Abstract
Purpose
An association between the INS VNTR polymorphisms and polycystic ovary syndrome (PCOS) susceptibility has been reported in previous studies, but the results were inconsistent. This study was conducted to explore this association using meta-analysis.
Methods
PubMed, Embase, and China National Knowledge Infrastructure (CNKI) were searched according to predefined criteria for all relevant studies published up to August 2013. Four genetic models, together with odds ratios (ORs) and 95 % confidence intervals (CI), were calculated. Subgroup analyses were performed by ethnicity, anovulatory PCOS, and Hardy–Weinberg equilibrium (HWE) in the controls.
Results
In total, 13 case–control studies, including 1,767 cases and 4,108 controls, were included. No significant association was detected in overall population in all models (III/III vs. I/I: OR = 1.200, 95%CI = 0.866–1.664, P = 0.277; I/III vs. I/I: OR = 1.041, 95%CI = 0.880–1.232, P = 0.637; III/III + I/III vs. I/I: OR = 1.191, 95%CI = 0.912–1.554, P = 0.199; III/III vs. I/III + I/I: OR = 1.100, 95%CI = 0.816–1.484, P = 0.531), the same as in Caucasian and Asian populations. When the studies were limited to conform to HWE, the results remained persistent and robust. The anovulation subgroup showed significantly elevated risk in the I/III vs. I/I (OR = 1.460, 95%CI = 1.017–2.095, P = 0.040).
Conclusions
This meta-analysis revealed no significant association between INS VNTR polymorphisms and the risk of PCOS in the overall population, while it supported that variance may be associated with susceptibility to PCOS with anovulation. Further confirmation is needed from more well-designed and larger studies.
Keywords: INS, Polymorphism, Polycystic ovary syndrome, Meta-analysis
Introduction
Polycystic ovary syndrome (PCOS), which is characterized by hyperandrogenism, polycystic ovarian morphology, chronic anovulation, hirsutism, and obesity, is one of the most common heterogeneous endocrine disorders. It affects 6–10 % of women of reproductive age according to 1990 NIH criteria [1, 2] and even more individuals according to the broader Rotterdam criteria [3]. It also has been tightly associated with insulin resistance, which has prevalence rates of 44 to 70 % reported using surrogate markers [4, 5]. In addition, human studies have demonstrated that disturbances in insulin secretion and action play an important part in sustaining the ovarian androgen hypersecretion [6] and cause compensatory hyperinsulinemia [7], which drives many of the phenotypic features of PCOS.
The presence of PCOS confers a substantially increased risk for type 2 diabetes and affected women have marked insulin resistance, independent of obesity [8, 9]. Therefore, insulin resistance and the associated hyperinsulinemia play a pathogenetic role in PCOS. The etiology of this condition remains controversial, but the genetic variations in some candidate genes that result in abnormal insulin secretion and activity may predispose an individual to the development of PCOS.
The minisatellite variable number of tandem repeats (VNTR) locus on chromosome 11p15.5 in the promoter of the INS gene is 596 base pairs (bp) upstream of the translation initiation site. The polymorphism arises from a tandem repetition of 14–15 bp. Allelic variations, which have a bimodal distribution in size, were classified according to the minisatellite length: class I (26–63 repeats; approximate frequency 30 %), class II (mean of 80 repeats; rare) and class III (141–209 repeats; approximate frequency 70 %) [10]. The variation in VNTR was shown to influence transcriptional activity of the gene in vitro and regulates INS transcription levels in cadaveric adult [11] and fetal human pancreas [12], and in pancreatic beta cells [13]. Le Fur S. et al. [14] revealed that the polymorphisms of INS VNTR are associated in vivo with a trend for lower insulin and higher glucose levels, while Bazaes et al. [15] reported an association between INS VNTR genotype and both insulin sensitivity and secretion in infancy. However, recent clinical studies that attempted to confirm these associations have produced conflicting results.
We considered the conflicting results reported in publications regarding associations of INS VNTR with PCOS risk and conducted a comprehensive meta-analysis of the published data to clarify the inconsistencies and identify potential sources of heterogeneity that might confound the conclusions.
Method
Search strategy and selection criteria
We searched Medline (PubMed), Embase, and China National Knowledge Infrastructure (CNKI), updated on August 2013, for all eligible genetic associated publications evaluating INS VNTR polymorphism and POS susceptibility. The combined search strategy used the terms (“polycystic ovary syndrome” or “polycystic ovarian syndrome” or “PCOS”) in combination with (“INS VNTR” or “insulin VNTR” or “insulin variable number of tandem repeats”) in combination with (“polymorphism” or “variation” or “SNP”). No language or country filters were applied. Manual retrieval was performed in all eligible studies to identify additional relevant publications. The inclusion criteria were as follows: (1) case–control or cohort studies design with evaluating the association between PCOS risk and INS VNTR polymorphism; (2) PCOS patients with any diagnosis criteria; the anovulation of anovulatory PCOS was defined as women with oligomenorrhoea (intermenstrual interval more than 6 weeks); (3) the papers had to provide the size of the samples, distribution of alleles and genotypes, or other information that could help to infer the OR and 95%CI; (4) when multiple publications reported on the same or overlapping data, we chose the one with most recent or largest population. When a study reported the results on different subpopulations, we treated it as a separate study in the meta-analysis. The exclusion criteria were as follows: (1) studies that contained overlapping data; (2) studies in which family members had been studied because their analyses are based on linkage considerations; and (3) reviews, comments, letters, and repeated literature.
Data extraction
Two reviewers (Xue Qin and Liu-ying Song) independently extracted the information from all eligible studies, as follows: first author, year of publication, country of origin, ethnicity of the population studied, total number of cases and controls, genotyping methods, diagnosis criteria for PCOS, source of control, genotype distribution in cases and controls, genotype distribution in the subgroup anovulatory PCOS. When different results appeared, both reviewers would check the data again and have a discussion to come to a consensus. If they could not reach an agreement, a third author (Shan Li) was invited to the discussion.
Statistical analysis
Data were analyzed mainly using the STATA version 12.0 (StataCorp LP, College Station, Texas, USA). Previous studies found that class III alleles relate to the disordered insulin secretion, the decreased pancreatic expression of insulin, and increased risk of glucose intolerance in elderly men, while all of included articles regard class III allele as a baseline allele, I/III and III/III genotypes was used as a baseline genotype. So, the association between INS VNTR polymorphism and PCOS was first determined using the per-allele approach, which compared PCOS patients against controls for the contrast of III versus I alleles. We estimated the association with PCOS risk under certain genotypic models, namely codominant (III/III vs. I/I; I/III vs. I/I), recessive (III/III vs. I/III + I/I), and dominant (III/III + I/III vs. I/I). The strength of association was assessed by calculating a summary odds ratio (OR) together with the corresponding 95 % confidence interval (CI). The inter-study heterogeneity was appraised using χ2-based Q statistics and I2 [16] for statistical significance of heterogeneity. If no heterogeneity was indicated, based on P > 0.10 and I2 < 50 %, a fixed-effect model using the Mantel-Haenszel (M-H) [17] method was used. Otherwise, the random-effects model using the DerSimonian-Laird (D-L) [18] method was employed. Publication bias was assessed by the funnel plots and Egger regression asymmetry test [19]. Prespecified subgroup analyses were performed by (1) ethnicity; (2) anovulatory PCOS; and (3) Hardy-Weinberg equilibrium (HWE) in the controls. Sensitivity analysis [20] was performed to assess the consistency of results and the influence of each study on the overall meta-analysis by sequential omission of individual studies. HWE was detected using the goodness-of-fit χ2 test. All significance tests of the standard test were bilateral P < 0.05.
Results
Eligible studies
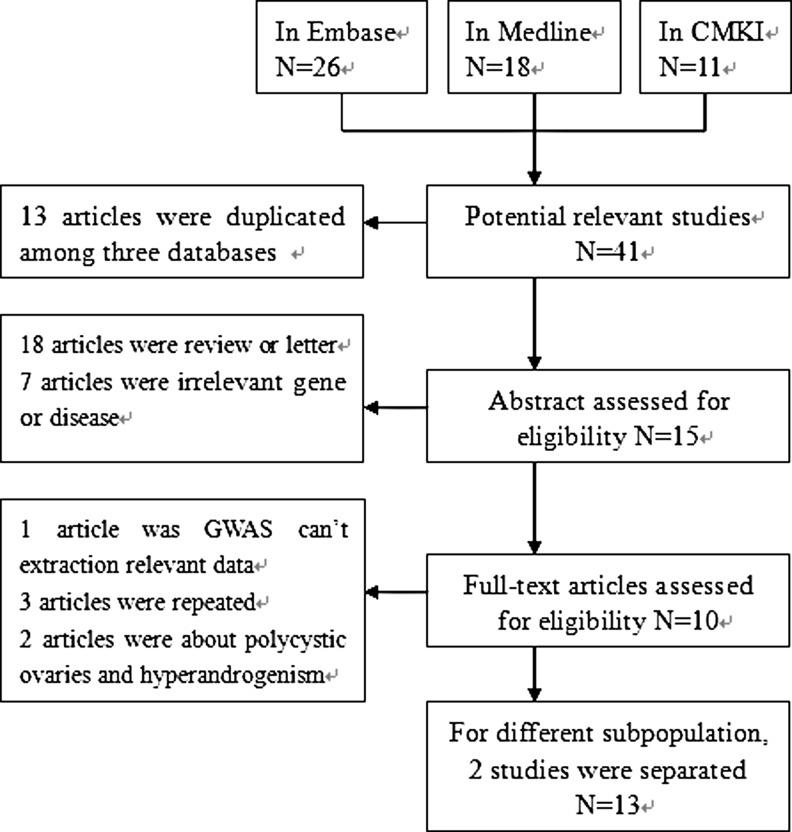
The literature retrieval identified 18 publications in Medline, 26 in Embase, and 11 in CNKI. Ultimately, 13 articles were included and the flow diagram is presented in Fig. 1.
Fig. 1.
Flow diagram of included studies for this meta-analysis
Baseline characteristics
In total, 13 case–control studies [21–30] including 1,767 cases and 4,181 controls, were included in the analysis. Waterworth DM [21] and Powell BL [23] were conducted on the anovulatory PCOS. The baseline characteristics are shown in Table 1.
Table 1.
The characteristics of eligible studies of meta-analysis
| First author (year) | Ethnicity (country) | Sample size (case/control) | Genotyping methods | Source of control | PCOS diagnosis | Matching criteria | HWE P value |
|---|---|---|---|---|---|---|---|
| Waterworth(1) (1997) | Caucasian (London) | 59/54 | NR | PB | Ultrasound symptoms | NR | 0.142 |
| Waterworth(2) (1997) | Caucasian (London) | 39/39 | NR | FB | Ultrasound symptoms | NR | 0.006 |
| Vankova (2002) | Caucasian (Česko) | 38/22 | PCR-RFLP | PB | NIH criteria | Age, Weight | 0.670 |
| Powell (1) (2005) | Caucasian (Britain) | 185/1062 | ARMS assay, Pyrosequencing | PB | Rotterdam criteria | Age | 0.321 |
| Powell (2) (2005) | Caucasian (Finland) | 72/1069 | ARMS assay, Pyrosequencing | PB | Ultrasound symptoms | Age, BMI, Weight | 0.564 |
| Powell (3) (2005) | Caucasian (Finland) | 458/1069 | ARMS assay, Pyrosequencing | PB | symptoms | Age, BMI, Weight | 0.564 |
| Kadri (2007) | Caucasian (Estonia) | 30/75 | PCR-RFLP | HB | Rotterdam criteria | Age, BMI | 0.182 |
| P Perk (2008) | Caucasian (Slovenia) | 117/108 | TaqMan assay | PB | Rotterdam criteria | Age | 0.811 |
| Y-P Xu (2009) | Asian (China) | 216/192 | PCR-RFLP | HB | Rotterdam criteria | Age | 0.982 |
| Q-F Zhang (2010) | Asian (China) | 130/130 | PCR-RFLP | HB | Rotterdam criteria | NR | 0.055 |
| Zhen Liu (2011) | Asian (China) | 55/50 | PCR-RFLP | HB | Rotterdam criteria | Age | 0.006 |
| J-H Yun (2012) | Asian (Korea) | 218/141 | PCR-RFLP | HB | Rotterdam criteria | BMI,Weight | 0.292 |
| L. Skrgati (2013) | Caucasian (Croatia) | 150/170 | TaqMan assay | PB | Rotterdam criteria | Age, BMI | 0.674 |
ARMS amplification refractory mutation system, PB population-base, HB hospital-base, FB family-base, NR not reported
Data in boldface represent P<0.05
Meta-analysis
Summary results for the association between INS VNTR Polymorphism and PCOS susceptibility are presented in Table 2.
Table 2.
Meta-analysis of the INS VNTR polymorphism on male infertile risk
| Comparison | Population | No. of studies | Sample size | Test of association | Test of heterogeneity | PEgger | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | OR | 95%CI | P value | M | PQ value | I2 (%) | ||||
| III vs. I | Overall | 13 | 1767 | 4181 | 1.108 | 0.910–1.351 | 0.307 | R | 0.007 | 55.8 | 0.495 |
| Caucasian | 9 | 1148 | 3668 | 1.209 | 0.993–1.471 | 0.058 | R | 0.047 | 49.1 | 0.051 | |
| Asians | 4 | 619 | 513 | 0.786 | 0.464–1.332 | 0.371 | R | 0.062 | 59.2 | 0.921 | |
| Anovulatory PCOS | 2 | 151 | 1116 | 1.662 | 0.664–4.163 | 0.278 | R | 0.014 | 83.4 | – | |
| HWE in control | 11 | 1673 | 4092 | 1.076 | 0.871–1.329 | 0.497 | R | 0.005 | 60.4 | 0.706 | |
| III/III vs. I/I | Overall | 12 | 1549 | 4040 | 1.200 | 0.866–1.664 | 0.277 | F | 0.114 | 34.5 | 0.003 |
| Caucasian | 9 | 1148 | 3668 | 1.362 | 0.967–1.919 | 0.077 | F | 0.186 | 29.1 | 0.032 | |
| Asians | 3 | 401 | 372 | 0.382 | 0.121–1.203 | 0.100 | F | 0.191 | 39.7 | 0.240 | |
| Anovulatory PCOS | 2 | 151 | 1116 | 1.349 | 0.716–2.541 | 0.354 | R | 0.005 | 87.5 | – | |
| HWE in control | 10 | 1455 | 3951 | 1.110 | 0.792–1.556 | 0.544 | F | 0.125 | 35.3 | 0.831 | |
| I/III vs. I/I | Overall | 12 | 1549 | 4040 | 1.041 | 0.880–1.232 | 0.637 | F | 0.180 | 26.9 | 0.000 |
| Caucasian | 9 | 1148 | 3668 | 1.063 | 0.897–1.259 | 0.480 | F | 0.270 | 19.5 | 0.085 | |
| Asians | 3 | 401 | 372 | 0.368 | 0.104–1.299 | 0.120 | F | 0.201 | 37.7 | 0.043 | |
| Anovulatory PCOS | 2 | 151 | 1116 | 1.460 | 1.017–2.095 | 0.040 | F | 0.241 | 27.4 | – | |
| HWE in control | 10 | 1455 | 3951 | 1.030 | 0.868–1.222 | 0.735 | F | 0.106 | 37.9 | 0.871 | |
| III/III + I/III vs. I/I | Overall | 12 | 1549 | 4040 | 1.191 | 0.912–1.554 | 0.199 | R | 0.086 | 38.3 | 0.004 |
| Caucasian | 9 | 1148 | 3668 | 1.080 | 0.917–1.272 | 0.358 | F | 0.116 | 37.9 | 0.033 | |
| Asians | 3 | 401 | 372 | 0.377 | 0.120–1.185 | 0.095 | F | 0.187 | 40.4 | 0.254 | |
| Anovulatory PCOS | 2 | 151 | 1116 | 1.895 | 0.679–5.286 | 0.222 | R | 0.072 | 69.2 | – | |
| HWE in control | 10 | 1455 | 3951 | 1.036 | 0.879–1.222 | 0.671 | R | 0.053 | 46.3 | 0.615 | |
| III/III vs. I/III + I/I | Overall | 13 | 1767 | 4181 | 1.100 | 0.816–1.484 | 0.531 | R | 0.074 | 39.0 | 0.227 |
| Caucasian | 9 | 1148 | 3668 | 1.283 | 0.995–1.654 | 0.054 | F | 0.263 | 20.2 | 0.273 | |
| Asians | 4 | 619 | 513 | 0.810 | 0.577–1.137 | 0.223 | F | 0.128 | 47.3 | 0.846 | |
| Anovulatory PCOS | 2 | 151 | 1116 | 2.181 | 0.209–22.758 | 0.515 | R | 0.011 | 84.7 | – | |
| HWE in control | 11 | 1673 | 4092 | 1.071 | 0.789–1.454 | 0.662 | R | 0.075 | 41.0 | 0.624 | |
Data in boldface represent P<0.05
Analysis of the overall population
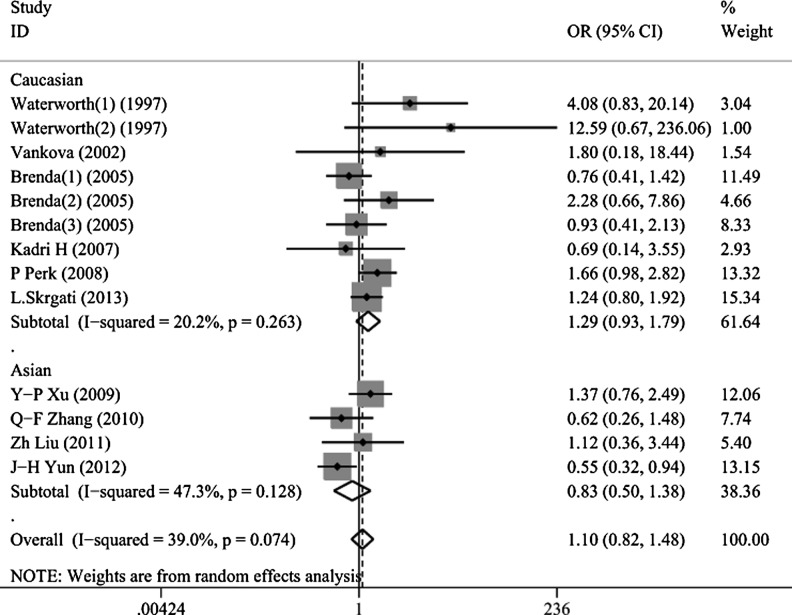
Thirteen studies (1,767 cases and 4,181 controls) were investigated in III/III vs. I/III + I/I; one study was excluded because the number of I/I was 0 in other models. Significant between-study heterogeneity was detected in the contrasts of III/III + I/III vs. I/I and III/III vs. I/III + I/I (PQ = 0.086, PQ = 0.074, repeatedly). No association was observed between the INS VNTR polymorphism and PCOS in the overall population in all models (III/III vs. I/I: OR = 1.200, 95%CI = 0.866–1.664, P = 0.277; I/III vs. I/I: OR = 1.041, 95%CI = 0.880–1.232, P = 0.637; III/III + I/III vs. I/I: OR = 1.191, 95%CI = 0.912–1.554, P = 0.199; III/III vs. I/III + I/I: OR = 1.100, 95%CI = 0.816–1.484, P = 0.531, Fig. 2).
Fig. 2.
Forest plots of INS VNTR polymorphisms and PCOS risk using a random-effect model (recessive model III/III vs. I/III + I/I). The squares and horizontal lines correspond to the study-specific OR and 95 % CI. The area of the squares reflects the study-specific weight (inverse of the variance). The diamond represents the summary OR and 95 % CI
Analysis in Caucasian and Asian populations
The meta-analysis included nine studies (1,148 cases and 3,668 controls) in the Caucasian population in all models and four studies (619 cases and 513 controls) in the Asian population in III/III vs. I/III + I/I, while three studies in other models had a number for I/I of 0, according to J-H Yun’s article. No positive result was detected either the text of between-study heterogeneity or the text of association in the Caucasian and Asian populations.
Analysis in the anovulatory population
The meta-analysis was conducted on two studies (151cases and 1,116 controls) in the anovulatory population. Between-study heterogeneity was found in III/III vs. I/I (PQ = 0.005, I2 = 87.5 %), III/III + I/III vs. I/I (PQ = 0.072, I2 = 69.2 %) and III/III vs. I/III + I/I (P = 0.011 I2 = 84.7 %). A statistically significant association was established for the INS VNTR polymorphism in the anovulatory population in I/III vs. I/I (OR = 1.460, 95%CI = 1.017–2.095, P = 0.040).
Analysis in the HWE population
The meta-analysis was carried out in 11 studies (1,673 cases and 4,092 controls) which conformed to HWE. Significant between-study heterogeneity was detected in the contrasts of III/III + I/III vs. I/I and III/III vs. I/III + I/I (PQ = 0.053, PQ = 0.075, repeatedly). We did not detect any association between the INS VNTR polymorphism and PCOS in the HWE population in all models
Sensitivity analysis and publication bias
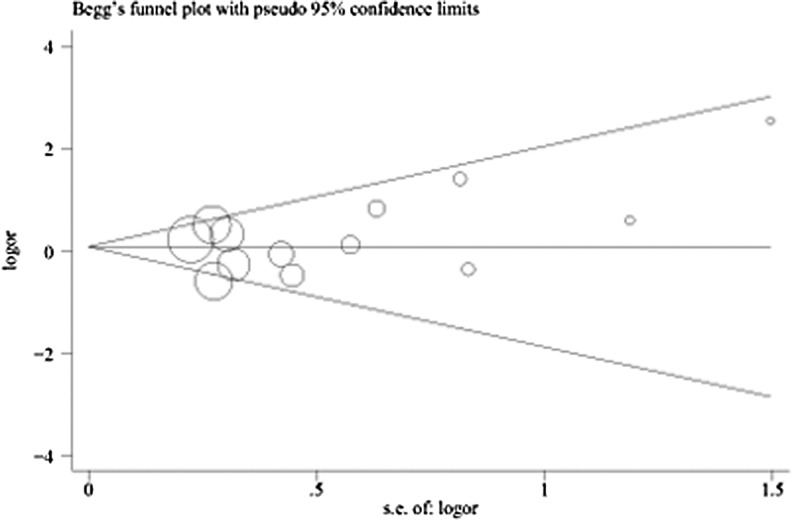
Sensitivity analysis confirmed that no single study influenced the overall results (data not shown). The results of Begg’s funnel plot and Egger’s tests indicated a lack of publication bias in recessive models (III/III vs. I/III + I/I: PEgger = 0.227, Fig. 3), but existence of publication bias using other models (III/III vs. I/I: PEgger = 0.003; I/III vs. I/I: PEgger = 0.000; III/III + I/IIIvs. I/I: PEgger = 0.004).
Fig. 3.
Begg’s funnel plots for publication bias in the studies of the meta-analysis on the association between INS VNTR and PCOS risk of the overall populations (recessive model III/III vs. I/III + I/I). Each point represents a separate study for the indicated association. Log [OR], natural logarithm of OR. Horizontal line means effect size
Discussion
The INS gene region is a susceptibility locus for several endocrine diseases. This association is modulated by the VNTR polymorphism, which is almost completely concordant with the adjacent A to T polymorphism at the −23 Hph1 site of the insulin gene (rs689). The latter is therefore used as surrogate for class I and III INS VNTR alleles. The INS VNTR has been mostly responsible for the association of autoimmune diabetes, T1DM, T2DM, gestational diabetes, and altered insulin release, but the findings remain controversial among the existing studies.
Waterworth et al. [21] first reported that the INS VNTR polymorphism had an effect on increasing the risk of PCOS. Since then, several genetic association studies on it have been performed, but the small sample sizes of these studies inevitably increased the risk that chance could be responsible for their conclusions. This, then, could lead to the apparently contradictory findings, as could the different study designs, various methodologies, insufficient power, and different population backgrounds [31]. However, meta-analysis is a good statistical method that has the advantage of increasing the effective sample size, reducing random error, and achieving precise estimates for potential genetic associations by combining data from all eligible studies [32]. To the best of our knowledge, no meta-analysis has yet evaluated the association between the INS VNTR polymorphisms and PCOS risk, so our meta-analysis is the first one on this issue. We have included thirteen individual case–control studies with 1,767 cases and 4,181 controls in our meta-analysis.
Our meta-analysis revealed no association between INS VNTR polymorphism and PCOS susceptibility. The majority of the included studies identified PCOS according to the Rotterdam criteria, where confirmed cases were based on the appearance of two of the phenotypes—hyperandrogenism and PCO with ovulatory cycles, or anovulation and PCO without hyperandrogenism—which have modest [33] or absent [34] evidence for insulin resistance using surrogate markers. In addition, Michelmore et al. [35] observed no association between INS VNTR genotype and the presence of PCO on ultrasound, while Rosa M. et al. [36] found that hyperandrogenism and the INS VNTR regulatory polymorphism were not associated. This may have influenced the results by potential selection bias on the criteria for PCOS. What’s more, it may possible that this genetic region play a role in insulin resistance when the beta cell function declines to a certain degree, as the absence of a difference in insulin secretion between the class I and class III allele groups in healthy adults [37].
Significant parent-of-origin effects have also been detected previously at the INS VNTR in relation to PCOS and these studies were not family-based. Possibly the absence of parental DNA prevented parent-of-origin effects from being tested, further contributing to the inconsistency [21]. The variations in matching criteria among the eligible studies also could potentially affect the negative results. Besides, this allelic variation could possibly relate to PCOS only at certain times during development, or in specific environmental or genetic backgrounds. Alternatively, INS VNTR polymorphism may simply not be a key factor in the etiology and the pathogenesis of PCOS.
Although our meta-analysis showed that the INS VNTR polymorphism had no exact effect on increased risk for PCOS overall, it did increase the risk of PCOS with anovulation in the I/III vs. I/I model. Some of the human studies [38, 39] have suggested that hyperinsulinemia/insulin resistance-mediated reduction in pituitary sensitivity to gonadotropin-releasing hormone (GnRH) may contribute to anovulation in PCO. Previous randomized controlled trials and meta-analysis found that anovulation improved during treatment with metformin, which is an insulin sensitizing agent, in women with PCOS [40, 41]. This indicated the presence of a certain relationship between anovulation and insulin related genes. Kadri et al. [24] also observed that the mean ovarian follicle number was lower in VNTR I/III and III/III individuals compared to VNTR I/I individuals, while Michelmore et al. [35] also declared that both III/III genotype and paternal class III allele transmission were significantly related to the increased number of PCOS features and to reduced insulin sensitivity among women with PCO.
All these findings therefore suggested positive correlations between INS VNTR polymorphism and anovulatory PCOS. We propose that this disease susceptibility locus be designated PCOS, but sufficient and robust evidence is still lacking and the mechanism is uncertain, so the further research should focus on this issue. The limited number of included studies, the significant inter-study heterogeneity, and the significant association only in the I/III vs. I/I model should dictate that the positive results be interpreted with caution.
Heterogeneity plays an important role in meta-analysis and finding the source of heterogeneity is very important for the final result of the meta-analysis. In the current study, obvious inter-study heterogeneity was found in the overall population in III/III + I/III vs. I/I and III/III vs. I/III + I/I for the P values were greater than 0.1. When stratified by ethnicity, no significant heterogeneity was found, which indicated that ethnicity may be the major source of the heterogeneity. The different genetic effects may be accounted for by differences in genetic backgrounds and in the environments in which the Asian and the Caucasian populations lived. It also indicated that PCOS may be an outcome of the interaction between genes and environment. However, other potential sources of heterogeneity should not be neglected.
Selection bias could also possibly have played a role as the genotype distribution of INS VNTR polymorphism among the control subjects did not conform to HWE in two studies. Departures from HWE, if not due to chance or violation of genetic reasons of no new mutations, no selection, and random mating assumptions, are widely recognized as pointing to methodological problems such as genotyping error or biased selection of subjects from the population [42]. We carried out subgroup analysis by HWE in controls, considering that the results of genetic association studies might be spurious if the distribution of genotypes in the controls deviated from HWE. Exclusion of the studies where the distribution of genotypes in the control groups were not in HWE, still gave results that were persistent and robust, suggesting that this factor probably had little effect on the overall estimates.
Some limitations of this meta-analysis require caution. First, the characteristics between PCOS and controls were not matched in almost all the studies, and the results were not adjusted for it in most cases. These types of confounding factors might mask the true relationship between INS VNTR and PCOS risk; statistical analysis indicated publication bias may have been present as perhaps some related unpublished studies that met the inclusion criteria were missed. Second, all the eligible studies were performed in Asian and Caucasian populations; therefore, studies on other ethnic groups including African Americans and Latinos will be required in order to capture the full range of possible ethnic differences in INS polymorphisms. Third, the lack of original study limited our further evaluation of the association between phenotype and genotype.
In spite of these shortcomings, our meta-analysis also had several advantages, as follows: First, a meta-analysis of the association of INS VNTR polymorphisms on PCOS risk is statistically more powerful than any other single study. Second, strict literature retrieval by a combination of computer-assisted and manual searches make the group of studies included as eligible as complete as possible. Third, the sensitivity analysis indicated the stability and credibility of the meta-analysis, which leads to a more convincing result. More important, the process of literature selection, data extraction, and data analysis in the meta-analysis was well designed and conducted to guarantee robust results.
Conclusions
This meta-analysis suggests that an association between INS VNTR polymorphism with PCOS risk is lacking in the overall population, while presents in the anovulatory. Well-designed and larger studies are still needed to validate our conclusions and to investigate the effects of gene-gene and gene-environment interactions.
Acknowledgement
No funding was provided for the analysis.
Conflict of interest
None Declared.
Footnotes
Capsule INS VNTR polymorphism implicate in PCOS by influence insulin sensitivity and secretion, but this meta-analysis found no association between INS VNTR polymorphism and PCOS risk.
Contributor Information
Shan Li, Phone: +86-771-5356052, FAX: +86-771-865353342, Email: lis8858@126.com.
Xue Qin, Phone: +86-771-5356052, FAX: +86-771-865353342, Email: qinxue919@126.com.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 3.Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, et al. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 4.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460. doi: 10.1016/j.fertnstert.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 5.de Paula Martins W, Santana LF, Nastri CO, Ferriani FA, de Sa MF, et al. Agreement among insulin sensitivity indexes on the diagnosis of insulin resistance in polycystic ovary syndrome and ovulatory women. Eur J Obstet Gynecol Reprod Biol. 2007;133:203–207. doi: 10.1016/j.ejogrb.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Tosi F, Negri C, Perrone F, Dorizzi R, Castello R, et al. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97:1712–1719. doi: 10.1210/jc.2011-2939. [DOI] [PubMed] [Google Scholar]
- 7.Semple RK, Savage DB, Cochran EK, Gorden P, O’Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32:498–514. doi: 10.1210/er.2010-0020. [DOI] [PubMed] [Google Scholar]
- 8.Pierpoint T, McKeigue P, Isaacs A, Wild S, Jacobs H. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51:581–586. doi: 10.1016/S0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 9.Margolin E, Zhornitzki T, Kopernik G, Kogan S, Schattner A, et al. Polycystic ovary syndrome in post-menopausal women—marker of the metabolic syndrome. Maturitas. 2005;50:331–336. doi: 10.1016/j.maturitas.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes melliitus. Diabetes. 1984;33:176–183. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 11.Bennett ST, Wilson AJ, Cucca F, Nerup J, Pociot F, et al. IDDM2-VNTR-encoded susceptibility to type 1 diabetes: dominant protection and parental transmission of alleles of the insulin gene-linked minisatellite locus. J Autoimmun. 1996;9:415–421. doi: 10.1006/jaut.1996.0057. [DOI] [PubMed] [Google Scholar]
- 12.Vafiadis P, Bennett ST, Colle E, Grabs R, Goodyer CG, et al. Imprinted and genotype-specific expression of genes at the IDDM2 locus in pancreas and leucocytes. J Autoimmun. 1996;9:397–403. doi: 10.1006/jaut.1996.0054. [DOI] [PubMed] [Google Scholar]
- 13.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 14.Le Fur S, Auffray C, Letourneur F, Cruaud C, Le Stunff C, et al. Heterogeneity of class I INS VNTR allele association with insulin secretion in obese children. Physiol Genomics. 2006;25:480–484. doi: 10.1152/physiolgenomics.00311.2005. [DOI] [PubMed] [Google Scholar]
- 15.Bazaes R, Petry C, Ong K, Avila A, Dunger D, et al. Insulin gene VNTR genotype is associated with insulin sensitivity and secretion in infancy. Clin Endocrinol. 2003;59:599–603. doi: 10.1046/j.1365-2265.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W Statistical aspects of the analysis of data from retrospective studies of disease. The challenge of epidemiology: issues and selected readings. 2004;1:533–53.
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1:247–262. doi: 10.1093/biostatistics/1.3.247. [DOI] [PubMed] [Google Scholar]
- 21.Waterworth DM, Bennett ST, Gharani N, McCarthy MI, Hague S, et al. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349:986–990. doi: 10.1016/S0140-6736(96)08368-7. [DOI] [PubMed] [Google Scholar]
- 22.Vanková M, Vrbíková J, Hill M, Cinek O, Bendlová B. Association of insulin gene VNTR polymorphism with polycystic ovary syndrome[J]. Ann NY Acad Sci. 2002;967:558–65. [DOI] [PubMed]
- 23.Powell BL, Haddad L, Bennett A, Gharani N, Sovio U, et al. Analysis of multiple data sets reveals no association between the insulin gene variable number tandem repeat element and polycystic ovary syndrome or related traits. J Clin Endocrinol Metab. 2005;90:2988–2993. doi: 10.1210/jc.2004-2485. [DOI] [PubMed] [Google Scholar]
- 24.Haller K, Laisk T, Peters M, Talving E, Karits P, et al. VNTR I/I genotype of insulin gene is associated with the increase of follicle number independent from polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2007;86:726–732. doi: 10.1080/00016340701322085. [DOI] [PubMed] [Google Scholar]
- 25.Ferk P, Perme MP, Gersak K. Insulin gene polymorphism in women with polycystic ovary syndrome. J Int Med Res. 2008;36:1180–1187. doi: 10.1177/147323000803600603. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Wei Z, Zhang Z, Xing Q, Hu P, et al. No association of the insulin gene VNTR polymorphism with polycystic ovary syndrome in a Han Chinese population. Reprod Biol Endocrinol. 2009;7:141. doi: 10.1186/1477-7827-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q-F, Han S-P, Cui Y-G, Li Y, Ma X, et al. Polymorphism of insulin gene variable number tandem repeats correlation with polycystic ovary syndrome. J Int Reprod Health/Fam Plan. 2010;29:252–255. [Google Scholar]
- 28.Zhen L. Genetic polymorphism of CYP17, INS and LHβ genes in patients with polycystic ovary syndrome. Master’s thesis Guangzhou Medical University. 2011.
- 29.Yun JH, Gu BH, Kang YB, Choi BC, Song S, et al. Association between INS-VNTR polymorphism and polycystic ovary syndrome in a Korean population. Gynecol Endocrinol. 2012;28:525–528. doi: 10.3109/09513590.2011.650658. [DOI] [PubMed] [Google Scholar]
- 30.Skrgatic L, Baldani DP, Gersak K, Cerne JZ, Ferk P, et al. Genetic polymorphisms of INS, INSR and IRS-1 genes are not associated with polycystic ovary syndrome in Croatian women. Coll Anthropol. 2013;37:141–146. [PubMed] [Google Scholar]
- 31.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 32.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Carmina E, Wong L, Chang L, Paulson RJ, Sauer MV, et al. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod. 1997;12:905–909. doi: 10.1093/humrep/12.5.905. [DOI] [PubMed] [Google Scholar]
- 34.Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol. 2007;66:513–517. doi: 10.1111/j.1365-2265.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 35.Michelmore K, Ken Ong SM, Bennett S, Perry L, Vessey M, et al. Clinical features in women with polycystic ovaries: relationships to insulin sensitivity, insulin gene VNTR and birth weight. Clin Endocrinol. 2001;55:439–446. doi: 10.1046/j.1365-2265.2001.01375.x. [DOI] [PubMed] [Google Scholar]
- 36.Calvo RM, Telleria D, Sancho J, San Millan JL, Escobar-Morreale HF. Insulin gene variable number of tandem repeats regulatory polymorphism is not associated with hyperandrogenism in Spanish women. Fertil Steril. 2002;77:666–668. doi: 10.1016/S0015-0282(01)03238-1. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed S, Bennett S, Huxtable S, Todd J, Matthews D, et al. INS VNTR allelic variation and dynamic insulin secretion in healthy adult non-diabetic Caucasian subjects. Diabet Med. 1999;16:910–917. doi: 10.1046/j.1464-5491.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- 38.Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, et al. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93:2089–2096. doi: 10.1210/jc.2007-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab. 2003;88:5158–5162. doi: 10.1210/jc.2003-030167. [DOI] [PubMed] [Google Scholar]
- 40.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 41.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JP. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol. 2006;163:300–309. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]