Abstract
Purpose
This study was conducted to determine if expression of the testis-specific phospholipase C Zeta1 (PLCZ1) correlated with low success or fertilization failure after ICSI in patients with normal parameters after standard semen analysis (SA).
Methods
Couples <43 years with one or two failed or low fertilization ICSI cycles. Standard Semen Analysis (SA) was performed to determine sperm parameters in male partners, whereas females were evaluated for antral follicle counts (AFC), day 3 FSH levels and peak Estradiol (E2) levels. The presence of PLCZ1 in sperm was ascertained using Western blotting and Immunofluorescence (IF) analysis. The ability of sperm to initiate changes in the intracellular concentrations of free calcium ([Ca2+]i), which is characteristic of mammalian sperm, was performed after injection of human sperm into mouse eggs loaded with the Ca2+ sensitive dye fura-2 AM.
Results
Male partners of couples with failed or low success ICSI fertilization but with normal SA parameters showed low expression levels of PLCZ1 as determined by western blotting and reduced fluorescent signal during IF studies. In addition, fewer of these males’ sperm showed PLCZ1 expression and were able to initiate robust [Ca2+]i oscillations upon injection into eggs.
Conclusion
Our data suggest that in patients with normal SA parameters but with repeated low fertilization or outright failed fertilization results after ICSI, abnormal PLCZ1 function should be considered as the underlying mechanism responsible for the failure of fertilization.
Keywords: Human, Sperm, Oocytes, PLCZeta1, Calcium, Fertilization
Introduction
Fertilization causes eggs to leave their meiotic arrest and initiate embryo development. The universal signal whereby the sperm induces the initiation of development is an increase in the intracellular concentration of free Ca2+ ([Ca2+]i). In mammals, this Ca2+ signal unfolds in a manner of repeated short-lived rises, which are also known as [Ca2+]i oscillations, and that last in excess of 4 h in the mouse and as long as 20 h in other species including the human [1, 2]. It is thought that activation of the phosphoinositide pathway (PI) in the egg by a sperm-derived phospholipase C (PLC) enzyme underlies the oscillations by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 promotes Ca2+ release by binding to the inositol 1,4,5- triphosphate receptors (IP3R1) located in the endoplasmic reticulum (ER) membrane, which is the Ca2+ store of the cell [3, 4]. The [Ca2+]i oscillations induce the exit from the meiotic arrest, the metaphase II stage (MII), and the initiation of the events of egg activation that render eggs into zygotes [5, 6]. Despite this pivotal role, a persistent question has been by what mechanism does the sperm initiate and sustain the [Ca2+]i oscillations?
Current evidence suggests that after fusion the sperm delivers into the egg’s cytosol a factor that is responsible for triggering [Ca2+]i oscillations. It is thought this factor is capable of activating the PI pathway and production of IP3. Evidence suggests that the factor is a male-specific PLC, Zeta1 (PLCZ1), which was first discovered following a screen for expression of sequence tags related to PLCs in testis [7]. It was subsequently shown that expression of cRNAs encoding for PLCZ1 in eggs initiated oscillations reminiscent of those induced by normal fertilization [7, 8]. Additional studies demonstrated expression of PLCZ1 in the testes of other mammals and PLCZ1 cRNAs were able to induce oscillations in eggs of homologous and heterologous species including human PLCZ1 into human eggs [7, 9, 10]. Further, injection of sperm extracts depleted of PLCZ1 failed to initiate oscillations [7, 8, 11]. Despite this evidence, demonstration of the unique and primary role of PLCZ1 on mammalian fertilization needs confirmation from genetic models where the gene is purposely eliminated.
While experimental genetic models of this kind are not yet available, human clinical studies have proven a valuable asset to gain insight into the role of PLCZ1 in fertilization and fertility. For example, approximately 1–3 % of ICSI procedures results in fertilization failure and nearly 80 % of these failures display “unsuccessful egg activation” [12, 13]. Examination of patients with repeated ICSI failure revealed that in the majority of cases their sperm were unable to consistently initiate [Ca2+]i responses and showed low expression levels of PLCZ1 [14]. Subsequent studies extended the association between PLCZ1 and infertility, as it was found that one case of failed ICSI was linked to point mutations in the catalytic domains of PLCZ1, which undermined the ability of the enzyme to initiate robust oscillations [15, 16]. Interestingly, in the initial studies, most of the PLCZ1-associated defects appeared linked to severe defects of sperm morphology, especially globozoospermia, which is characterized by round-headed sperm that lack most of the acrosomal content [17]. Nevertheless, in our present observations we noted a few cases of ICSI failure in patients with grossly normal semen analysis (SA) including normal morphology. Thus, in this study we examined the association of PLZ1 expression and ICSI failure in patients with normal SA parameters. We also examined whether the sperm of these patients showed uniform or inconsistent ability to initiate [Ca2+]i oscillations. We found that sperm of patients with normal SA but failed fertilization (FF) after ICSI express low levels of PLCZ1 and have dissimilar [Ca2+]i-inducing oscillatory ability; a minority of sperm are capable of initiating robust responses, whereas the majority of sperm are capable of only inducing mediocre or no responses at all. Invariably, in these sperm, the equatorial distribution of PLCZ1 was compromised. Thus, our results show in cases of ICSI failure with normal SA values only a few sperm are capable of initiating robust [Ca2+]i responses and their selection will likely yield better fertilization outcomes.
Materials and methods
Patients
Following institutional IRB approval, infertile couples undergoing Assisted Reproductive Technologies for intracytoplasmic sperm injection (ICSI) were consented for PLCZ1 analysis of the husbands’ sperm after one or two failed or low fertilization ICSI cycles. Clinical information regarding antral follicle count (AFC), day 3 FSH levels and peak Estradiol (E2) levels for the three patients were also obtained and are presented in Table 1. Patients underwent either a long luteal protocol using the GnRH-analogue (GnRH-a), leuprolide acetate (LA) (Lupron; TAP Pharmaceuticals Inc., Chicago, IL) or a microdose stimulation protocol using the same anologue [18, 19].
Table 1.
Fertilization, pregnancy outcomes, stimulation and sperm parameters in patients with successful (SF1) and unsuccessful (FF1-3 and OAT) fertilization ICSI outcomes
| Female parameters | Sperm parametersc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Age (years) | Day3 FSH (IU) | Peak E2 (pmol/l) | AFCa | Fertilization (#2PN/#OOCb) | Pregnancy (Y/N) | Motility (%) | Concentration (X 106) | Morphology (%) |
| SF1 | 29 | 5.3 | 1526 | 21 | 10/11 | Y | 74 | 45.75 | 5 |
| FF1 | 39 | 8.5 | 2303 | 7 | 0/6;2/8 | N | 47 | 23 | 4.5 |
| FF2 | 36 | 5.9 | 2102 | 19 | 0/6 | N | 55 | 73 | 5 |
| FF3 | 30 | 8.7 | 1167 | 11 | 1/5 | N | 49 | 41 | 6 |
| OATd | 42 | 6.2 | 3132 | 18 | 1/13 | N | 17 | 4.2 | 2 |
aAFC. Antral follicular count
bPN stands for pronuclei. Ooc stands for oocytes
cSperm parameters were evaluated according to WHO standard
dOAT stands for Oligoasthenoteratozoospermia
Semen analysis
Freshly ejaculated semen was collected and analyzed for motility, concentration, viability, and morphology as previously described by us [14]; morphology was evaluated according to strict Kruger criteria. Sperm were then washed by centrifugation on a 40 %/80 % PureCeption Percoll gradient (Sage BioPharma, Bedminster, NJ) and the recovered pellet was washed in three times in PBS, after which sperm were prepared for the different functional or biochemical assays.
Detection of PLCZ1 immunoblotting
Western blotting was performed as previously published by our laboratory [14]. Patients’ sperm were diluted to appropriate concentrations, 2X sample buffer added and samples kept at −80 °C until use. Thawed samples were boiled for 3 min, mixed well and loaded onto 7.5 % SDS-PAGE gels and resolved polypeptides were transferred onto PVDF membranes (Millipore, Billerica, MA) using a Mini Trans-Blot Cell (Bio-Rad, Hercules, CA). The membranes were blocked in 6 % nonfat dry milk in PBS–0.1 % Tween and incubated overnight at 4 °C with the MI-305 antibody (1:500); this was followed by 1 h of incubation with a horseradish peroxidase–labeled secondary antibody (Bio-Rad). Immunoreactivity was detected using chemiluminescence per manufacturer’s instructions (PerkinElmer, Waltham, MA) using a Kodak Image Station 440CF. Western blotting procedures were repeated at least 2 times per sample.
[Ca2+]i monitoring
[Ca2+]i monitoring was carried out as described previously [14]. In brief, mouse eggs were loaded with fura-2-acetoxymethyl ester (Fura 2-AM, Molecular Probes; Invitrogen) prior to performing the ICSI procedure after which eggs were transferred to a monitoring dish (Mat- Tek Corp., Ashland, MA) containing microdrops of TL-HEPES medium under mineral oil. Eggs were monitored simultaneously using a 20X objective on an inverted microscope (Nikon) outfitted for fluorescence measurements and with a temperature-controlled stage. Excitation wavelengths were alternated between 340 and 380 nm using a filter wheel (Ludl Electronic Products Ltd., Hawthorne, NY) and fluorescence ratios were taken every 20 s. After passing through a 510-nm barrier filter, the emitted light was collected by a CoolSNAPES digital camera (Roper Scientific, Tucson, AZ). SimplePCI imaging software was used to run all the hardware and capture images (Hamamatsu, Sewickley, PA). [Ca2+]i values are reported as the ratio of 340 nm:380 nm fluorescence in the whole egg. [Ca2+]i oscillatory activity of human sperm was scored from 0 to +++ according to the number of [Ca2+]i rises detected within 1 h of initiating the monitoring [14].
Immunofluorescence
Patients’ sperm were fixed in freshly made 3.7 % PFA-DPBS and kept at 4 °C in DPBS until use, when the suspension was spotted onto 0.1 % poly l-lysine (Sigma-Aldrich, St. Louis, MO) pre-coated multi-well slides (Thermo Scientific, Waltham, MA). Attached sperm were permeabilized with 0.1 % (v/v) Triton X-100–DPBS (Triton X-100; Sigma-Aldrich) for 5 min at 4 °C. Slides were blocked in DPBS + 5 % normal goat serum (GIBCO, Invitrogen) and incubated overnight at 4 °C with the primary anti-PLCZ1 antibody MI-305, (1:30) in 5 % normal goat serum [14]. Washes were performed with 0.1 % (v/v) Tween 20–DPBS followed by 1 h incubation at room temperature in an Alexa Fluor 555–labeled secondary goat anti-rabbit antibody (1:200) (Invitrogen). Samples were counterstained with 5 μg/ml Hoechst 33342 (Sigma-Aldrich) and mounted using mounting media (Vector Laboratories, Burlingame, CA). Fluorescence images were captured with a Zeiss Axiovert 200 M microscope outfitted with a × 63 oil immersion objective and a Hamamatsu Orca-AG cooled CCD Camera controlled by AxioVision software 4.6 (Zeiss, Maple Gove, MN). Figures were assembled in PowerPoint (Microsoft, Redmond, WA).
Preparation of mouse eggs
MII eggs were obtained from superovulated 6- to 10-week-old B6D2F1 (C57BL/6 J × DBA/2 J) female mice [20]. Eggs were collected in a HEPES-buffered Tyrode’s lactate solution (TL-HEPES) supplemented with 5 % heat-treated FCS (GIBCO; Invitrogen, Carlsbad, CA). Cumulus cells were removed with 0.1 % bovine testes hyaluronidase (Sigma-Aldrich) and eggs incubated in CZB medium at 36.5 °C under 5 % CO2 until the time of injection. All animal studies were approved by the Animal Care and Use Committee.
ICSI of mouse eggs
MII eggs were injected with patient sperm as described by Kimura and Yanagimachi [21] and [22] using a piezo micropipette-driven unit (PiezoDrill; Burleigh Instruments Inc., Rochester, NY). Spare sperm from patients as well as routine sperm analysis samples were washed with microinjection buffer (MIB) and frozen at −80 °C until injection, which was performed within a week of collection. Prior to ICSI, human sperm were thawed and mixed 1:1 with MIB containing 12 % PVP. ICSI was performed in HEPES-buffered CZB medium supplemented with 0.1 % polyvinyl alcohol (MW = 30–80 kDa).
Results
Clinical parameters of failed-fertilization (FF) ICSI patients
Four couples seeking infertility treatment during 2010–2012 had either one or two failed ICSI cycles or low (<25 %) fertilization after this procedure. Our clinic performs ~500 A.R.T cycles per year and therefore these three cases represent approximately 1 % of the patient population, which is in line with national statistics for failed fertilization cases after ICSI [23]. Table 1 summarizes the main female clinical and SA parameters information regarding the couples under consideration. Clinical information is also presented as a form of control from a contemporaneous couple that underwent successful fertilization (SF) after ICSI and from another contemporaneous patient with abnormal semen parameters, oligoasthenoteratozoospermia (OAT), which failed ICSI. Female clinical parameters are limited to a few values indicative of their response to stimulation protocols, such as AFC, peak E2 levels before day 10 of stimulation, day 3 FSH and numbers of eggs retrieved. The obtained values were mostly unremarkable, which suggested that the cause of unsuccessful fertilization might be of male origin. Male parameters from SA examination were also unremarkable, as parameters for FF1-FF3 patients were comparable to those of the control patient SF1; sperm parameters for the OAT male were as expected abnormal. Therefore, the only consistent and significant finding was the low fertilization rates after ICSI for males FF1, FF2 and FF3. In light of these results and knowing the close association between failed ICSI and defects in PLCZ1 expression [14, 15, 24], we examined the patients’ sperm for PLCZ1 expression and localization and ability to initiate [Ca2+]i responses.
Morphology and [Ca2+]i-inducing oscillatory activity of sperm of FF patients
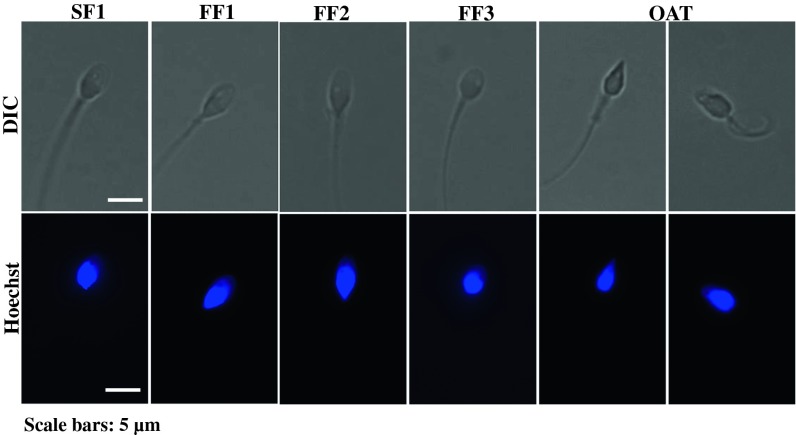
As previously discussed and as shown in Fig. 1, the sperm of patients FF1, FF2 and FF3 displayed unremarkable morphology, meaning that it was similar to that displayed by the sperm of a cohort control patient, SF1; in contrast, the sperm morphology of the OAT patient was abnormal, showing a combination of pyriform heads and amorphous shapes (Fig. 1, OAT, left and right panels, respectively).
Fig. 1.
DIC and DNA staining images of sperm from successful, SF1, and unsuccessful, FF1-3 and OAT, fertilization ICSI cases. DIC images, top panels, and same samples stained with Hoechst to assess nuclear DNA, lower panels, of sperm from FF1-3 and OAT patients, which failed ICSI, and from a control, SF1, patient whose sperm successfully activated eggs during ICSI
To elucidate whether these sperm with grossly normal morphology but that were unable to fertilize following ICSI were capable of initiating [Ca2+]i oscillations, we performed ICSI using the sperm of each patient into mouse eggs and monitored [Ca2+]i responses. While all spermatozoa from the control patient, SF1, initiated [Ca2+]i responses (13/13), only 50 to 70 % of the FF patients’ sperm initiated responses (Fig. 2). Importantly, while 8/13 sperm from the SF1 patient initiated the expected vigorous, +++, [Ca2+]i responses, only 1/8, 2/10 and 0/24 sperm from FF1, FF2, and FF3 patients, respectively, initiated these type of responses. In the case of the SF1 patient, the rest of the sperm initiated moderate, ++, responses, whereas all the rest of FF1-3 sperm initiated moderate to low, ++, [Ca2+]i responses. Expectedly, sperm from the OAT patient were only capable of initiating these moderate to low [Ca2+]i responses (3/8; Fig. 2). Altogether, the results show that whereas the morphology of sperm associated with ICSI failure is highly variable, these sperm share a common defect in their ability to initiate robust [Ca2+]i oscillations. It is noteworthy nevertheless that a small minority, ~10 to 20 %, of the sperm from patients that failed ICSI were capable of initiating robust [Ca2+]i responses in mouse eggs.
Fig. 2.
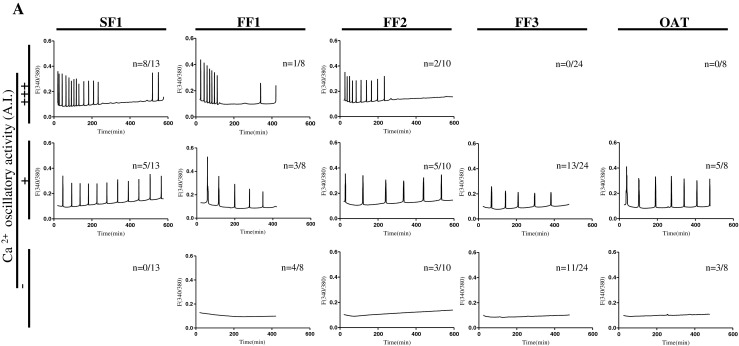
[Ca2+]i response-inducing ability of sperm from patients with successful (SF1) and unsuccessful, FF1-3 and OAT, ICSI. (A) Ca++ oscillations in mouse eggs injected with sperm of control the control patient SF1, or with sperm from patients that failed ICSI, FF1, FF2, FF3 and OAT. n, numerator indicates the number of eggs that displayed the [Ca2+]i profile shown in each panel, whereas the denominator denotes the total number of eggs tested
Expression and localization of PLCZ1 in the sperm of FF patients
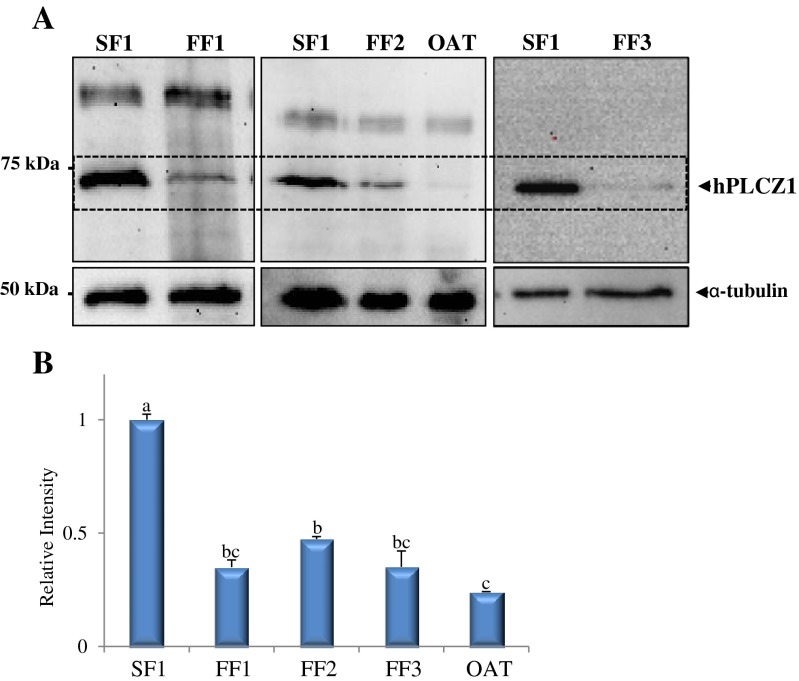
To determine whether the low [Ca2+]i -oscillatory activity of FF patients correlated with low expression levels of PLCZ1 and/or mislocalization of PLCZ1, we performed western blotting and IF studies using an specific anti-PLCZ1 antibody. Immunoblots studies revealed that all patients that repeatedly failed ICSI displayed reduced expression of PLCZ1, which was especially noticeable in sperm from the FF3 and OAT patients (Fig. 3); these 2 patients’ sperm were the only ones unable to initiate robust [Ca2+]i responses.
Fig. 3.
PLCZ1 expression in sperm of control, SF1, and failed fertilization, FF1-3 and OAT, ICSI patients. a Western blot displaying PLCZ1 IB expression in sperm of a control, SF1, patient and in the sperm of patients that failed ICSI, FF1, FF2, FF3 and OAT. The MW of human PLCZ1 is ~ 70 kDa and is denoted by an arrowhead on the right side of the panel. α-tubulin was used as a loading control, and immunoblots against it show ~ equal loading (lower panels) b Bar graph displaying the relative intensity of the PLCZ1 band in the upper panel. Bars with different superscript are significantly different (P < 0.05). Please note that western blots were run on different dates, as samples became available. The SF1 sample was prepared once and used for all Immunoblots
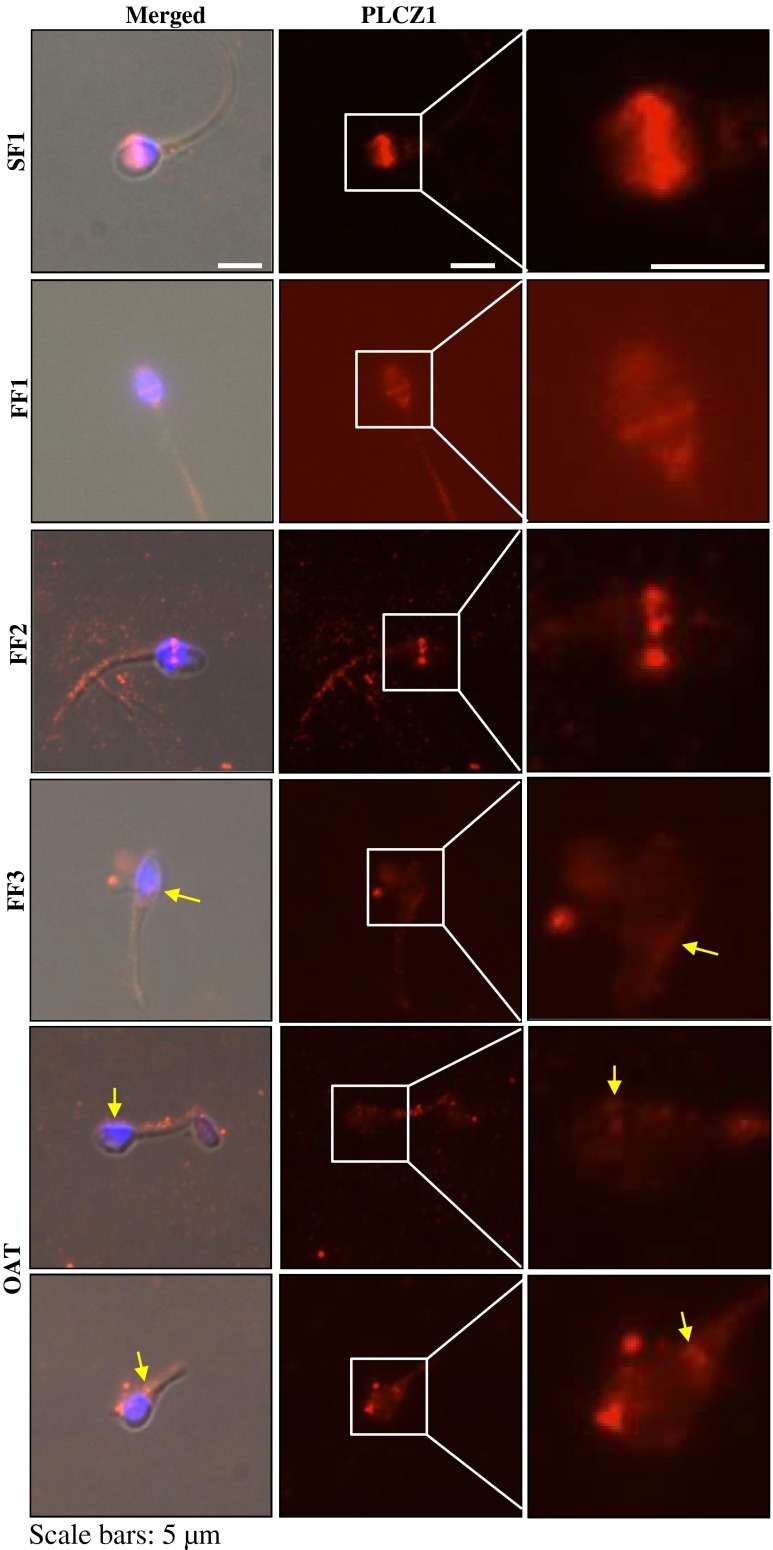
Examination of the localization of PLCZ1 in the same patients revealed that the majority of SF1 sperm (63 %, 41/65) showed the expected equatorial localization of PLCZ1 in human sperm (Fig. 4) [14]. Remarkably, the PLCZ1 signal was detectable in only 25 % (12/47) of FF1 sperm, 21 % (18/86) of FF2 sperm and 12 % (2/17) of FF3 sperm. As expected, only 5 % (2/36) of the OAT sperm showed PLCZ1 reactivity. In addition, the intensity and distribution of PLCZ1 was highly abnormal in FF sperm (Fig. 4). For instance, while FF1 sperm displayed PLCZ1 distribution consistent with its uniform equatorial localization, albeit of low intensity, PLCZ1 localization in FF2 sperm was arranged in discontinuous patches along the equatorial band, whereas in FF3 sperm the presence of PLCZ1 in the equatorial region was negligible, although some reactivity was observed on the base of the head, which is denoted by an arrowhead. Sperm from the OAT patient, as expected, showed atypical distribution of PLCZ1 (Fig. 4; bottom two rows).
Fig. 4.
PLCZ1 localization in control sperm, SF1, or in sperm that failed ICSI, FF1-FF3. Using, immuno fluorescence (IF), PLCZ1 is shown to adopt the expected equatorial localization, whereas in FF1-FF3 sperm, the intensity of PLCZ1 reactivity is highly decreased or mis-localized, or both. Images on the left panels are a combination of bright field, DNA staining and fluorescence, whereas middle panel show IF images, and the right panels show higher magnifications of the IF images. In the lower three panels, yellow arrows denote the abnormal localization and weak presence of PLCZ1
Discussion
In this study we investigated the underlying functional defects of sperm from three patients that either had low or failed fertilization after ICSI during cycles performed in calendar years 2010 and 2012. None of these patients conceived during this period, despite normal SA parameters. We found that these sperm largely failed to initiate robust [Ca2+]i oscillations, although 2 of these 3 patients had ~10 % of sperm capable of initiating moderate [Ca2+]i responses in mouse eggs. The expression levels of PLCZ1 were severely reduced in all patients and, more importantly, in the few sperm that expressed PLCZ1 the distribution and intensity were affected. Therefore, our results suggest that abnormal PLCZ1 expression is likely to underlie most cases of ICSI failure, even when SA parameters are grossly normal.
ICSI failure and sperm morphology
ICSI is a highly successful technique that overcomes the most severe cases of male infertility. Nevertheless, low fertilization or fertilization failures after ICSI occur in ~4 % of ICSI cases [12, 17], and the great majority of those cases show a consistent phenotype; namely, absence of egg activation [25]. ICSI failure is also observed in a disproportionate number of patients with globoozoospermia [17, 26]. While the precise molecular defect(s) that underlie these patients’ infertility remains to be resolved, these sperm tend to have defects in the organization of the sperm head, including the perinuclear theca and equatorial regions [27, 28], which are the sites where purportedly PLCZ1 is located [14, 29]. In this vein, it was first reported that globozoospermic sperm from patients that failed ICSI showed reduced or absent expression of PLCZ1 [14], and a recent study has extended the association of globozoospermia and infertility to the mouse, as the loss or point mutations in the DPY19L2 gene, which encodes for an inner nuclear membrane protein, is associated with disruption of the sperm perinuclear area, globozoospermia and infertility in this species [30]. Thus, it appears that abnormal sperm head organization, especially in the perinuclear theca and equatorial regions, which may affect the anchoring and/or the stability of PLCZ1, are associated with inability of the sperm to initiate [Ca2+]i responses and egg activation. This association is supported in our study, as the OAT patient that was used as a negative control fell in this category.
ICSI failure has also been reported to occur in a few patients with apparent normal gross sperm morphology [17, 15]. In one case, after careful analysis of the PLCZ1 gene sequence, point mutations that undermined the activity of the enzyme were found in the catalytic region of PLCZ1, providing a molecular explanation for the lack of egg activation and infertility observed in the patient [31, 15]. Here, we found 3 patients that despite displaying normal gross sperm morphology and SA parameters failed or had low fertilization after ICSI. The sperm of these patients displayed greatly reduced PLCZ1 expression, which might underlie the failure to initiate [Ca2+]i responses regardless of whether or not point mutations were present in PLCZ1; nevertheless, we cannot exclude the possibility that mutations in the enzyme of those patients might further compromise the function of the enzyme. Also, because we did not carry out EM studies, we cannot exclude the possibility of abnormal perinuclear theca organization in these sperm might contribute to the abnormal expression and/or distribution of PLCZ1 in those patients. A recent study also found reduced expression of PLCZ1 in patients that fail ICSI, although it also reported similar low PLCZ1 expression in some control, fertile patients [32]. Quantification of PLCZ1 expression in that study was performed by immunofluorescence, whereas in our previous and current study quantification was performed by immunoblotting, and the differences in methodology may account for the large variation observed in that study [32]. Further, it is worth pointing out that in the aforementioned study, PLCZ1 expression was low in all infertile patients and the variability of PLCZ1 expression levels among patients that failed ICSI was considerably lower than among fertile patients [32]. Therefore, in the presence of failed fertilization after ICSI, abnormal PLCZ1 levels remain highly predictive of repeated fertilization failure. Collectively, our results suggest that regardless of sperm morphology, patients that repeatedly fail ICSI or have low fertilization rates, especially those where acceptable numbers of eggs are retrieved, are likely to exhibit defects on the ability to induce [Ca2+]i oscillations associated with defects in PLCZ1 expression and/or function.
[Ca2+]i responses, PLCZ1 expression and low ICSI success
In a previous study it was found that sperm from patients with repeated ICSI failure when injected into mouse oocytes were largely unable to initiate [Ca2+]i oscillations; in all cases, this was associated with reduced expression of PLCZ1 [14]. Nevertheless, in that study, the sperm of the patients examined displayed severe globozoospermia or obvious morphology defects that, as was reported then and it was later confirmed by other studies, it correlates with loss of PLCZ1 [14, 15]. In the present study, we focused on three patients that despite showing grossly normal sperm morphology and SA parameters failed ICSI. Whether or not these types of ICSI failure are also associated with reduced ability to induce [Ca2+]i responses and defective PLCZ1 expression has not been closely investigated. We found that ~20 % of these sperm were capable of initiating robust [Ca2+]i responses and another 20 % had the capacity to initiate moderate to low [Ca2+]i responses, while 30 to 50 % of the sperm were unable to initiate [Ca2+]i responses. None of the sperm of the single patient in the study with abnormal morphology were able to initiate robust responses, although ~60 % of the sperm induced moderate to low responses. Collectively, these data fit nicely with western blots results showing that the levels of PLCZ1 expression in these patients were ~40 to 80 % lower than those of control sperm. It is worth noting that [Ca2+]i monitoring was performed in mouse eggs, which are believed to be more sensitive to IP3 than human eggs, and therefore only those sperm capable of initiating robust responses in mouse eggs would be capable of initiating [Ca2+]i oscillations in human eggs.
We next addressed the question of whether the low expression of PLCZ1 was uniform in every sperm or rather due to a few sperm expressing normal amounts of PLCZ1 and others expressing none. To accomplish this, we examined PLCZ1 expression in individual sperm using IF. In patients FF1, FF2 and FF3, less than 30 % of the sperm showed reactivity after IF, which was in marked contrast to the nearly 70 % of the sperm that displayed reactivity in the control patient. In the OAT patient with abnormal morphology less than 5 % of the sperm displayed reactivity. Thus, our results suggest that in patients that have low fertilization after ICSI, not only is the overall expression of PLCZ1 reduced, but also the number of sperm expressing PLCZ1 is affected. Similar results have recently been reported in a larger study evaluating PLCZ1 expression in sperm from control and failed fertilization patients [32]. Given that single sperm are selected and injected during the ICSI procedure, the possibility of identifying and using those sperm that express PLCZ1 and are capable of initiating [Ca2+]i responses is intriguing and may increase the chances of successful fertilization. Selection of these sperm could be aided by approaches such as motile sperm organelle morphology examination (MSOME) that enables evaluation of nuclear morphology in motile spermatozoa in real time and under high magnification, and the selected sperm could then be used for intracytoplasmic injection [16, 33]; a recent manuscript showed that such a selection may be associated with higher levels of PLCZ1 expression [34].
In summary, we found that low fertilization and failed fertilization after ICSI can occur in patients with normal gross sperm morphology and defects in PLCZ1 expression and/or function and should be considered in the diagnosis of these patients. Remarkably, despite that the proportion of sperm expressing PLCZ1 is severely reduced in these patients, some sperm retain PLCZ1 expression and are capable of initiating robust [Ca2+]i responses. These findings may have clinical implications, as selection of the [Ca2+]i oscillation-competent sperm may overcome the severe fertilization defects observed in patients with repeated low fertilization or failed ICSI.
Footnotes
Capsule Sperm with normal semen parameters from male partner of couples with repeat low or failed fertilization results after ICSI, showed low PLCZ1 expression and initiated decreased calcium release upon injection into eggs. Our data suggests PLCZ1 function could be the underlying cause and should be assessed in cycles with low or failed fertilization.
References
- 1.Jones K. Ca2+ oscillations in the activation of the egg and development of the embryo in mammals. Int J Dev Biol. 1998;42(1):1–10. [PubMed] [Google Scholar]
- 2.Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100(5):545–52. doi: 10.1254/jphs.CPJ06003X. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M, Irvine R. Inositol phosphates and cell signalling. Nature. 1989;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32(5–6):235–49. doi: 10.1016/S0143416002001823. [DOI] [PubMed] [Google Scholar]
- 5.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz R, Kopf G, et al. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002;250(2):280–91. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 6.Ducibella T, Fissore R. The roles of Ca2+ downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315(2):257–79. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders C, Larman M, Parrington J, Cox L, Royse J, Blayney L, et al. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129(15):3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa M, Sato K-I, Wu H, He C, Malcuit C, Black S, et al. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285(2):376–92. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Kurokawa M, Sato K-I, Fissore R. Mammalian fertilization: from sperm factor to phospholipase Czeta. Biol Cell Auspices Eur Cell Biol Organ. 2004;96(1):37–45. doi: 10.1016/j.biolcel.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Rogers N, Hobson E, Pickering S, Lai F, Braude P, Swann K. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction. 2004;128(6):697–702. doi: 10.1530/rep.1.00484. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa M, Yoon S, Alfandari D, Fukami K, Sato K-I, Fissore R. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev Biol. 2007;312(1):407–18. doi: 10.1016/j.ydbio.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sousa M, Tesarik J. Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Hum Reprod. 1994;9(12):2374–80. doi: 10.1093/oxfordjournals.humrep.a138455. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty S, Payne D, Matthews C. Fertilization failures and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13(Suppl 1):155–64. doi: 10.1093/humrep/13.suppl_1.155. [DOI] [PubMed] [Google Scholar]
- 14.Yoon S-Y, Jellerette T, Salicioni A, Lee H, Yoo M-S, Coward K, et al. Human sperm devoid of PLC zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Investig. 2008;118(11):3671–81. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod. 2009;24(10):2417–28. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- 16.Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee H, Hamer R, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012;27(1):222–31. doi: 10.1093/humrep/der384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20(8):2237–41. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 18.Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S. Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertil Steril. 2005;84(3):555–69. doi: 10.1016/j.fertnstert.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Hsu A, Arny M, Knee A, Bell C, Cook E, Novak A, et al. Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril. 2011;95(2):474–9. doi: 10.1016/j.fertnstert.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Wakai T, Vanderheyden V, Yoon S-Y, Cheon B, Zhang N, Parys J, et al. Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. J Cell Physiol. 2012;227(2):705–17. doi: 10.1002/jcp.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121(8):2397–405. doi: 10.1242/dev.121.8.2397. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa M, Fissore R. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod. 2003;9(9):523–33. doi: 10.1093/molehr/gag072. [DOI] [PubMed] [Google Scholar]
- 23.Esfandiari N, Javed M, Gotlieb L, Casper R. Complete failed fertilization after intracytoplasmic sperm injection–analysis of 10 years data. Int J Fertil Women's Med. 2005;50(4):187–92. [PubMed] [Google Scholar]
- 24.Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Updat. 2010;16(6):690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- 25.Terada Y, Hasegawa H, Takahashi A, Ugajin T, Yaegashi N, Okamura K. Successful pregnancy after oocyte activation by a calcium ionophore for a patient with recurrent intracytoplasmic sperm injection failure, with an assessment of oocyte activation and sperm centrosomal function using bovine eggs. Fertil Steril. 2009;91(3). doi:10.1016/j.fertnstert.2008.09.043. [DOI] [PubMed]
- 26.Taylor S, Yoon S, Morshedi M, Lacey D, Jellerette T, Fissore R, et al. Complete globozoospermia associated with PLCζ deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod Biomed Online. 2010;20(4):559–64. doi: 10.1016/j.rbmo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escalier D. Failure of differentiation of the nuclear-perinuclear skeletal complex in the round-headed human spermatozoa. Int J Dev Biol. 1990;34(2):287–97. [PubMed] [Google Scholar]
- 28.Coutton C, Zouari R, Abada F, Ben Khelifa M, Merdassi G, Triki C, et al. MLPA and sequence analysis of DPY19L2 reveals point mutations causing globozoospermia. Hum Reprod. 2012;27(8):2549–58. doi: 10.1093/humrep/des160. [DOI] [PubMed] [Google Scholar]
- 29.Sutovsky P, Oko R, Hewitson L, Schatten G. The removal of the sperm perinuclear theca and its association with the bovine oocyte surface during fertilization. Dev Biol. 1997;188(1):75–84. doi: 10.1006/dbio.1997.8618. [DOI] [PubMed] [Google Scholar]
- 30.Pierre V, Martinez G, Coutton C, Delaroche J, Yassine S, Novella C, et al. Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development. 2012;139(16):2955–65. doi: 10.1242/dev.077982. [DOI] [PubMed] [Google Scholar]
- 31.Kashir J, Konstantinidis M, Jones C, Heindryckx B, De Sutter P, Parrington J, et al. Characterization of two heterozygous mutations of the oocyte activation factor phospholipase C zeta (PLCζ) from an infertile man by use of minisequencing of individual sperm and expression in somatic cells. Fertil Steril. 2012;98(2):423–31. doi: 10.1016/j.fertnstert.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Kashir J, Jones C, Mounce G, Ramadan W, Lemmon B, Heindryckx B, et al. Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril. 2013;99(1):107–17. doi: 10.1016/j.fertnstert.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Setti A, Paes de Almeida Ferreira Braga D, Iaconelli A, Aoki T, Borges E. Twelve years of MSOME and IMSI: a review. Reprod Biomed Online. 2013;27(4):338–52. doi: 10.1016/j.rbmo.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Kashir J, Sermondade N, Sifer C, Oo S, Jones C, Mounce G, et al. Motile sperm organelle morphology evaluation-selected globozoospermic human sperm with an acrosomal bud exhibits novel patterns and higher levels of phospholipase C zeta. Hum Reprod. 2012;27(11):3150–60. doi: 10.1093/humrep/des312. [DOI] [PubMed] [Google Scholar]