based on Magosso & Ursino25 who chose the parameters in their model such that it will fit the isocapnic hypoxia response with 7% O2 published in Reynolds & Milhorn16. This is perhaps an illustration that the integrated behaviour of the system is different than the behaviour of isolated components.
The sheer size of the Khoo/PNEUMA model and the computational platform it was implemented on make it difficult to analyze this model in the same way the Grodins model was analyzed (for example, by reducing the model using scaling and asymptotic analysis as was done in Fowler et al.17). Nevertheless earlier versions of the model were analyzed in isolation (see, for example, references5, 26).
The Ben-Tal and Smith model
There have been several attempts to link neural dynamics to models of the lungs. For example, Eldridge27 coupled a limit cycle oscillator (the Fitzhugh-Van der Pol-Bonhoeffer) to a lung model which incorporates volume change and gas exchange, and Longobardo et al.28 included a respiratory pattern generator in their model. The Ben-Tal & Smith model29 which we will review here in more detail relied less than previous models on empirical input-output relationships and provided unique insights into the physiological system. The model consists of nine ordinary differential equations. The controlled system represents the lungs as a single compartment with a moving plate and takes into account lung mechanics, gas exchange and gas transport. The controlled quantities are the blood partial pressures of CO2 and the saturation value of hemoglobin at the end of the capillaries (i.e. where blood exit the lungs). The controlling quantity is a phrenic nerve signal (ramp signal in the model) that leads to changes in the intrapleural pressure (via a differential equation representing the diaphragm displacement). The control system consists of an oscillator, representing the activity of the pre Bötzinger complex (pre-BötC, an area of the brainstem considered to be vital for the breathing rhythm generation during the inspiratory phase) and a leaky, resetting integrator representing ramp generation in the ventral respiratory group (VRG, also located in the brainstem) which is driven by the pre-BötC and drives the phrenic nerve motor output to produce diaphragm displacement. The model makes the assumption (based on experimental observations) that amplitude is controlled at the level of the VRG and frequency is controlled at the level of the pre-BötC (note however that changes in frequency will also affect the amplitude of respiration due to the integration of the pre-BötC signal).
Ben-Tal & Smith (2008)29 investigated the dynamic response of the model to 2 min exposure to hypercapnia or hypoxia when the controlling system was affected by a signal proportional to an error (the difference between the setting value and the controlled quantity) and/or to the integral of the error (known as a P-controller and a PI-controller, respectively). The experiments that this study attempted to mimic were performed on anesthetized dogs and are described in Figures 4–10 and 5-1A of Comroe2. The effect of an additional circulatory transport delay on these dynamic responses and on the appearance of periodic breathing were studied in Ben-Tal & Smith (2010)30. In these two studies the authors concluded that the PI-controller gave results that fit better with experimental data and that as a consequence of a PI-type behavior, the system cannot reach steady state under hypercapnia once a certain level of CO2 is inhaled (unless other mechanisms, that have not been taken into account in the model, start to operate). This prediction is consistent with Figure 3 and 4 above where minute ventilation does not reach steady state after 30 min when 7% CO2 is inhaled, while the hypocapnic hypoxia response stabilizes after less than 2 min (although the minute ventilation starts to drop later on, an observation also predicted by the Ben-Tal & Smith model). This conclusion of the Ben-Tal & Smith model brings into question some of the methodology used to create Figure 3 (although otherwise the experiment in Reynolds et al.14 was carefully done). Why the mean of 14 human subjects was used to describe the response to 7% inhaled CO2 and not the mean of 10 subjects as was done in the other cases (was it to smooth the data?) and how the subjects were chosen out of the entire pool of 15 subjects? In comparison (as pointed out in Ben-Tal & Smith, 200829), Dripps & Comroe31 noted that only 27 of 42 individuals reached steady state when 7.6% CO2 was inhaled and only 13 of 31 subjects when 10.4% CO2 was inhaled (the experiment continued until steady state was reached or until the subject became too uncomfortable, when steady state was reached it was in less than 10 min). Reynolds et al.14 noted that subjects with erratic CO2 responses were eliminated from the experiment.
MODELS OF RESPIRATORY NEURAL CONTROL
“A heart may continue to beat rhythmically for many hours after it has been removed from the body… The muscles of respiration possess no inherent rhythm”. J.H. Comroe2.
The quest for finding the sites and mechanisms involved in respiratory rhythm generation can be traced back to 1812 when Legallois identified a small part of the medulla as the origin of breathing2, 32. Several regions within the medulla have now been identified as essential for rhythm generation and control of breathing but there is no agreement over the details of how the different regions are connected and interact3, 33, 34. Mathematical and computational models of the respiratory network have been developed at the level of a single neuron, small and large networks of neurons, and networks of populations of neurons; these models have helped to form hypotheses and guide experimental studies (see a recent review by Lindsey et al.35). Most studies (experimental and theoretical) have focused more on the mechanism of respiratory pattern generation and less on the neural response to changes in blood partial pressures of O2 and CO2. Some models have taken into account mechanical feedback from the lungs (sent from the stretch receptors via the vagus nerve36) and other signals associated with cough37. While activation of the pulmonary stretch receptors during inspiration shortens the inspiratory phase in rats, cats, dogs and rabbits, it has little or no effect on humans1, 2, 38. This difference between humans and laboratory animals highlights one of the difficulties in coupling neural models to lung models: measurements of lung function which are used to develop lung models are routinely done on humans while measurements at the neural level are performed on laboratory animals. Some of the lung models (for example, Ben-Tal39) can be scaled to represent another mammal and hence can be coupled to neural models developed for that animal. However, developing a detailed neural model that truly represents the human neural system is more difficult and remains a challenge.
A recent example where the effects of hypercapnia and hypoxia have been taken into account in a detailed model of neural network is described in Molkov et al.40. The model is based on Rybak et al.41 and Smith et al.42, and includes eight interacting neuronal populations, each represented by 50 heterogeneous neurons. The aim of the study was to examine the interaction between neurons that reside in a region of the brainstem called the Bötzinger complex (BötC) and pre-BötC, and neurons that reside in the retrotrapezoid nucleus (RTN)/parafacial respiratory group (pFRG) region. The BötC/pre-BötC is considered to be responsible for generating oscillations that drive the diaphragm during inspiration while the RTN/pFRG region contains central chemoreceptors43 and is considered to be a second oscillator that drives the abdominal muscles in response to hypercapnia to produce forced expiration. The neural system response to changes in O2 and CO2 concentrations was studied in this context. Experimental results from Abdala et al.44 and within Molkov et al.40 show that when hypercapnia or hypoxia is strong enough, the activity of the abdominal nerve increases over time with a step-wise increase in the ratio between the phrenic nerve frequency and the abdominal nerve frequency (changing from 1:5 and 1:4 to 1:3 and 1:2 and, finally, to 1:1). The model in Molkov et al.40 reproduced these results (with hypercapnia and hypoxia taken as tonic drives). The same results were reproduced and analyzed using a reduced model in Rubin et al.45. The interaction between the pFRG and pre-BötC was also investigated in Lal et al.46 and Wittmeier et al.47.
MODELS OF THE LUNGS
”One of the foremost problems is how to ventilate with air and perfuse with blood a surface about the size of a tennis court – and to do that efficiently, homogeneously, and with perfusion and ventilation well matched. This is a demanding task of engineering.” E.R. Weibel48
The lungs are the central organ of external respiration, acting as the interface between air and blood. Achieving effective oxygen uptake and carbon dioxide elimination requires bringing inhaled air into close proximity to blood, along with a reasonable spatial and temporal matching of air and blood delivery to all areas of the gas exchange surface. Mathematical models of external respiration therefore require consideration of at least minute ventilation, right ventricular output, and gas exchange. When a model of these components is considered independent from the rest of the respiratory system, the specification of boundary conditions is also required to represent interaction with other system components as illustrated in Figure 1. Here we consider some examples of models for external respiration from the literature that could be (or have been) used as a component of a full respiratory system model and we consider their respective advantages and disadvantages in this context.
Single-unit lung models
External respiration can be approached as a steady-state problem by assuming complete equilibration of alveolar and arterial partial pressures of O2 and CO2 and specifying only the rates of alveolar ventilation and pulmonary capillary perfusion49. This mass-balance formulation has been used in many models of the entire respiratory system (for example in the Grodins model described above) but limits any understanding of the mechanics of breathing and the interactions between lung, chest wall, airway resistance, and gas exchange. Dynamic (sometimes called ‘breathing’) single-unit ‘compartment’ models are sufficient for understanding the interaction between total ventilation, cardiac output, and gas exchange, and can easily be driven by external models or simple boundary conditions that represent the rest of the respiratory system. Each compartment represents a component of the lung or chest wall, with spatially-distributed and/or complex function lumped into relatively simple models. For example, Ben-Tal39 and Liu et al.50 used similar approaches to modeling dynamic lung and chest wall mechanics and gas exchange, using single-unit compartment lung models with an equation of motion to relate the rate of change of intrapleural pressure (Ppl) to the pressures required to overcome airway resistance and functional lung tissue (i.e. parenchymal) elasticity. Ben-Tal39 presented a ‘simplified’ whole lung model for external respiration that can be reduced to a simple mass balance (under the assumption of full equilibration of gas exchange and time-averaged steady-state flows), or can be scaled to smaller spatial units than the whole lung51, 52, or can be integrated into a respiratory system model to provide dynamically changing gas partial pressures to a neural control model which in turn dynamically controls the frequency and amplitude of breathing29, as described above. Ben-Tal39 assumed constant resting-level values for lung and chest wall compliance, and rigid airways; in contrast the goal of Liu et al.50 was to derive a model that satisfactorily described the dynamics of airway and lung mechanics over the full range of lung volumes from residual volume (RV) to total lung capacity (TLC), hence they included approximations for nonlinear lung compliance and airway resistance. The alveolar region in this model was a nonlinear time-varying viscoelastic lumped parameter unit, with a semi-empirical definition of the pressure-volume relationship for the lung including an imposed hysteresis. As in Ben-Tal39, the upper airway (anatomical deadspace) in Liu et al.50 was assumed rigid, but a compliant ‘collapsible segment’ was included to adequately represent pressure-flow data during simulation of forced expiration. PO2 and PCO2 were simulated as functions of residence time and location within the capillary bed in both models, including equations for the biophysics of O2 binding to hemoglobin. In each model the lung was enclosed in a rigid-walled thoracic cage, with ventilation driven by a time-varying intrapleural pressure (Ppl). In the Ben-Tal39 model the Ppl followed a prescribed sinusoid; in Liu et al.50 the Ppl was set to follow measured Ppl waveforms during the forced expiration. Liu et al.50 used a nonlinear parameter-estimation algorithm to vary sensitive model parameters to obtain reasonable least squares fits to experimental data. This differs from the more biophysical approach of Ben-Tal39, which based model parameters on measured quantities. This relates to an important advantage of the Ben-Tal39 model and its predecessors, which is its scalability. That is, the model is equally relevant to a lung, a lobe, or an arbitrary unit of gas exchange with appropriate change of model parameters. The same scaling is more difficult using the nonlinear model of Liu et al.50 because of its empirical parameter fitting. Swan et al.52 demonstrated scale translation of the Ben-Tal39 model in a study of gas exchange within the pulmonary microstructure, where each ‘unit’ for gas exchange was an individual alveolar duct of the multi-branching acinus (the original model treated the whole lung alveolar volume as a ‘unit’); Swan51 further showed how the model can be scaled to represent each individual acinar ventilation/perfusion (V/Q) unit (~32,000) in the human lung, as will be discussed later.
Dynamic single-unit models such as the two above exemplify the current state-of-the art in terms of integration with the full respiratory system. When models for the biophysics of O2-hemoglobin binding are included, these models provide significant advantage over the assumption of full equilibration, they are fast to compute, are amenable to mathematical analysis, and relatively easy to couple with more complex control system models. A limitation is that they cannot be used to capture the contribution of ventilation/perfusion (V/Q) mismatch to gas exchange. That is, an inherent assumption in the single-unit models is that the lungs are homogeneous. However, the branching structure of the airways and vasculature, the shape of the lungs, inherent regional variations in tissue properties, gravitational effects, and interaction with external organs such as the diaphragm are all factors that lead to spatial heterogeneity of function, which requires a multi-unit model.
Multiple-unit models for ventilation/perfusion matching
Both ventilation (V) and perfusion (Q) have a preferentially gravitational distribution in the normal lung (flow increases on average in the direction of gravity), overlaid with marked iso-gravitational heterogeneity53. Virtually all diseases of the lung that impair gas exchange affect V/Q matching. For example, in asthmatic bronchoconstriction the ventilation is reduced to some regions of tissue; and in pulmonary embolism (occlusion of the pulmonary arteries, typically by a blood clot embolus) the perfusion to specific regions is reduced. Less obvious are the effects of pulmonary edema (fluid in the alveolar space) which increases the diffusion barrier for gas exchange and effectively stiffens the alveolar tissue hence reducing ventilation; or pulmonary arterial hypertension where arterial wall remodeling could theoretically increase the heterogeneity of perfusion. When compensatory mechanisms - either local or system-level – are not sufficient to improve the spatial or temporal matching, this results in obvious gas exchange dysfunction. Understanding the full respiratory system interaction during chronic alteration of V/Q has relevance to the older lung, where a myriad of changes to lung structure and elasticity can alter the V/Q distribution and its heterogeneity, in parallel with a reduction in sensitivity to change in respiratory blood gases.
Models with prescribed V/Q
Many studies have used multi-unit models of, or statistical analyses with, prescribed V/Q distributions to study, for example, the effect of V/Q mismatch on gas exchange54, gas exchange with inhaled N2O55, emphysema and embolism56, hepatopulmonary syndrome57, acute respiratory distress syndrome58, and lung inflammation59. While some of these models are not suitable for integration into a dynamic respiratory system model, others have been designed with this as a long-term goal. For example, Reynolds et al.59 developed a multi-compartment model for gas exchange with focus on inflammation in acute lung injury (ALI, an inflammatory process that typically requires mechanical ventilation). Model compartments comprised the alveolar air space, ‘lung tissue’, and capillary blood. The lung tissue compartment represented the interstitial space, where inflammation could lead to accumulation of interstitial fluid. Each respiratory unit in their model comprised on the order of 25 alveoli and their capillaries, and the functional model comprised equations for O2, oxyhemoglobin, CO2, and bicarbonate (i.e. consistent with the single-unit models of Ben-Tal39 and Liu et al.50), and a relatively simple model for inflammation and the movement of water between compartments. To represent the interaction of the respiratory units with the rest of the lung, the authors assumed the lengths of inspiration and expiration, blood velocity, and inspired partial pressures of O2 and CO2. A ‘lung scale’ model was constructed by linking several respiratory units, each with a different V/Q. The longer-term goal of this work is to understand how to provide interventions to a lung that is under inflammatory stress without triggering further inflammation. This is clearly a multi-scale problem that requires interaction with the full system behavior: the authors noted that their approach to V/Q was simplistic, and the assumption of fixed boundary conditions for pulmonary arterial blood gases and acid-base status was limiting. In a multi-scale approach the prescribed V/Q would be replaced by dynamic simulation of regional ventilation and perfusion in response to local inflammation stiffening the lung tissue; full system interaction would provide variable rates of ventilation and perfusion, and pulmonary arterial blood gases. The detailed focus on inflammation would of course not be suitable or necessary for all studies of respiratory system interaction, and the very small spatial scale of the model means that it cannot be included directly and explicitly for all alveolar units in the intact lung. Its inclusion at a higher spatial scale requires model reduction, by using the detailed behavior of the inflammation model to parameterize a lumped model that represents the averaged behavior of alveoli within a larger spatial unit.
Biophysical models for V/Q matching
The Reynolds et al.59 study highlights the need for predictive models for V/Q that respond to local alterations in the lung, and that interact with the rest of the respiratory system. Predictive models for V/Q require inclusion of the important passive mechanisms that are known to determine V/Q matching. Distributions of ventilation and perfusion throughout the lung exhibit significant heterogeneity, and both are influenced by the branching geometry of the airways and blood vessels, gravity, fluid properties, regional tissue properties, and pathology. Active mechanisms also contribute to V/Q matching. Perhaps the best understood of these is hypoxic pulmonary vasoconstriction (HPV), which is a sustained constriction of the pulmonary arterial smooth muscle in response to low local alveolar PO2, and which acts to divert blood away from poorly ventilated regions of the lung tissue60. The relative importance of active in comparison to passive mechanisms in matching V/Q is not fully understood, however active mechanisms clearly become important when the lung is under stress, and will be necessary to include in models of pathology.
Gravity contributes to V/Q matching and mis-matching in several ways. Hydrostatic pressure leads to greater intravascular pressures in the dependent lung, and deformation of the lung tissue under its own weight (the ‘Slinky effect’) establishes a greater density, on average, - of vessels per unit volume in this region, and hence more flow per unit volume of tissue. Hopkins et al.61 proposed that the perfusion gradient became insignificant when flow per unit of tissue was normalized by density, hence postulating that the gravitational gradient in perfusion was largely due to the Slinky effect. Clark et al.62 studied this hypothesis using a computational model that included both tissue deformation under gravity, and the effect of a hydrostatic pressure gradient on pulmonary blood flow. A finite deformation elasticity model for deformation of compressible lung tissue63 was used to simulate the displacement of the lung tissue under gravity, and the elastic recoil pressures acting on an anatomically-structured model of the pulmonary arteries, veins64, and intra-acinar microcirculation65. Perfusion and elastic vessel deformation were simulated under steady-state boundary conditions for right ventricular output and left atrial pressure. The steady-state assumption allows simulation of perfusion using Poiseuille flow equations, rather than the more time-consuming solution of one-dimensional Navier Stokes equations (which are necessary for pulsatile flow). The study concluded that the hydrostatic pressure gradient contributes at least as much as the tissue deformation to the gravitational gradient, disputing the Hopkins hypothesis. A biophysical model for perfusion distribution therefore requires adequate consideration of hydrostatic effects in parallel with the ‘Slinky effect’.
Gravity also plays an important role in determining the ventilation distribution. The deformation of the lung under its own weight establishes a gravitationally directed gradient in tissue expansion at functional residual capacity (FRC, the lung volume at the end of a restful exhalation), with tissue in the dependent region less expanded than tissue in the non-dependent region (i.e. the base and apex, respectively, of the upright lung). The lung is nonlinearly elastic, so this distribution of tissue volumes results in a nonlinear distribution of tissue compliance at FRC. A uniform decrease in the intrapleural pressure around the lung therefore expands the lung tissue at different rates, depending on the local compliance, and hence when inhaling a normal tidal volume from FRC the ventilation is weighted more towards the dependent than the non-dependent tissue. Multiple-unit models for the lung have been used to simulate this ventilation non-uniformity; for example, Chang & Yu66 used a five-lobe model to study the contribution of compliance and airway resistance to ventilation distribution under quiet breathing, at high flow rates, and using gases of different physical properties. The model was derived on the basis of balancing the change in pleural pressure during breathing with the change in pressures due to elastic deformation of each lobe, and resistive pressure drop along the airways. The model was parameterized using some measured quantities and some empirical estimates from the literature. The five-lobe model can reproduce several features of the ventilation distribution: a preferential distribution of ventilation to the dependent tissue during quiet breathing, reversal of the distribution above a high flow threshold, and sensitivity of the distribution to gas density and viscosity. A multi-unit model was also presented by Swan et al.67, who extended the Ben-Tal39 model to simulate flow to each of the ~32,000 acini of the human lung. The Ben-Tal39 model was adapted by explicitly including a full multi-branching tree, with each terminal branch coupled to an independent compliant acinus. Using the same approach as Clark et al.62, an anatomically-structured airway tree model68 was embedded within a compressible finite elasticity model for the lung63. Deformation of the lung tissue model was used to initialize the compliance of each acinus. As in Ben-Tal39, ‘breathing’ was simulated by specifying a sinusoidally-varying pleural pressure. The model predicted ventilation distribution under resting breathing that was consistent with experimental studies, and iso-gravitational heterogeneity as a consequence of variation in material properties in the model. In agreement with the five-lobe model, resistance was found to play an insignificant role in distributing the ventilation during baseline conditions. The five-lobe model is a convenient approach that provides advantages over the single-unit models in terms of its ability to predict a non-uniform ventilation distribution, however it lacks the dynamics of the single-unit models. The Swan et al.67 model provides a more comprehensive prediction of ventilation distribution that is time-dependent, and that can be controlled by modifying the sinusoidal intrapleural pressure boundary condition to one that is controlled by the respiratory system interactions. A further advantage is that the model can be reduced to a lesser number of units (by appropriate series and parallel summation of tissue compliances and airway resistances) as is required.
Swan51 integrated the perfusion and ventilation models of Clark et al.62 and Swan et al.67, respectively, with the gas exchange model in Ben-Tal39 to simulate the spatial distribution of gas exchange in the full upright human lung. The time-averaged spatial distribution was remarkably consistent with values estimated by West et al.69 based on measured gravitational gradients for perfusion and ventilation, exactly the same as the steady-state distribution from the Kapitan & Hempelman49 model under resting conditions in a healthy lung model, and with an alveolar-arterial partial pressure difference that indicated slightly more efficient gas exchange than is expected for the healthy intact lung. Swan51 used the model to study the sensitivity of gas exchange to diffusion barrier thickening and lung tissue stiffening in ALI, which is similar to the study of Reynolds et al.59 but on a different spatial scale and without a model for the inflammatory process. The study concluded that gas exchange is far more sensitive to lung tissue stiffening than to diffusion barrier thickening, due to the effect of stiffening on V/Q disturbance. Because this model is an extension of the Ben-Tal39 single-unit, which has already been extended to dynamic interaction with neural control29, 30, it should in theory be merely a matter of implementation to substitute the single-unit model for the multiple-unit model in a full respiratory system model.
Iso-gravitational heterogeneity in the ventilation and perfusion distributions has already been mentioned in brief. The asymmetric pulmonary arterial tree geometry has been shown to be an important source of heterogeneity in the perfusion distribution62. In contrast, the low viscosity of gas in the airway tree means that the airway tree geometry is not likely to contribute significantly to the ventilation distribution and hence to its heterogeneity under resting breathing, and in this situation normal variation in the material properties of the lung is likely to be the important mechanism. On the other hand, when air flow increases the resistance to flow becomes significant, and tree geometry is likely to contribute to heterogeneity. Capturing the contribution of airway and vascular tree geometry to these distributions requires models with anatomically-based and spatially-distributed geometry. One approach is to adopt a fractal model, where each bifurcation in the tree is assumed to be scale-independent, and the blood or air flow is divided asymmetrically according to a prescribed ratio. Using this approach Glenny et al.70 have shown how small asymmetries in flow division can lead to perfusion heterogeneity on the order of what is observed experimentally. However this approach lacks any connection to the biophysics of fluid flow and its interaction with the deforming lung tissue. Clark et al.62 and Swan et al.67 used spatially distributed models for the pulmonary vascular and airway trees, respectively, that were dependent on the forces transmitted to them via the lung tissue model in which they were embedded. Using this approach one could readily examine the effect of a localized change in lung tissue material properties on perfusion and ventilation distributions, gas exchange, and feedback from respiratory control (e.g. the effect of increased tissue compliance as in emphysema).
Conclusion
Models of the respiratory system have been developed at different levels and at high degree of sophistication but so far detailed models for lung function have not yet been integrated with detailed models for neural control. The current challenge is to bring these models together in a tractable way, and to integrate the system- and organ-level models with models that operate at the tissue or cellular level, to enable good understanding of the system function in health and disease. Integrating all of the important functions of the lung with neural control of ventilation into a full respiratory system model requires models of individual components that are scalable. The need for scalability has been illustrated in this article for models of both neural control and the lungs. Scalability improves model sophistication, enables coupling of models that would otherwise be too cumbersome to bring together, and is important for the integration of models that were originally developed using data from different species. Models that can scale in complexity allow us to interrogate the function of a whole organ or neural circuitry when it is represented in full detail, and to determine the exact features that can be reduced using simple assumptions of structure (e.g. symmetry or regular asymmetry of airway or vascular branching) or function (e.g. homogeneous perfusion and/or ventilation). That is, this approach enables testing of the degree of complexity that is necessary in each component before it is integrated into the full system model. With the current trend towards developing multi-scale models that integrate function across spatial scales from sub-cellular through to tissue and whole organ, this ability to understand the characteristic responses of a model system and its reducible features becomes particularly important. Integration of local small spatial-scale behavior or inflammation brings further challenges of increased model complexity and may require the addition of local control mechanisms, but is likely to give rise to emergent behavior that cannot be predicted or understood without a full integrated system model.
Acknowledgements
Thanks to Sophie S. Shamailov for her help in extracting the data in Figures 3 and 4. This work was partly supported by NIH grants R01 NS069220 and HL103405.
Footnotes
No conflicts of interest
Contributor Information
Alona Ben-Tal, Institute of Natural and Mathematical Sciences, Massey University, Albany, Auckland, New Zealand.
Merryn H. Tawhai, Email: m.tawhai@auckland.ac.nz, Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand.
References
- 1.Guyton AC, Hall JE. Textbook of medical physiology. 10th ed. Philadelphia: Saunders; 2000. [Google Scholar]
- 2.Comroe JH. Physiology of respiration : an introductory text. 2nd ed. Chicago: Year Book Medical Publishers; 1977. [Google Scholar]
- 3.Smith JC, Abdala APL, Borgmann A, Rybak IA, Paton JFR. Brainstem respiratory networks: building blocks and microcircuits. Trends in neurosciences. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weibel ER. The Pathway for Oxygen - Structure and Function of the Mammalian Respiratory System. USA: Harvard University Press; 1984. [Google Scholar]
- 5.Khoo MC. Quantitative Models of Periodic Breathing and Cheyne-Stokes Respiration. In: Bradley TD, Floras JS, editors. Sleep Apnea Implications in Cardiovascular and Cerebrovascular Disease. 2nd ed. Informa healthcare; 2010. pp. 275–301. [Google Scholar]
- 6.Khoo MC, Yamashiro SM. Models of Control of Breathing. In: Chang HK, Paiva M, editors. Respiratory Physiology: An Analytical Approach. Vol. 40. Marcel Dekker Inc; 1989. pp. 799–829. [Google Scholar]
- 7.Ben-Tal A. Computational models for the study of heart-lung interactions in mammals. Wiley interdisciplinary reviews. Systems biology and medicine. 2012;4:163–170. doi: 10.1002/wsbm.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batzel JJ, Kappel F, Schneditz D, Tran TH. Cardiovascular and respiratory systems : modeling, analysis, and control. Philadelphia: Society for Industrial and Applied Mathematics; 2007. [Google Scholar]
- 9.Grodins FS, Gray JS, Schroeder KR, Norins AL, Jones RW. Respiratory responses to CO2 inhalation; a theoretical study of a nonlinear biological regulator. Journal of applied physiology. 1954;7:283–308. doi: 10.1152/jappl.1954.7.3.283. [DOI] [PubMed] [Google Scholar]
- 10.Haldane JS, Priestley JG. The regulation of the lung-ventilation. The Journal of physiology. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grodins FS. Control theory and biological systems. New York: Columbia University Press; 1963. [Google Scholar]
- 12.Gray JS. The multiple factor theory of the control of respiratory ventilation. Science (New York, N.Y.) 1946;103:739–744. doi: 10.1126/science.103.2687.739. [DOI] [PubMed] [Google Scholar]
- 13.Grodins FS, Buell J, Bart AJ. Mathematical analysis and digital simulation of the respiratory control system. Journal of applied physiology. 1967;22:260–276. doi: 10.1152/jappl.1967.22.2.260. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds WJ, Milhorn HT, Holloman GH. TRANSIENT VENTILATORY RESPONSE TO GRADED HYPERCAPNIA IN MAN. Journal of applied physiology. 1972;33:47-&. doi: 10.1152/jappl.1972.33.1.47. [DOI] [PubMed] [Google Scholar]
- 15.ENGAUGE. Engauge Digitizer. Available at: http://sourceforgenet/projects/digitizer/files/Engauge%20Digitizer/digitizer-41/. [Google Scholar]
- 16.Reynolds WJ, Milhorn HT. TRANSIENT VENTILATORY RESPONSE TO HYPOXIA WITH AND WITHOUT CONTROLLED ALVEOLAR PCO2. Journal of applied physiology. 1973;35:187–196. doi: 10.1152/jappl.1973.35.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Fowler AC, Kalamangalam GP, Kember G. A mathematical analysis of the Grodins model of respiratory control. IMA Journal of Mathematics Applied in Medicine and Biology. 1993;10:249–280. doi: 10.1093/imammb/10.4.249. [DOI] [PubMed] [Google Scholar]
- 18.Fowler AC, Kalamangalam GP. Periodic breathing at high altitude. Ima Journal of Mathematics Applied in Medicine and Biology. 2002;19:293–313. [PubMed] [Google Scholar]
- 19.Topor ZL, Pawlicki M, Remmers JE. A computational model of the human respiratory control system: responses to hypoxia and hypercapnia. Annals of Biomedical Engineering. 2004;32:1530–1545. doi: 10.1114/b:abme.0000049037.65204.4c. [DOI] [PubMed] [Google Scholar]
- 20.Saunders KB, Bali HN, Carson ER. A breathing model of the respiratory system; the controlled system. Journal of Theoretical Biology. 1980;84:135–161. doi: 10.1016/s0022-5193(80)81041-1. [DOI] [PubMed] [Google Scholar]
- 21.Fincham WF, Tehrani FT. A mathematical model of the human respiratory system. Journal of biomedical engineering. 1983;5:125–133. doi: 10.1016/0141-5425(83)90030-4. [DOI] [PubMed] [Google Scholar]
- 22.Duffin J. The role of the central chemoreceptors: a modeling perspective. Respiratory Physiology & Neurobiology. 2010;173:230–243. doi: 10.1016/j.resp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respiration Physiology. 2000;120:13–26. doi: 10.1016/s0034-5687(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Ivanova O, Fan H-H, Khoo MCK. An integrative model of respiratory and cardiovascular control in sleep-disordered breathing. Respiratory Physiology & Neurobiology. 2010;174:4–28. doi: 10.1016/j.resp.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magosso E, Ursino M. A mathematical model of CO2 effect on cardiovascular regulation. American journal of physiology. Heart and circulatory physiology. 2001;281:H2036–H2052. doi: 10.1152/ajpheart.2001.281.5.H2036. [DOI] [PubMed] [Google Scholar]
- 26.Batzel JJ, Tran HT. Stability of the human respiratory control system. I. Analysis of a two-dimensional delay state-space model. Journal of mathematical biology. 2000;41:45–79. doi: 10.1007/s002850000044. [DOI] [PubMed] [Google Scholar]
- 27.Eldridge FL. The North Carolina Respiratory Model. A multipurpose model for studying the control of breathing. In: Khoo MC, editor. Bioengineering Approaches to Pulmonary Physiology and Medicine. Plenum Press; 1996. pp. 25–49. [Google Scholar]
- 28.Longobardo G, Evangelisti CJ, Cherniack NS. Introduction of respiratory pattern generators into models of respiratory control. Respiratory Physiology & Neurobiology. 2005;148:285–301. doi: 10.1016/j.resp.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Tal A, Smith JC. A model for control of breathing in mammals: coupling neural dynamics to peripheral gas exchange and transport. Journal of Theoretical Biology. 2008;251:480–497. doi: 10.1016/j.jtbi.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Tal A, Smith JC. Control of breathing: two types of delays studied in an integrated model of the respiratory system. Respiratory Physiology & Neurobiology. 2010;170:103–112. doi: 10.1016/j.resp.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dripps RD, Comroe JH., Jr The respiratory and circulatory response of normal man to inhalation of 7.6 and 10.4 per cent CO2 with a comparison of the maximal ventilation produced by severe muscular exercise, inhalation of CO2 and maximal voluntary hyperventilation. The American journal of physiology. 1947;149:43–51. doi: 10.1152/ajplegacy.1947.149.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Gesell R, Bricker J, Conway M. Structural and functional organization of the central mechanism controlling breathing. American Journal of Physiology. 1936;31:423–452. [Google Scholar]
- 33.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nature reviews. Neuroscience. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsey BG, Rybak IA, Smith JC. Computational Models and Emergent Properties of Respiratory Neural Networks. Comprehensive Physiology. 2012;2:1619–1670. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molkov YI, Bacak BJ, Dick TE, Rybak IA. Control of breathing by interacting pontine and pulmonary feedback loops. Frontiers in Neural Circuits. 2013;7:18. doi: 10.3389/fncir.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, Davenport PW, Lindsey BG. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Frontiers in physiology. 2012;3:264. doi: 10.3389/fphys.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology - the Nervous System. Vol. IV. Bethesda: American Physiological Society; 1986. pp. 463–524. [Google Scholar]
- 39.Ben-Tal A. Simplified models for gas exchange in the human lungs. Journal of Theoretical Biology. 2006;238:44–495. doi: 10.1016/j.jtbi.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Molkov YI, Abdala APL, Bacak BJ, Smith JC, Paton JFR, Rybak IA. Late-Expiratory Activity: Emergence and Interactions With the Respiratory CPG. Journal of Neurophysiology. 2010;104:2713–2729. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC. Cisek P, Drew T, Kalaska JF, editors. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Computational Neuroscience: Theoretical Insights into Brain Function. 2007;Vol. 165 doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. Journal of Neurophysiology. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guyenet PG, Stornetta RL, Abbott SBG, Depuy SD, Kanbar R. The retrotrapezoid nucleus and breathing. Advances in experimental medicine and biology. 2012;758:115–122. doi: 10.1007/978-94-007-4584-1_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdala APL, Rybak IA, Smith JC, Paton JFR. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. The Journal of physiology. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin JE, Bacak BJ, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Interacting oscillations in neural control of breathing: modeling and qualitative analysis. Journal of Computational Neuroscience. 2011;30:607–632. doi: 10.1007/s10827-010-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lal A, Oku Y, Hulsmann S, Okada Y, Miwakeichi F, Kawai S, Tamura Y, Ishiguro M. Dual oscillator model of the respiratory neuronal network generating quantal slowing of respiratory rhythm. Journal of Computational Neuroscience. 2011;30:225–240. doi: 10.1007/s10827-010-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wittmeier S, Song G, Duffin J, Poon C-S. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weibel ER. Symmorphosis; on form and function in shaping life. Cambridge, Massachusetts: Harvard University Press; 2000. [Google Scholar]
- 49.Kapitan KS, Hempelman SC. Computer simulation of mammalian gas-exchange. Computers in Biology and Medicine. 1986;16:91–101. doi: 10.1016/0010-4825(86)90034-x. [DOI] [PubMed] [Google Scholar]
- 50.Liu CH, Niranjan SC, Clark JW, Jr, San KY, Zwischenberger JB, Bidani A. Airway mechanics, gas exchange, and blood flow in a nonlinear model of the normal human lung. Journal of Applied Physiology. 1998;84:1447–1469. doi: 10.1152/jappl.1998.84.4.1447. [DOI] [PubMed] [Google Scholar]
- 51.Swan AJ. A Multi-scale Computational Model of Pulmonary Gas Exchange. Auckland Bioengineering Institute. 2011;Vol. PhD [Google Scholar]
- 52.Swan AJ, Tawhai MH. Evidence for minimal oxygen heterogeneity in the healthy human pulmonary acinus. Journal of applied physiology. 2011;110:528–537. doi: 10.1152/japplphysiol.00888.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glenny RW, Bernard S, Robertson HT, Hlastala MP. Gravity is an important but secondary determinant of regional pulmonary blood flow in upright primates. Journal of Applied Physiology. 1999;86:623–632. doi: 10.1152/jappl.1999.86.2.623. [DOI] [PubMed] [Google Scholar]
- 54.West JB. Ventilation-perfusion inequality and overall gas exchange in computer models of the lung. Respiration Physiology. 1969;7:88–110. doi: 10.1016/0034-5687(69)90071-1. [DOI] [PubMed] [Google Scholar]
- 55.Peyton PJ, Robinson GJB, Thompson B. Ventilation-perfusion inhomogeneity increases gas uptake: theoretical modeling of gas exchange. Journal of Applied Physiology. 2001;91:3–9. doi: 10.1152/jappl.2001.91.1.3. [DOI] [PubMed] [Google Scholar]
- 56.Yem JS, Turner MJ, Baker AB, Young IH, Crawford ABH. A tidally breathing model of ventilation, perfusion and volume in normal and diseased lungs. British Journal of Anaesthesia. 2006;97:718–731. doi: 10.1093/bja/ael216. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty S, Balakotaiah V, Bidani A. Multiscale model for pulmonary oxygen uptake and its application to quantify hypoxemia in hepatopulmonary syndrome. Journal of Theoretical Biology. 2007;244:190–207. doi: 10.1016/j.jtbi.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 58.Brook BS, Murphy CM, Breen D, Miles AW, Tilley DG, Wilson AJ. Theoretical models for the quantification of lung injury using ventilation and perfusion distributions. Computational and Mathematical Methods in Medicine. 2009;10:139–154. [Google Scholar]
- 59.Reynolds A, Ermentrout GB, Clermont G. A mathematical model of pulmonary gas exchange under inflammatory stress. Journal of Theoretical Biology. 2010;264:161–173. doi: 10.1016/j.jtbi.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swenson ER, Domino KB, Hlastala MP. Physiological effects of oxygen and carbon dioxide on VA/Q heterogeneity. In: Hlastala MP, Robertson HT, editors. Complexity in structure and function of the lung. Marcel Dekker, Inc.; 1998. pp. 511–547. [Google Scholar]
- 61.Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai T, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. Journal of Applied Physiology. 2007;103:240–248. doi: 10.1152/japplphysiol.01289.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark AR, Tawhai MH, Hoffman EA, Burrowes KS. The interdependent contributions of gravitational and structural features to perfusion distribution in a multiscale model of the pulmonary circulation. Journal of Applied Physiology. 2011;110:943–955. doi: 10.1152/japplphysiol.00775.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tawhai MH, Nash MP, Lin CL, Hoffman EA. Supine and prone differences in regional lung density and pleural pressure gradients in the human lung with constant shape. Journal of Applied Physiology. 2009;107:912–920. doi: 10.1152/japplphysiol.00324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burrowes KS, Hunter PJ, Tawhai MH. Anatomically-based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. Journal of Applied Physiology. 2005;99:731–738. doi: 10.1152/japplphysiol.01033.2004. [DOI] [PubMed] [Google Scholar]
- 65.Clark AR, Burrowes KS, Tawhai MH. Contribution of serial and parallel microperfusion to spatial variability in pulmonary inter- and intra-acinar blood flow. Journal of Applied Physiology. 2010;108:1116–1126. doi: 10.1152/japplphysiol.01177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang YH, Yu CP. A model of ventilation distribution in the human lung. Aerosol Science and Technology. 1999;30:309–319. doi: 10.1080/027868299304660. [DOI] [PubMed] [Google Scholar]
- 67.Swan AJ, Clark AR, Tawhai MH. A computational model of the topographic distribution of ventilation in healthy human lungs. Journal of Theoretical Biology. 2012;300:222–231. doi: 10.1016/j.jtbi.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tawhai MH, Hunter P, Tschirren J, Reinhardt J, McLennan G, Hoffman EA. CT-based geometry analysis and finite element models of the human and ovine bronchial tree. Journal of Applied Physiology. 2004;97:2310–2321. doi: 10.1152/japplphysiol.00520.2004. [DOI] [PubMed] [Google Scholar]
- 69.West JB. Regional differences in gas exchange in the lung of erect man. Journal of Applied Physiology. 1962;17:893–898. doi: 10.1152/jappl.1962.17.6.893. [DOI] [PubMed] [Google Scholar]
- 70.Glenny RW, Robertson HT. Fractal modeling of pulmonary blood flow heterogeneity. Journal of Applied Physiology. 1991;70:1024–1030. doi: 10.1152/jappl.1991.70.3.1024. [DOI] [PubMed] [Google Scholar]