Abstract
The influence of seminal plasma on the cytokine and immune uterine environment is well characterised in mice and humans, while the effects of disruption to uterine seminal plasma exposure on pregnancy and offspring health is becoming more clearly understood. The cellular and molecular environment of the uterus during the pre- and peri-implantation period of early pregnancy is critical for implantation success and optimal foetal and placental development. Perturbations to this environment not only have consequences for the success of pregnancy and neonatal health and viability, but can also drive adverse health outcomes in the offspring after birth, particularly the development of metabolic disorders such as obesity, hypertension and insulin resistance. It is now reported that an absence of seminal plasma at conception in mice promotes increased fat accumulation, altered metabolism and hypertension in offspring. The evidence reviewed here demonstrates that seminal plasma is not simply a transport medium for sperm, but acts also as a key regulator of the female tract environment providing optimal support for the developing embryo and benefiting future health of offspring.
Keywords: Seminal fluid, Programming, Inflammation, Fertility
Introduction
The process of insemination is no longer viewed simply as the delivery of sperm into the female reproductive tract [1]. Although this might be the primary purpose of insemination, there is now evidence that semen can act directly on tissues in the female reproductive tract to elicit accessory functions which in turn may influence implantation, development of the pre-implantation embryo and future health of the offspring. Over the past decade specific compounds which can exert biological activities in female tract cells have been identified within seminal plasma. However, it is clear that whole semen is not an absolute requirement for a viable pregnancy outcome, as shown by the advent of embryo transfer and artificial insemination. Thus it appears that seminal factors act primarily to optimise the likelihood of successful pregnancy. This has been supported by studies showing the addition of seminal plasma or seminal factors to assisted reproductive procedures can markedly improve pregnancy success in mice, rats, hamsters, sheep and humans [2–6].
Studies using mouse models have demonstrated the importance of cytokines present in semen. In particular, seminal transforming growth factor-beta (TGFβ) [7] has been described to interact with uterine epithelial cells to induce the expression of an array of pro-inflammatory cytokines, resulting in a classic inflammatory response that culminates in an influx of leukocytes into the endometrial tissues [8]. This inflammatory response to semen and the associated change in the cytokine environment has been hypothesised to mediate several downstream effects in the female reproductive tract [9]. More recently these studies have been recapitulated in the human, particularly the cervix following intercourse [10–12]. Recently we have reported that pregnancies initiated in the absence of seminal plasma in mice give rise to progeny with altered metabolic profiles, obesity and hypertension due in part to perturbations in the peri-conceptional environment and oviduct expression of embryotrophic cytokines [13].
The inflammatory changes induced at insemination may be linked with the immune changes necessary to accommodate pregnancy. Introduction of allogeneic material into a host is usually met with a rapid rejection of the tissue by the host immune system. The survival of a semi-allogeneic conceptus within its mother is the exception to this general rule. The process of embryo survival through implantation and pregnancy is thought to be mediated by maternal immune suppression or immune deviation, a process where the cytokine environment can direct the quality of an immune response to achieve functional immune tolerance. The events leading to these immune changes in pregnancy are unknown, but are hypothesised to be initiated at insemination, when the maternal immune system is first exposed to paternal transplantation antigens, some of which are shared by the conceptus. Thus, insemination may comprise a ‘priming’ event leading to the maternal immune tolerance required for the survival of the semi-allogeneic conceptus [14, 9].
The inflammatory events known to occur at insemination may also have subsequent effects on processes important in embryo development, implantation and trophoblast invasion. The substantial changes in the cytokine environment of the oviduct and uterus, as a consequence of seminal plasma exposure, may also contribute to enhancing pre-implantation embryo development and facilitating the changes in both the uterine epithelium and stroma necessary for embryo attachment and implantation. These early steps in establishing pregnancy are crucial to the ongoing success of a pregnancy.
Here we summarise the current understanding of the events of early pregnancy and discuss their possible modulation by uterine exposure to seminal factors. Many of the processes occurring in early pregnancy appear to be affected by cytokines or leukocytes activated by seminal factor signalling in the female tract (Fig. 1). As a consequence, disruption of seminal exposure may have negative effects on events occurring in early pregnancy, resulting in detrimental outcomes for both the pregnancy and offspring.
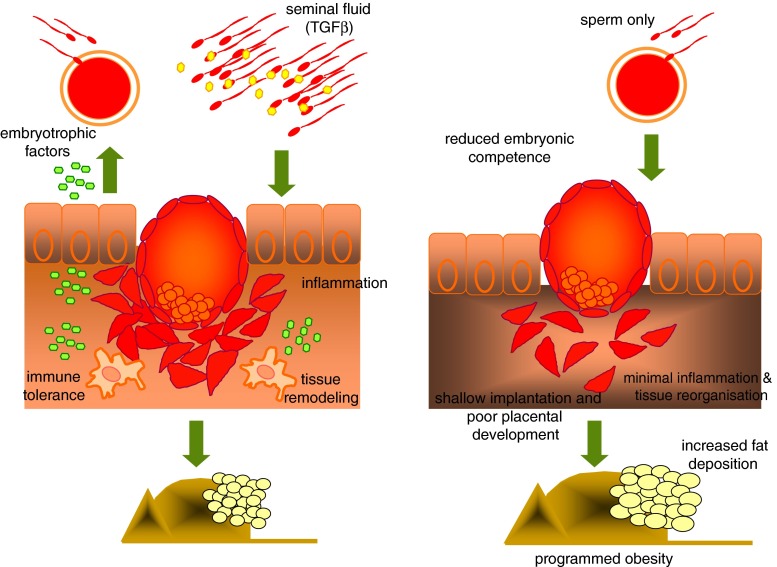
Fig. 1.
Schematic representation of the early events following insemination. Seminal fluid components interact with the endometrial epithelium to produce embryotrophic factors to support embryonic development, and cytokines and chemokines to initiate an acute inflammatory reaction. Infiltration of leukocytes and antigen presenting cells follow to initiate the immune response for successful pregnancy establishment and tissue remodelling for embryo implantation. All of these events are critical to the establishment and maintenance of a healthy pregnancy and are initiated by exposure to seminal fluid. The right panel demonstrates our hypothesized series of events leading to altered pregnancy outcomes in the absence of seminal fluid. An absence of seminal fluid induced inflammation reduces the production of embryothrophic factors, immunological tolerance toward the conceptus, minimal tissue remodelling to facilitate implantation and placental development. These factors combined result in compromised foetal programming and development of adult metabolic syndrome
Early origins of adult disease and foetal programming
Increasingly, perturbations in the in utero environment have been linked with altered metabolic status and increased risk of disease in adult life. Since the late 1980s a clear association between low birth weight in humans and the development of adult hypertension has been defined by David Barker and his colleagues [15]. Since these initial studies a series of epidemiological studies have shown associations between low birth weight and the onset of adult diseases including hypertension and extending to obesity, type II diabetes mellitus, heart disease, kidney disease and the insulin resistance syndrome (reviewed in [16]). The relationship between birth weight and the development of adult disease has been termed the “foetal origins of adult disease”. Foetal growth potential is controlled at many levels; however one very critical factor is that of the in utero maternal environment. Maternal feed restriction, through experimental approaches or food shortage, like that of the Dutch famine during World War II, has been correlated with low birth weight and subsequent changes in endocrine, metabolic and reproductive parameters in these offspring later in life, as well as predisposition to certain cancers [17–26]. It is hypothesised that these changes in endocrine and metabolic parameters are in essence programming the offspring for the development of endocrine associated adult diseases.
The molecular mechanisms underpinning foetal programming are not clearly defined. However, recently it is become more evident that epigenetic regulation of the genome may be playing some role [27]. At the very early stages of embryonic development epigenetic modification of the conceptus can be modulated by endogenous, environmental and dietary factors. Mitochondrial abnormalities have also been suggested as mechanisms affecting foetal programming, as suggested by studies investigating mitochondrial abnormalities arising due to in vitro embryo culture [28, 29]. Now it has been shown the offspring of rats fed on a high fat diet not only have impaired glucose homeostasis, but also have a decreased mitochondrial number accompanied by a decrease in the expression of mitochondrial specific genes [29].
The prenatal programming of endocrine axes is also a keg regulator of the future health and metabolic status of subsequent progeny. The hypothalamic/pituitary/adrenal (HPA), somatotropic and insulin axes are all thought to contribute to the differential regulation of key endocrine regulators of metabolism that can influence the metabolic status of an individual. These highly regulated, tissue specific endocrine regulators are known to be influenced by programming mechanisms to exert differential development of the foetus [30–32]. More recently a direct contribution of the father has been shown to influence the metabolism of offspring. Male rats fed a high fat diet give rise to female offspring with β-cell dysfunction [33], while our own studies provide evidence that male mice devoid of seminal plasma give rise to male offspring with altered metabolic profiles and increased fat accumulation [13].
Pre-implantation embryo development
The pre-implantation embryo is extremely susceptible to environmental stressors such as reactive oxygen species, ammonium and metabolic substrate supply, therefore small perturbations in the oviductal/uterine environment in which the embryo develops can have a long term impact on the health and viability of the organism [34–36]. The progression from a single cell zygote to a differentiated, highly organised competent blastocyst is controlled both by maternal and embryonic factors.
Specific growth factors and cytokines expressed by the epithelium of the oviduct and uterus have been shown to be of critical importance in optimal embryonic development. The primary mechanism for the control of expression of these molecules is proposed to be that of ovarian steroid hormones [37]. More recently, regulation of these factors has also been attributed to male factors derived from semen [38, 39]. A wide range of molecules including granulocyte macrophage-colony stimulating factor (GM-CSF) [40], leukaemia inhibitory factor (LIF) [41], IL-6, TGFβ, TNFα [42], TGFα [43], insulin, insulin-like growth factor (IGF)-I and II [44], epidermal growth factor (EGF) [45], and heparin binding-epidermal growth factor-like growth factor (HB-EGF) [46] have all been identified as having potential roles in embryonic development, and are in part regulated in the oviductal epithelium by seminal plasma exposure. In female mice mated to males devoid of seminal plasma expression of embryotrophic factors in the oviduct is reduced while the pro-apoptotic factor Trail is increased. In addition embryos derived from these matings display perturbations in blastocyst formation which can be partially rescued using in vitro culture [13]. A definitive study by Sjoblom et al. has demonstrated that addition of GM-CSF into culture media can alleviate some of the detrimental effects of embryo culture on pregnancy outcomes, such as altered placental morphogenesis [47]. The addition of many these embryotrophic growth factors to in vitro embryo culture media is now being more closely investigated for their use in human assisted reproduction to allow closer mimicry of the in vivo environment.
Seminal plasma
Insemination
Insemination is the delivery of sperm into the female reproductive tract. Upon ejaculation men produce approximately 4 ml of semen that contains up to 600 million sperm. After coitus these sperm must then traverse the female tract to the oocyte where fertilisation can occur. Ejaculated semen contains a number of constituents, both cellular and acellular. Primarily, semen is regarded as a concentrated fluid of mature sperm. However, semen also contains a number of epithelial and myeloid cells derived from the testis and urogenital lining. The predominant constituent of semen is the seminal fluid termed seminal plasma. Seminal plasma is derived from the male accessory sex glands including the seminal vesicle glands, prostate, epididymis and bulbourethral (or Cowpers) gland. The fluid in which sperm are transported through the male urogenital tract and into the female reproductive tract is protein-rich and contains a number of compounds which assist in sustaining sperm viability. Seminal plasma is rich in fructose, which is the predominant metabolic substrate that facilitates the journey of sperm through the male urogenital tract across the cervix and into the oviduct. However, seminal fluid also contains a number of micronutrients and amino acids to aid in meeting the high metabolic requirements of the sperm. Seminal plasma is also a rich source of both oxidative and anti-oxidative agents. Sperm have been shown to be highly susceptible to oxidative DNA damage due to the small degree of buffering their diminished cytoplasm offers. Oxidative stress occurs due to the presence of hydroxyl radicals, superoxide anions and hydrogen peroxide present in either the ejaculate or the female reproductive tract fluids. In the event of sperm DNA damage, including that acquired from oxidative stress, pregnancy failure or pathologies can ensue due to embryonic loss [48, 49]. To counteract the detrimental effects of these oxidative agents seminal plasma is rich in powerful antioxidant agents such as catalase and superoxide dismutase [50]. Seminal plasma has a powerful buffering capacity required to counteract the harsh acidic environment of the female reproductive tract [51, 52].
Seminal plasma is rich in a number of active protein moieties. Various signalling molecules including many originally described for their immunological activities have been shown to be present in seminal plasma of both humans and rodents, including TGFβ, interferon-gamma (IFNγ), prostaglandin E2 (PGE2), tumour necrosis factor-alpha (TNFα) and members of the interleukin (IL) family of cytokines, including IL-1b, IL-6, IL-8, IL-10 and IL-12. TGFβ is produced predominately by the seminal vesicles and is present in ejaculated seminal plasma at concentration of approximately 200 ng/ml, substantially higher concentrations than that of blood plasma [9]. The seminal vesicles of men are also known to produce vast quantities of PGE2 resulting in seminal plasma levels 100 000 times greater than those seen in tissues during an acute inflammatory responses [53]. It has recently become apparent the function of these molecules is to elicit strong molecular and cellular responses within the female reproductive tract after insemination.
For decades it has been proposed the sole purpose of seminal plasma has been as a transport medium for sperm to traverse the female reproductive tract. However, more recently accessory functions of seminal plasma during pregnancy establishment and progression have been described, specifically the modulation of the maternal immune response to pregnancy. The impact of seminal vesicle removal on fertility has been partially documented in the house mouse and golden hamster [54–56]. Peitz et al. have demonstrated that removal of the seminal vesicles severely diminishes the capacity of these mice to fertilise oocytes efficiently, therefore significantly reducing the pregnancy rate of mated females. Similarly male hamsters in which the accessory glands have been removed have been shown to have detrimental effects on embryonic development [4, 57, 58].
Post-insemination inflammation
Induction of pro-inflammatory cytokines is the first step in elicitation of a transient inflammatory response. The inflammatory response occurring at insemination can be attributed to factors within seminal plasma, as opposed to sperm. This conclusion is based in findings that show the lack of a uterine inflammatory response after mating mice with seminal vesicle deficient stud males [8], suggesting that the factors initiating this inflammatory cascade originate in the seminal vesicle, where the majority of seminal plasma is produced. The up-regulation of cytokines within the human cervix and murine uterus and has been specifically linked with the seminal plasma cytokine TGFβ [7, 11].
The inflammatory response initiated within the human cervix and murine uterus is very similar to a classical inflammatory response, for example seen after chemical insult or injury [59]. After uterine epithelial cells are exposed to seminal factors, the surge of pro-inflammatory cytokines and chemokines (as stated above) causes an infiltration of inflammatory leukocytes. In addition to neutrophils a large number of antigen presenting cells (APCs) are recruited into the uterine endometrium, including macrophages and dendritic cells (DCs) expressing high levels of MHC class II [60, 8]. This response has now been well characterised in other species including rabbit, horse, pig, and human [61–64, 12].
Immunological actions of seminal plasma
As previously stated seminal plasma itself has immune deviating properties, some of which may be more important than sperm protection alone. It has been known for several decades that seminal plasma from various species can directly affect the immunological functions of T cells, B cells, NK cells and macrophages in vitro [65–67]. While it does not directly influence the generation of tolerogenic lymphocytes, seminal fluid has been shown to impair complement dependent antibody cell lysis and cell mediated killing of pathogenic bacteria [68]. Anderson et al. tested the effects of seminal plasma on the extent of humoral responses to antigen challenge in mice and showed that all fractions of seminal plasma; prostate, seminal vesicle, and epididymis, were able to suppress both the primary and secondary humoral responses to venous administered washed sperm [69]. Kelly has also demonstrated that human seminal plasma creates a cytokine switch in blood lymphocytes in vitro, resulting in an up-regulation of IL-10 and a down-regulation of IL-12, effectively creating an immunosuppressive environment [70].
More recently it has demonstrated that seminal plasma, predominately the bioactive factor TGFβ, has the capability of interacting with the female tract. Seminal fluids target the epithelial cell layer of the cervix and uterus, inducing the expression of pro-inflammatory cytokines such as GM-CSF, CSF-1 (colony stimulating factor 1), IL-1α, IL-6, IL-8, LIF, RANTES and MIP-1α [9]. The up-regulation of these cytokines by the epithelium has multiple consequences. These pro-inflammatory cytokines are responsible for establishing the post-insemination inflammatory response, while many of the cytokines are also potent immune-deviating signals which act to drive the establishment of a Th2 immune environment conducive to pregnancy success [71]. Other cytokines up-regulated by the epithelium of the female tract as a consequence of seminal plasma exposure are known to be potent embryotrophic molecules.
Studies in mice suggest that semen plays a role in the induction of systemic immune tolerance in early pregnancy. Females inseminated after uterine ligation become transiently tolerant to male transplantation antigens as measured by survival of paternal tumour challenge, suggesting that semen, as opposed to the embryo itself is a key requirement for inducing tolerance [9]. The cytokine environment at the site of antigen exposure is also a critical factor for the future development of tolerance, and seminal plasma elicits a cascade of events leading to the induction of a unique cytokine microenvironment with the uterine epithelium.
Antigenic characteristics of semen
Semen carries a multitude of paternal antigens expressed on the sperm itself, genital tract epithelial cells and seminal leukocytes some of which are also expressed by the conceptus [72, 73]. The antigens expressed on trophoblast cells and found in semen are limited, however there are a number of transplantation antigens found within semen, or expressed on the sperm itself. Mouse sperm has been shown to express both class I and class II MHC [74], as well as H-Y [75], all of which have the potential to interact with reactive lymphocytes, and are commonly expressed by the conceptus. In the case of human sperm, it remains controversial whether MHC antigens are expressed on the sperm surface [76–78]. However, it is evident that a number of leukocytes and epithelial cells are also present in the ejaculate, and these have been shown to express MHC antigens [73].
Whether APCs recruited after insemination can traffic from the uterus to the LN remains to be formally demonstrated. However, there is a small body of evidence to suggest this does occur. In the mid-1970s Beer and Billingham showed that the PALN of mice transiently enlarge after allogeneic insemination occurs, suggesting a cellular influx into the PALN [79]. Watson et al. showed that radio-labelled sperm not involved in the fertilisation process are phagocytosed by macrophages expressing high levels of class II MHC [80]. Watson also showed that these APCs could traffic to the MLN and spleen; however the draining LN of the uterus were not examined in this study. Parr et al. conducted similar studies using the introduction of FITC-conjugated proteins to trace the uptake of antigen within the vagina and uterus of mice [81]. Parr et al. studies indicated intravaginal administered proteins were later localised in dendritic and Langerhan-like cells within the vaginal epithelium and stroma, whereas labelled whole lymphocytes could be seen to pass across the epithelium and into maternal tissue after introduction into the murine uterine tract [82].
Lymphocyte activation following insemination
The studies mentioned above suggest the PALN and ileac LN as the draining LN of the uterus. It is therefore feasible to assume that DCs and macrophages exiting the uterus after insemination would arrive at these LN and perhaps become involved in lymphocyte activation. More recently it has been shown that the PALN increase in size is due to a cellular influx or proliferation, and that activation of T cells, B cells and NK cells occurs after insemination [83]. Alloreactive T cells have also been shown to increase in number within the PALN of mice in early pregnancy [84]. Although the precise phenotype of these activated lymphocytes is unknown, they are known to be recruited back into uterine tissues around the time of implantation, suggesting a role in the preparation of uterine immune environment for an ensuing pregnancy [83]. To further support the conclusion that maternal tolerance toward paternal antigen originates within these LN, O'Hearn demonstrated that PALN cells taken from allogeneic pregnant mice are specifically hypo-responsive toward paternal antigen, compared to those from virgin or syngeneically mated mice [85]. Suppression of alloreactive T cells taken from the PALN during pre-implantation and implantation stages of pregnancy has also been demonstrated, further supporting insemination as a possible start point for maternal T cell tolerance [86]. TGFβ producing suppressor cells have also been identified within the PALN during implantation [87]. Experiments conducted by Beer et al. [88] showed that the removal of the PALN before mating reduced the size of the feto-placental unit in mice, while splenectomy did not, consistent with the PALN being an important inductive site of maternal tolerance toward the semi-allogeneic conceptus. More recently the importance of antigen specific T-regulator lymphocytes (FoxP3+/CD25+) has become a focus in maintaining pregnancy. Initial studies depleting T-regulator lymphocytes showed a failure of pregnancy due to immune rejection of the conceptus [89]. Our studies have now shown that exposure to seminal plasma at insemination drives expansion of T-regulatory lymphocytes in the PALN and uterus [90].
Other mechanisms for the activation of alloreactive lymphocytes have been proposed, including interaction between lymphocytes and antigen in the peripheral circulation due to free trophoblast cells in blood [91]. A mechanism of trophoblast cells themselves activating alloreactive lymphocytes has also been proposed [92].
Tissue remodelling and seminal plasma
The myeloid immune cells recruited into the uterus after exposure to seminal plasma, predominately macrophages and neutrophils may have roles in regulation of epithelial and stromal remodelling during early pregnancy. These cells play a key role in the production of molecules critical for the breakdown and regeneration of specific ECM components and the generation of new blood vessels known to be required at the time of implantation.
Uterine macrophages and neutrophils produce a range of factors which contribute to ECM breakdown and rebuilding, namely MMPs and TIMPs. Neutrophils have been identified as the key source of MMP-9, a collagen specific protease during the peri-implantation period [93], while macrophages are known to produce a range of MMPs. A comprehensive study by Chow et al. [94] in the golden hamster showed that expression of molecules involved in ECM remodelling are controlled in a temporal and spatial manner and that seminal plasma is implicated in their regulation. VEGF, its receptors (VEGF-R1 and -R2) and MMP-2 expression in the uterus during the peri-implantation period were all up-regulated by seminal plasma exposure at the time of conception. Another study has further implicated seminal plasma exposure in regulating uterine artery remodelling in early mouse pregnancy, showing that uterine artery remodelling occurred comparably in mice mated with vasectomised studs or intact stud males [95].
The surface characteristics of epithelial cells are paramount for embryo attachment to occur successfully. One study has demonstrated that macrophages can contribute to the regulation of the embryo adhesive properties of epithelial cells in vitro [96]. This suggests a further important role for macrophages recruited into uterine tissues following seminal plasma exposure in events as early as embryo attachment.
Seminal plasma and pathologies of pregnancy
It is apparent that abnormal maternal immune responses, particularly insufficient immune tolerance are detrimental to pregnancy success. Immune disturbances can be most conclusively linked to implantation failure and trophoblast rejection in women [97], both of which are the basis of pregnancy pathologies such as pre-eclampsia and recurrent spontaneous abortion. There is good evidence that a strong Th1 response leading to detrimental cell-mediated immunity is deleterious to pregnancy outcome [98], where as a Th2 response resulting in a humoral immune response is seen as more favourable for a positive outcome [99]. The overall phenotype and proportion of lymphocytes is also thought to play a role in pathologies such as spontaneous abortion. For example, NK cells of the Th1 lineage are linked with foetal resorption in mice [98]. Interestingly, an aberrant immune response can be redirected; for example, foetal resorption in the abortion prone mouse model is significantly reduced by pre-immunisation of females with maternally matched class II MHC spleen cells [100].
Other immunological factors influencing pregnancy appear to be linked with semen exposure in a partner specific manner. Human studies show that both acute exposure to semen at the beginning of a pregnancy, as well as cumulative exposure over time, can protect against recurrent miscarriage and pre-eclampsia, in a partner specific manner [101]. Studies have also shown that semen exposure in women is advantageous to pregnancy outcome, the use of barrier contraception methods and the period of cohabitation between couples suggests that chronic semen exposure by an individual can be beneficial to subsequent pregnancies [102]. There is now a large body of evidence to suggest that preeclampsia has male factors, derived from semen, involved in its aetiology [103, 104].
Seminal plasma and assisted reproduction
Assisted reproductive techniques (ART), such as in vitro fertilisation (IVF), have become a widely utilised resource in the treatment of infertility in humans. Currently the consequences of such treatments on the health of offspring remain controversial. However, several studies now suggest that pregnancies derived from IVF/ICSI result in babies who are at an increased risks of cerebral palsy [105], premature birth [106, 107], low and very low birth weight [108], complications during delivery [109] and serious birth defects [110]. It is important however to note the controversy in the current literature, where opposing studies have found no association between the use of ART and various health outcome in children [111, 112]. The mechanisms by which these changes arise are as yet undefined; however epigenetic gene alterations of the placenta and foetus resulting from perturbations of the peri-conceptual environment are hypothesised to be significant factors [113].
Some applications of ART are relatively non-invasive and in part mimic natural conception, such as intra-uterine insemination. However, high tech approaches to ART are far more invasive and are dramatically different from the process of natural conception, particularly in regards to the handling of gametes and embryos. The circumstances involved in ART vary greatly from those of natural conception, including the utilisation of non-ejaculated sperm and forced fertilisation by intra-cytoplasmic sperm injection as well as the in vitro culture of embryos for extended periods of time. Another confounding factor in ART is the absence of cervical semen exposure both at the time of fertilisation and subsequent implantation.
Studies in animal models have begun to investigate the impact that semen exposure can have on altering the outcome of embryo transfer. Carp et al. demonstrated that mechanically induced pseudopregnant rats had a greater implantation rate after embryo transfer when exposed to semen at the time of implantation [114]. Artificial insemination in sheep has also been shown to benefit from cervical seminal plasma exposure, resulting in an increased percentage of pregnant ewes [6]. Removal of the accessories sex glands in the golden hamster has shown significant reductions in the expression of angiogenic and embryotrophic factors within the peri-implantation endometrium [94]. Our own studies have demonstrated the susceptibility to the very early embryo in generating offspring with metabolic perturbations. When in vivo derived 2-cell embryos are transferred to an oviduct not exposed to seminal plasma metabolic phenotypes develop in offspring, whereas in vivo derived blastocyst transferred to the same environment do not [13]. More recently this work has been carried over into the human ART program. It has been shown that semen exposure around the time of embryo transfer increases the rates of embryo implantation and possible subsequent foetal development [5, 115, 116]. Bellinge et al. has previously shown that seminal exposure as early as the time of oocyte retrieval can increase the incidence of pregnancy in women undergoing IVF [117]. However the effect is inconsistent as similar clinical trials found no relationship between high vaginal insemination at the time of oocyte pick up and pregnancy rates [118].
Summary
A large body of evidence now exists to suggest the importance of seminal exposure in driving multi factorial changes within the maternal uterus to establish an environment conducive to optimal embryo implantation and pregnancy outcome. Many of the processes known to be influenced by seminal exposure are critical in the processes of early embryonic development, implantation and trophoblast invasion. It has been clearly defined in the current literature that perturbation to these processes can have detrimental consequences in progeny not only during pregnancy but also after birth, with an increased risk of developmental and metabolic disorders. Although many studies have been conducted in animal models, epidemiological evidence exists suggesting an important role for seminal plasma exposure in the prevention of pregnancy related pathologies such as preeclampsia and spontaneous recurrent miscarriage. These are pathologies with unclear aetiologies; however both have links to the failure of the immune changes and other critical events occurring very early in pregnancy, at the time when seminal exposure is known to have its strongest influence in the maternal reproductive tract.
Acknowledgments
I would like to thank Sarah Robertson who pioneered this field of investigation and has been an supportive mentor. I would also like to thank David Albertini for his support and critical evaluation of the manuscript.
Footnotes
Capsule Seminal fluid exposure promotes healthy pregnancy outcomes.
References
- 1.Queen K, Dhabuwala CB, Pierrepoint CG. The effect of the removal of the various accessory sex glands on the fertility of male rats. J Reprod Fertil. 1981;62(2):423–6. doi: 10.1530/jrf.0.0620423. [DOI] [PubMed] [Google Scholar]
- 2.Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, et al. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165(9):5077–83. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 3.Cukierski MA, Sina JL, Prahalada S, Robertson RT. Effects of seminal vesicle and coagulating gland ablation on fertility in rats. Reprod Toxicol. 1991;5(4):347–52. doi: 10.1016/0890-6238(91)90093-u. [DOI] [PubMed] [Google Scholar]
- 4.Chan OC, Chow PH, O WS. Total ablation of paternal accessory sex glands curtails developmental potential in preimplantation embryos in the golden hamster. Anat Embryol (Berl). 2001;204 (2):117–22. [DOI] [PubMed]
- 5.Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod. 2000;15(12):2653–8. doi: 10.1093/humrep/15.12.2653. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell WM, Evans G, Mortimer ST, Gillan L, Gellatly ES, McPhie CA. Normal fertility in ewes after cervical insemination with frozen-thawed spermatozoa supplemented with seminal plasma. Reprod Fertil Dev. 1999;11(2):123–6. doi: 10.1071/rd99046. [DOI] [PubMed] [Google Scholar]
- 7.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58(5):1217–25. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 8.Robertson SA, Mau VJ, Tremellen KP, Seamark RF. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil. 1996;107(2):265–77. doi: 10.1530/jrf.0.1070265. [DOI] [PubMed] [Google Scholar]
- 9.Robertson SA, Mau VJ, Hudson SN, Tremellen KP. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol. 1997;37(6):438–42. doi: 10.1111/j.1600-0897.1997.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69(4):315–30. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol. 2012;189(2):1024–35. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 13.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci U S A. 2014;111(6):2200–5. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremellen KP. Seminal priming for successful mammalian pregnancy. In: Gupta S, editor. Reproductive Immunology. New Delhi: Narosa Publishing House; 1999. pp. 88–96. [Google Scholar]
- 15.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Bmj. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker DJ. Mothers, Babies and Health in Later Life. Edinburgh, NY: Churchill Livingstone; 1998. [Google Scholar]
- 17.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–8. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 18.Kind KL, Roberts CT, Sohlstrom AI. Katsman A. Robinson JS et al. Chronic maternal feed restriction impairs growth but increases adiposity of the fetal guinea pig. Am J Physiol Regul Integr Comp Physiol: Clifton PM; 2004. [DOI] [PubMed] [Google Scholar]
- 19.Desai M, Gayle D, Babu J, Ross MG. Programmed Obesity in Intrauterine Growth Restricted Newborns: Modulation by Newborn Nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R91–6. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JS, Falconer J, Owens JA. Intrauterine growth retardation: clinical and experimental. Acta Paediatr Scand Suppl. 1985;319:135–42. doi: 10.1111/j.1651-2227.1985.tb10123.x. [DOI] [PubMed] [Google Scholar]
- 21.Elias SG, Peeters PH, Grobbee DE, van Noord PA. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96(7):539–46. doi: 10.1093/jnci/djh087. [DOI] [PubMed] [Google Scholar]
- 22.Elias SG, Peeters PH, Grobbee DE, van Noord PA. The 1944–1945 Dutch famine and subsequent overall cancer incidence. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1981–5. doi: 10.1158/1055-9965.EPI-04-0839. [DOI] [PubMed] [Google Scholar]
- 23.Elias SG, van Noord PA, Peeters PH, den Tonkelaar I, Grobbee DE. Childhood exposure to the 1944–1945 Dutch famine and subsequent female reproductive function. Hum Reprod. 2005;20(9):2483–8. doi: 10.1093/humrep/dei090. [DOI] [PubMed] [Google Scholar]
- 24.Kind KL, Clifton PM, Katsman AI, Tsiounis M, Robinson JS, Owens JA. Restricted fetal growth and the response to dietary cholesterol in the guinea pig. Am J Physiol. 1999;277(6 Pt 2):R1675–82. doi: 10.1152/ajpregu.1999.277.6.R1675. [DOI] [PubMed] [Google Scholar]
- 25.Belobrajdic D, McIntosh G, Owens J. The effects of dietary protein on rat growth, body composition and insulin sensitivity. Asia Pac J Clin Nutr. 2003;12 Suppl:S42.
- 26.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127(19):4195–202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134(9):2169–72. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 28.McConnell JM, Petrie L. Mitochondrial DNA turnover occurs during preimplantation development and can be modulated by environmental factors. Reprod Biomed Online. 2004;9(4):418–24. doi: 10.1016/s1472-6483(10)61277-1. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R134–9. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sayer AA, Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005;81(9):735–44. doi: 10.1016/j.earlhumdev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127(5):515–26. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 32.Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81(9):723–34. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–6. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 34.Gardner DK, Lane M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod Fertil Dev. 2005;17(3):361–70. doi: 10.1071/rd04103. [DOI] [PubMed] [Google Scholar]
- 35.Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69(4):1109–17. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- 36.Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005;17(3):371–8. doi: 10.1071/rd04102. [DOI] [PubMed] [Google Scholar]
- 37.Robertson SA, Mayrhofer G, Seamark RF. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol Reprod. 1996;54(1):183–96. doi: 10.1095/biolreprod54.1.183. [DOI] [PubMed] [Google Scholar]
- 38.Robertson SA. Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF): A Paracrine Regulator in the Pre-Implantation Mouse Uterus. Adelaide: University of Adelaide; 1993. [Google Scholar]
- 39.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67(6):1817–23. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- 40.Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001;64(4):1206–15. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- 41.Lavranos TC, Rathjen PD, Seamark RF. Trophic effects of myeloid leukaemia inhibitory factor (LIF) on mouse embryos. J Reprod Fertil. 1995;105(2):331–8. doi: 10.1530/jrf.0.1050331. [DOI] [PubMed] [Google Scholar]
- 42.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172(2):221–36. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 43.Schultz GA, Heyner S. Growth factors in preimplantation mammalian embryos. Oxf Rev Reprod Biol. 1993;15:43–81. [PubMed] [Google Scholar]
- 44.Harvey MB, Kaye PL. Mediation of the actions of insulin and insulin-like growth factor-1 on preimplantation mouse embryos in vitro. Mol Reprod Dev. 1992;33(3):270–5. doi: 10.1002/mrd.1080330306. [DOI] [PubMed] [Google Scholar]
- 45.Brice EC, Wu JX, Muraro R, Adamson ED, Wiley LM. Modulation of mouse preimplantation development by epidermal growth factor receptor antibodies, antisense RNA, and deoxyoligonucleotides. Dev Genet. 1993;14(3):174–84. doi: 10.1002/dvg.1020140304. [DOI] [PubMed] [Google Scholar]
- 46.Tamada H, Higashiyama C, Takano H, Kawate N, Inaba T, Sawada T. The effects of heparin-binding epidermal growth factor-like growth factor on preimplantation-embryo development and implantation in the rat. Life Sci. 1999;64(22):1967–73. doi: 10.1016/s0024-3205(99)00128-9. [DOI] [PubMed] [Google Scholar]
- 47.Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146(5):2142–53. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 48.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7(1):65–70. doi: 10.1016/s1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 49.Sanocka D, Miesel R, Jedrzejczak P, Chelmonska-Soyta AC, Kurpisz M. Effect of reactive oxygen species and the activity of antioxidant systems on human semen; association with male infertility. Int J Androl. 1997;20(5):255–64. doi: 10.1046/j.1365-2605.1997.00050.x. [DOI] [PubMed] [Google Scholar]
- 50.Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl. 2004;6(1):59–65. [PubMed] [Google Scholar]
- 51.Wolters-Everhardt E, Dony JM, Lemmens WA, Doesburg WH, De Pont JJ. Buffering capacity of human semen. Fertil Steril. 1986;46(1):114–9. doi: 10.1016/s0015-0282(16)49468-9. [DOI] [PubMed] [Google Scholar]
- 52.Wolters-Everhardt E, Dony JM, Peters WH, De Pont JJ. Buffering substances of human semen. Fertil Steril. 1987;48(1):159–61. doi: 10.1016/s0015-0282(16)59309-1. [DOI] [PubMed] [Google Scholar]
- 53.Kelly RW. Prostaglandins in primate semen: biasing the immune system to benefit spermatozoa and virus? Prostaglandins Leukot Essent Fatty Acids. 1997;57(2):113–8. doi: 10.1016/s0952-3278(97)90000-4. [DOI] [PubMed] [Google Scholar]
- 54.Peitz B, Olds-Clarke P. Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod. 1986;35(3):608–17. doi: 10.1095/biolreprod35.3.608. [DOI] [PubMed] [Google Scholar]
- 55.Ying Y, Chow PH. Cheung MP. O WS. Effects of male accessory sex glands on sperm decondensation and oocyte activation during in vivo fertilization in golden hamsters. Int J Androl. 1999;22:68–76. doi: 10.1046/j.1365-2605.1999.00146.x. [DOI] [PubMed] [Google Scholar]
- 56.Pang SF, Chow PH, Wong TM. The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil. 1979;56(1):129–32. doi: 10.1530/jrf.0.0560129. [DOI] [PubMed] [Google Scholar]
- 57.WS O, Chen HQ, Chow PH. Effects of male accessory sex gland secretions on early embryonic development in the golden hamster. J Reprod Fertil. 1988;84(1):341–4. doi: 10.1530/jrf.0.0840341. [DOI] [PubMed] [Google Scholar]
- 58.Jiang HY, O WS, Lee KH, Tang PL, Chow PH. Ablation of paternal accessory sex glands is detrimental to embryo development during implantation. Anat Embryol (Berl). 2001;203 (4):255–63. [DOI] [PubMed]
- 59.De M. Determination of the number and distribution of macrophages, lymphocytes and granulocytes in the mouse uterus from mating through implantation. J Leukoc Biol. 1991;50:252–62. doi: 10.1002/jlb.50.3.252. [DOI] [PubMed] [Google Scholar]
- 60.Thompson LA, Barratt CL, Bolton AE, Cooke ID. The leukocytic reaction of the human uterine cervix. Am J Reprod Immunol. 1992;28(2):85–9. doi: 10.1111/j.1600-0897.1992.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 61.Phillips DM, Mahler S. Leukocyte emigration and migration in the vagina following mating in the rabbit. Anat Rec. 1977;189(1):45–59. doi: 10.1002/ar.1091890104. [DOI] [PubMed] [Google Scholar]
- 62.O'Leary S, Jasper MJ, Warnes GM, Armstrong DT, Robertson SA. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction. 2004;128(2):237–47. doi: 10.1530/rep.1.00160. [DOI] [PubMed] [Google Scholar]
- 63.Pandya IJ, Cohen J. The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril. 1985;43(3):417–21. doi: 10.1016/s0015-0282(16)48442-6. [DOI] [PubMed] [Google Scholar]
- 64.Troedsson MH, Loset K, Alghamdi AM, Dahms B, Crabo BG. Interaction between equine semen and the endometrium: the inflammatory response to semen. Anim Reprod Sci. 2001;68(3–4):273–8. doi: 10.1016/s0378-4320(01)00164-6. [DOI] [PubMed] [Google Scholar]
- 65.Anderson DJ, Tarter TH. Immunosuppressive effects of mouse seminal plasma components in vivo and in vitro. J Immunol. 1982;128(2):535–9. [PubMed] [Google Scholar]
- 66.Fahmi HA, Hunter AG, Markham RJ, Seguin BE. Immunosuppressive activity of bovine seminal plasma on bovine lymphocytes in vitro. J Dairy Sci. 1985;68(9):2315–21. doi: 10.3168/jds.S0022-0302(85)81105-X. [DOI] [PubMed] [Google Scholar]
- 67.Saxena S, Jha P, Farooq A. Immunosuppression by human seminal plasma. Immunol Invest. 1985;14(3):255–69. doi: 10.3109/08820138509076149. [DOI] [PubMed] [Google Scholar]
- 68.James K. Immunosuppression by seminal plasma and its possible clinical significance. Immunol Today. 1984;5(12):357–63. doi: 10.1016/0167-5699(84)90079-3. [DOI] [PubMed] [Google Scholar]
- 69.Anderson DJ, Tarter TH. Immunosuppressive effects of mouse seminal plasma components in vivo and in vitro. J Immunol. 1982;128(2):535–9. [PubMed] [Google Scholar]
- 70.Kelly RW, Carr GG, Critchley HO. A cytokine switch induced by human seminal plasma: an immune modulation with implications for sexually transmitted disease. Hum Reprod. 1997;12(4):677–81. doi: 10.1093/humrep/12.4.677. [DOI] [PubMed] [Google Scholar]
- 71.Robertson S, Sharkey D. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13(4):243–54. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 72.Thaler CJ. Immunological role for seminal plasma in insemination and pregnancy. Am J Reprod Immunol. 1989;21(3–4):147–50. doi: 10.1111/j.1600-0897.1989.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 73.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176(4):960–8. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 74.Uyenoyama MK. Coevolution of the major histocompatibility complex and the t-complex in the mouse. I. Generation and maintenance of high complementarity associations. Genetics. 1989;121(1):139–51. doi: 10.1093/genetics/121.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iyer SV, Nandedkar TD, Hegde UC. Production of H-Y antibody in the ascites fluid of mouse and localization of the antigen on cells and tissues. Gamete Res. 1989;22(1):37–49. doi: 10.1002/mrd.1120220105. [DOI] [PubMed] [Google Scholar]
- 76.Anderson DJ, Bach DL, Yunis EJ, DeWolf WC. Major histocompatibility antigens are not expressed on human epididymal sperm. J Immunol. 1982;129(2):452–4. [PubMed] [Google Scholar]
- 77.Hutter H, Dohr G. HLA expression on immature and mature human germ cells. J Reprod Immunol. 1998;38(2):101–22. doi: 10.1016/s0165-0378(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 78.Yao GD, Shu YM, Shi SL, Peng ZF, Song WY, Jin HX, et al. Expression and Potential Roles of HLA-G in Human Spermatogenesis and Early Embryonic Development. PloS one. 2014;9(3):e92889. doi: 10.1371/journal.pone.0092889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beer AE, Billingham RE. Host responses to intra-uterine tissue, cellular and fetal allografts. J Reprod Fertil, Suppl. 1974;21:59–88. [Google Scholar]
- 80.Watson JG, Chaykin S, Carroll J. Repoduction in mice: The fate of sprematozoa not involved in fertilisation. Gamete Res. 1983;7:75–84. [Google Scholar]
- 81.Parr MB, Parr EL. Antigen recognition in the female reproductive tract: I. Uptake of intraluminal protein tracers in the mouse vagina. J Reprod Immunol. 1990;17(2):101–14. doi: 10.1016/0165-0378(90)90029-6. [DOI] [PubMed] [Google Scholar]
- 82.Parr EL, Parr MB, Zheng LM, Young JD. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod. 1991;44(5):834–41. doi: 10.1095/biolreprod44.5.834. [DOI] [PubMed] [Google Scholar]
- 83.Johansson M, Bromfield JJ, Jasper MJ, Robertson SA. Semen activates the female immune response during early pregnancy in mice. Immunology. 2004;112(2):290–300. doi: 10.1111/j.1365-2567.2004.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piazzon I, Matusevich M, Deroche A, Nepomnaschy I, Pasqualini CD. Early increase in graft-versus-host reactivity during pregnancy in the mouse. J Reprod Immunol. 1985;8(2–3):129–37. doi: 10.1016/0165-0378(85)90036-1. [DOI] [PubMed] [Google Scholar]
- 85.O'Hearn M, Hilgard HR. Pregnancy-induced alterations in graft-versus-host responsiveness of uterine-draining and peripheral lymph node cells toward fetal alloantigens. Transplantation. 1981;32(5):389–91. doi: 10.1097/00007890-198111000-00009. [DOI] [PubMed] [Google Scholar]
- 86.Kapovic M, Rukavina D. Kinetics of lymphoproliferative responses of lymphocytes harvested from the uterine draining lymph nodes during pregnancy in rats. J Reprod Immunol. 1991;20:93–101. doi: 10.1016/0165-0378(91)90026-m. [DOI] [PubMed] [Google Scholar]
- 87.Clark D. Contoversies in reproductive immunology. Crit Rev Immunol. 1991;11 (3,4):215–47. [PubMed]
- 88.Beer AE, Scott JR, Billingham RE. Histoincompatibility and maternal immunological status as determinants of fetoplacental weight and litter size in rodents. J Exp Med. 1975;142(1):180–96. doi: 10.1084/jem.142.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 90.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod. 2011;85(2):397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 91.Vacchio MS, Jiang SP. The fetus and the maternal immune system: pregnancy as a model to study peripheral T-cell tolerance. Crit Rev Immunol. 1999;19(5–6):461–80. [PubMed] [Google Scholar]
- 92.Billingham R. Transplantation immunity and the maternal-fetal relation. N Engl J Med. 1964;270(13):667–71. doi: 10.1056/NEJM196403262701306. [DOI] [PubMed] [Google Scholar]
- 93.Daimon E, Wada Y. Role of neutrophils in matrix metalloproteinase activity in the preimplantation mouse uterus. Biol Reprod. 2005;73(1):163–71. doi: 10.1095/biolreprod.104.038539. [DOI] [PubMed] [Google Scholar]
- 94.Chow PH, Jiang HY, Poon HK, Lee KH, O WS. Embryos sired by males without accessory sex glands induce failure of uterine support: a study of VEGF, MMP and TGF expression in the golden hamster. Anat Embryol (Berl). 2003;206 (3):203–13. [DOI] [PubMed]
- 95.van der Heijden OW, Essers YP, Spaanderman ME, De Mey JG, van Eys GJ, Peeters LL. Uterine Artery Remodeling in Pseudopregnancy Is Comparable to that in Early Pregnancy. Biol Reprod. 2005;73(6):1289–93. doi: 10.1095/biolreprod.105.044438. [DOI] [PubMed] [Google Scholar]
- 96.Kosaka K, Fujiwara H, Tatsumi K, Yoshioka S, Higuchi T, Sato Y, et al. Human peripheral blood mononuclear cells enhance cell-cell interaction between human endometrial epithelial cells and BeWo-cell spheroids. Hum Reprod. 2003;18(1):19–25. doi: 10.1093/humrep/deg002. [DOI] [PubMed] [Google Scholar]
- 97.Hill JA. T-helper 1-type immunity to trophoblast: evidence for a new immunological mechanism for recurrent abortion in women. Hum Reprod. 1995;10(Suppl 2):114–20. doi: 10.1093/humrep/10.suppl_2.114. [DOI] [PubMed] [Google Scholar]
- 98.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18(10):478–82. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 99.Kanbour A, Ho HN, Misra DN, MacPherson TA, Kunz HW, Gill TJ. Differential expression of MHC class I antigens on the placenta of the rat. A mechanism for the survival of the fetal allograft. J Exp Med. 1987;166(6):1861–82. doi: 10.1084/jem.166.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiger N, Chaouat G, Kolb JP, Wegmann TG, Guenet JL. Immunogenetic studies of spontaneous abortion in mice. Preimmunization of females with allogeneic cells. J Immunol. 1985;134(5):2966–70. [PubMed] [Google Scholar]
- 101.Klonoff-Cohen HS, Savitz DA, Celafo RC, McCann MF. An epidemiologic study of contraception and preeclampsia. Journal of the American Medical association. 1989;262:3143–7. [PubMed] [Google Scholar]
- 102.Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. The Lancet. 1995;344:973–5. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 103.Dekker GA, Robillard PY, Hulsey TC. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. ObstetGynecolSurv. 1998;53(6):377–82. doi: 10.1097/00006254-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 104.Robertson SA, Bromfield JJ, Tremellen KP. Seminal 'priming' for protection from pre-eclampsia-a unifying hypothesis. J Reprod Immunol. 2003;59(2):253–65. doi: 10.1016/s0165-0378(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 105.Lambert RD. Safety issues in assisted reproductive technology: aetiology of health problems in singleton ART babies. Hum Reprod. 2003;18(10):1987–91. doi: 10.1093/humrep/deg361. [DOI] [PubMed] [Google Scholar]
- 106.Perri T, Chen R, Yoeli R, Merlob P, Orvieto R, Shalev Y, et al. Are singleton assisted reproductive technology pregnancies at risk of prematurity? J Assist Reprod Genet. 2001;18(5):245–9. doi: 10.1023/A:1016614217411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang JX, Norman RJ, Kristiansson P. The effect of various infertility treatments on the risk of preterm birth. Hum Reprod. 2002;17(4):945–9. doi: 10.1093/humrep/17.4.945. [DOI] [PubMed] [Google Scholar]
- 108.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346(10):731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 109.Ochsenkuhn R, Strowitzki T, Gurtner M, Strauss A, Schulze A, Hepp H, et al. Pregnancy complications, obstetric risks, and neonatal outcome in singleton and twin pregnancies after GIFT and IVF. Arch Gynecol Obstet. 2003;268(4):256–61. doi: 10.1007/s00404-003-0518-5. [DOI] [PubMed] [Google Scholar]
- 110.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–30. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 111.Marchand E, Poncelet C, Carbillon L, Pharisien I, Tigaizin A, Chanelles O. Is there more complications with pregnancies from the assisted reproductive technology than spontaneous pregnancies? A retrospective study over 6 years. Journal de gynecologie, obstetrique et biologie de la reproduction. 2011;40(6):522–8. doi: 10.1016/j.jgyn.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 112.Berry KA, Baron IS, Weiss BA, Baker R, Ahronovich MD, Litman FR. In vitro fertilization and late preterm preschoolers' neuropsychological outcomes: the PETIT study. Am J Obstet Gynecol. 2013;209 (4):356 e1-6. doi:10.1016/j.ajog.2013.06.041. [DOI] [PubMed]
- 113.Thompson JG, Kind KL, Roberts CT, Robertson SA, Robinson JS. Epigenetic risks related to assisted reproductive technologies: short- and long-term consequences for the health of children conceived through assisted reproduction technology: more reason for caution? Hum Reprod. 2002;17(11):2783–6. doi: 10.1093/humrep/17.11.2783. [DOI] [PubMed] [Google Scholar]
- 114.Carp HJ, Serr DM, Mashiach S, Nebel L. Influence of insemination on the implantation of transferred rat blastocysts. Gynecol Obstet Invest. 1984;18(4):194–8. doi: 10.1159/000299080. [DOI] [PubMed] [Google Scholar]
- 115.Coulam CB, Stern JJ. Effect of seminal plasma on implantation rates. Early Pregnancy. 1995;1(1):33–6. [PubMed] [Google Scholar]
- 116.Marconi G, Auge L, Oses R, Quintana R, Raffo F, Young E. Does sexual intercourse improve pregnancy rates in gamete intrafallopian transfer? Fertil Steril. 1989;51(2):357–9. doi: 10.1016/s0015-0282(16)60507-1. [DOI] [PubMed] [Google Scholar]
- 117.Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O‶Neil G, Cohen MJ. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil Steril. 1986;46(2):252–6. doi: 10.1016/s0015-0282(16)49521-x. [DOI] [PubMed] [Google Scholar]
- 118.Fishel S, Webster J, Jackson P, Faratian B. Evaluation of high vaginal insemination at oocyte recovery in patients undergoing in vitro fertilization. Fertil Steril. 1989;51(1):135–8. doi: 10.1016/s0015-0282(16)60442-9. [DOI] [PubMed] [Google Scholar]