Abstract
Purpose
Both vascular endothelial growth factor A (VEGFA) and endocrine gland-derived vascular endothelial growth factor (EG-VEGF) systems play major roles in angiogenesis. A body of evidence suggests VEGFs regulate critical processes during pregnancy and have been associated with recurrent pregnancy loss (RPL). However, little information is available regarding the interaction of these two major major angiogenesis-related systems in early human pregnancy. This study was conducted to investigate the association of gene polymorphisms and gene-gene interaction among genes in VEGFA and EG-VEGF systems and idiopathic RPL.
Methods
A total of 98 women with history of idiopathic RPL and 142 controls were included, and 5 functional SNPs selected from VEGFA, KDR, EG-VEGF (PROK1), PROKR1 and PROKR2 were genotyped. We used multifactor dimensionality reduction (MDR) analysis to choose a best model and evaluate gene-gene interactions. Ingenuity pathways analysis (IPA) was introduced to explore possible complex interactions.
Results
Two receptor gene polymorphisms [KDR (Q472H) and PROKR2 (V331M)] were significantly associated with idiopathic RPL (P < 0.01). The MDR test revealed that the KDR (Q472H) polymorphism was the best loci to be associated with RPL (P = 0.02). IPA revealed EG-VEGF and VEGFA systems shared several canonical signaling pathways that may contribute to gene-gene interactions, including the Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3 signaling pathways.
Conclusion
Two receptor gene polymorphisms [KDR (Q472H) and PROKR2 (V331M)] were significantly associated with idiopathic RPL. EG-VEGF and VEGFA systems shared several canonical signaling pathways that may contribute to gene-gene interactions, including the Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3.
Keywords: VEGFA, EG-VEGF (PROK1), Recurrent pregnancy loss, Gene-gene interaction, Ingenuity pathways analysis
Introduction
Angiogenesis and a sufficient blood supply are critical for a successful early pregnancy for endometrial preparation, implantation, and placental and fetal vasculature development [1, 2]. An inadequate blood supply and angiogenesis defect may contribute to several adverse pregnancy outcomes, such as infertility, miscarriage, intrauterine fetal distress/growth restriction, preeclampsia, and even intrauterine fetal death [1, 3]. In patients with idiopathic recurrent pregnancy losss (RPL), angiogenesis-related gene polymorphisms have been proposed as susceptibility factors that increase the risk of miscarriages compared with otherwise healthy women [4].
Vascular endothelial growth factor A (VEGFA) is a potent angiogenic factor and is a survival factor for endothelial cells during physiological and tumor angiogenesis, and functions in vasodilatation, vascular permeability and anti-apoptosis [5]. Establishment of pregnancy requires extensive angiogenesis both in the chorionic villi and during embryogenesis. VEGF and its receptors (VEGFR1/Flt-1, VEGFR2/KDR/Flk-1) play essential roles in oocyte maturation, embryo implantation, fetal development and placentation [6, 7]. Mice lacking the expression of VEGFA or either of the 2 receptors died in utero due to inadequate vascular formation [8, 9]. Aberrant placental VEGFA expression and vascular abnormalities may result in adverse pregnancy outcomes, including pregnancy loss, intrauterine fetal death, intrauterine fetal growth restriction and pre-eclampsia [10, 11].
Endocrine gland-derived vascular endothelial growth factor (EG-VEGF), also known as prokineticin 1 (PROK1), is a tissue-specific angiogenic factor. Its expression is restricted mainly to the steroidogenic glands, especially to the tissues of the ovary, testis, adrenal gland, and placenta, and it induces cell proliferation, migration, and fenestration in capillary endothelial cells [12]. EG-VEGF acts through the G-protein coupled receptors prokineticin receptor 1 (PROKR1) and prokineticin receptor 2 (PROKR2), which are involved in the regulation of male and female reproduction [13–16]. EG-VEGF was suggested to be involved in embryo implantation and third trimester parturition through regulating multiple inflammatory-related cytokines in the endometrium and myometrium [17, 18], and was regarded as a key endocrine factor in placental development [19].
In view of the importance of angiogenesis in human pregnancy and the fact that little information is available regarding the interaction between VEGFA and EG-VEGF systems in early gestation, a study was conducted to investigate the association of gene polymorphisms and gene-gene interaction among genes in the 2 major angiogenesis-related systems and idiopathic RPL. The second aim was to explore the possible complex pathways linking these 2 systems, using ingenuity pathways analysis (IPA).
Materials and methods
Subjects
The present study was approved by the Institutional Review Board of National Cheng Kung University Hospital, and informed consent was obtained from all patients and controls in this study. Ninety-eight women who had experienced at least 2 consecutive spontaneous abortions (SA) were recruited from outpatient clinics of our hospital. All women had conceived naturally without the aid of assisted reproductive technologies (ART). SA included both embryonic and anembryonic losses before 12 weeks of gestational age. Cases of biochemical pregnancy were excluded from the study. All subjects had undergone a comprehensive examination as described in our previous publications, including a detailed history, physical examination, chromosome analysis of peripheral blood lymphocytes, and trans-vaginal 3-dimensional ultrasound to detect uterine anomalies and endometrial defects. They also underwent the 75-g oral glucose tolerance test, and were checked for thyroid functions (T3, T4, and TSH), anti-cardiolipin antibodies (IgG, IgM and β-glycoprotein), lupus anticoagulant, anti-thrombin III, protein S, protein C, and endocrinology profiles on day 3 of the menstrual cycle [follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), and testosterone (T)] [20, 21]. Women with any identifiable cause of RPL, history of infertility, or gynecological disorders which might interfere with implantation or placentation were excluded from the study. We also recruited 142 women from our delivery room as control subjects. They had delivered at least one full-term healthy baby without the aid of ART and had not experienced miscarriage or pregnancy complications.
Genotyping
Five functional SNPs were selected from VEGFA and EG-VEGF gene systems (Table 1) based on our previous studies [20, 21]: VEGFA (−1154G/A, rs1570360), KDR (Q472H, rs1870377), EG-VEGF (V67I, rs7514102), PROKR1 (I379V, rs34715748) and PROKR2 (V331M, rs117106081). VEGFA (−1154G/A, rs1570360) and KDR (Q472H, rs1870377) were detected by primer extension analysis using end-point TaqMan assays (Applied Biosystems, Warrington, UK). EG-VEGF (V67I), PROKR1 (I379V) and PROKR2 (V331M) were detected by the Sanger sequence method [21].
Table 1.
Genes and variants analyzed in this study
| Gene name | Chromosome | Functional loci | Single nucleotide polymorphism (rs#) |
|---|---|---|---|
| Vascular Endothelial Growth factor A (VEGFA) | 6p12 | −1154 G > A | rs1570360 G/A |
| Kinase Insert Domain Receptor (KDR) | 4q11-q12 | Q472H | rs1870377 A/T |
| Prokineticin 1 (EG-VEGF) | 1p21 | V67I | rs7514102 G/A |
| Prokineticin Receptor 1 (PROKR1) | 2p13.1 | I379V | rs34715748 A/G |
| Prokineticin Receptor 2 (PROKR2) | 20p12.3 | V331M | rs117106081 G/A |
Statistical analysis
Comparisons of clinical information between the 2 groups (age, number of successful pregnancies) and the association with single markers between RPL patients and normal controls were performed using a χ2 test or Fisher’s exact test. A P value of <0.05 was considered statistically significant. In comparing genotype/allele frequencies between 2 groups, stringent statistics was introduced using the Bonferroni–Dunn method for multiple testing corrections and a P value of <0.01 was considered statistically significant. The relative risk of habitual abortion was estimated from logistic odds ratios (OR) with a 95 % confidence interval (CI) in multivariate analysis. Hardy-Weinberg equilibrium was calculated in accordance with standard procedures using χ2 analysis.
Individuals that carried more than one risk allele or genotype may have a higher risk of RPL; therefore, gene-gene interactions were explored. We analyzed gene-gene interactions among 5 polymorphisms using the multifactor dimensionality reduction (MDR) method (MDR software, version 2.0) [22], and a P value of less than 0.05 was considered statistically significant.
Ingenuity pathway analysis (IPA)
We also used the commercial software Ingenuity Pathway Analysis (Ingenuity® Systems; IPA) (http://www.ingenuity.com/) to identify enriched pathways and functional themes, as reported previously [23]. Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base (IPKB). The IPKB, containing a large network of curated molecular interactions and pathways, was searched to find the shortest paths enriched in genes of interest (VEGFA, KDR, PROK1, PROKR1, and PROKR2). Graphical representations of these sub-networks, containing direct and indirect molecular relationships, were generated.
Results
All the patients and controls were of Taiwanese Han descent and their ages at enrollment were, respectively, 30.95 ± 4.05 years (mean ± standard deviation [SD]) and 29.80 ± 4.49 years, which was not significantly different. The time interval between enrollment and previous miscarriage was less than 6 months. The number of previous pregnancy losses in the study group was 2.61 ± 0.99 (mean ± SD); 56 and 42 women, respectively, experienced 2 and >2 consecutive pregnancy losses. We selected one polymorphism each from the VEGFA, KDR, EG-VEGF, PROKR1 and PROKR2 genes to genotype in each subject (Table 1). The genotypic and allelic frequencies of VEGFA (−1154G > A), KDR (Q472H), EG-VEGF (V67I), PROKR1 (I379V) and PROKR2 (V331M) in the women with RPL and the control subjects are shown in Table 2. The genotypic frequency of KDR (Q472H) and PROKR2 (V331M) was significantly different between patients and controls using stringent statistical method for multiple testing (P < 0.01). The protective allele of PROKR2 (A allele) was significantly associated with RPL (P < 0.01).
Table 2.
Allele and genotype frequencies of 5 functional gene polymorphisms in RM patients and controls
| Location | Allele frequency | Genotype frequency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Allele | Case (n = 196) | Control (n = 284) | P value | Genotype | Case (n = 98) | Control (n = 142) | P value | Odds ratio (95%CI) | |
| VEGFA (−1154 G > A) rs1570360 G/A | G | 156 (79.6 %) | 235(82.7 %) | 0.382 | GG | 62 (63.3 %) | 100(70.4 %) | 0.245 | 1.38(0.80–2.39) |
| A | 40 (20.4 %) | 49 (17.3 %) | GA | 32 (32.7 %) | 35 (24.6 %) | ||||
| AA | 4 (4 %) | 7 (5 %) | |||||||
| KDR (Q472H) rs1870377 A/T | A | 103 (52.6 %) | 157 (55.3 %) | 0.555 | AA | 34 (34.7 %) | 39 (27.4 %) | 0.007 | 2.07(1.11–3.83) |
| T | 93 (47.4 %) | 127 (44.7 %) | AT | 35 (35.7 %) | 79 (55.6 %) | ||||
| TT | 29 (29.6 %) | 24 (16.9 %) | |||||||
| EG-VEGF (V67I) rs7514102 G/A | G | 109 (55.6 %) | 177 (62.3 %) | 0.141 | GG | 41 (41.8 %) | 72 (50.7 %) | 0.176 | 1.43(0.85–2.40) |
| A | 87 (44.4 %) | 107 (37.7 %) | GA | 27(27.6 %) | 33 (23.2 %) | ||||
| AA | 30(30.6 %) | 37 (26.1 %) | |||||||
| PROKR1 (I379V) rs34715748 A/G | A | 186 (94.9 %) | 256 (90.1 %) | 0.040 | AA | 88 (89.8 %) | 114 (80.3 %) | 0.047 | 0.46(0.21–1.00) |
| G | 10 (5.1 %) | 28 (9.9 %) | AG | 10 (10.2 %) | 28 (19.7 %) | ||||
| GG | 0 (0 %) | 0 (0 %) | |||||||
| PROKR2 (V331M) rs117106081 G/A | G | 196 (100 %) | 273(96.1 %) | 0.005 | GG | 98 (100 %) | 132 (93 %) | 0.007 | NA |
| A | 0 (0 %) | 11 (3.9 %) | GA | 0 (0 %) | 9 (6.3 %) | ||||
| AA | 0 (0 %) | 1 (0.7 %) | |||||||
Statistical significance P values were corrected for multiple testing by Bonferroni–Dunn method, and a P value of <0.01 is considered statistically significant. Significant P values were shown in boldface
Gene-gene interactions were analyzed among 5 polymorphisms of these 5 genes using the MDR method. The KDR (Q472H) polymorphism was regarded as the best fit model, with an accuracy of 60.0 %, to be associated with RPL (P = 0.02), and a maximum cross-validation (CV) consistency of 10 out of 10 (Table 3). KDR (Q472H) and PROKR2 (V331M) were shown to be the best 2-locus models with an accuracy of 53.4 %; however, gene-gene interactions were not significant in these 2 loci (P = 0.24, Table 3).
Table 3.
Summary of mutifactorical dimension reduction (MDR) analysis
| Best model | Testing results | 10-fold cross-validation | |||
|---|---|---|---|---|---|
| Accuracy (%) | P-valuea | Consistency | P-valuea | ||
| 1. VEGFA(−1154G/A) | 2 | 60.0 | 0.02 | 10/10 | 0.74 |
| 2. KDR(Q472H) | 2, 5 | 53.4 | 0.24 | 7/10 | 0.95 |
| 3. EG-VEGF(V67I) | 1, 2, 3 | 48.5 | 0.61 | 8/10 | 0.91 |
| 4. PROKR1(I379V) | 1, 2, 3, 4 | 49.1 | 0.60 | 10/10 | 0.74 |
| 5. PROKR2(V331M) | – | – | – | – | – |
aEmpirical P-values were calculated by permutation analysis, and significant P values were shown in boldface
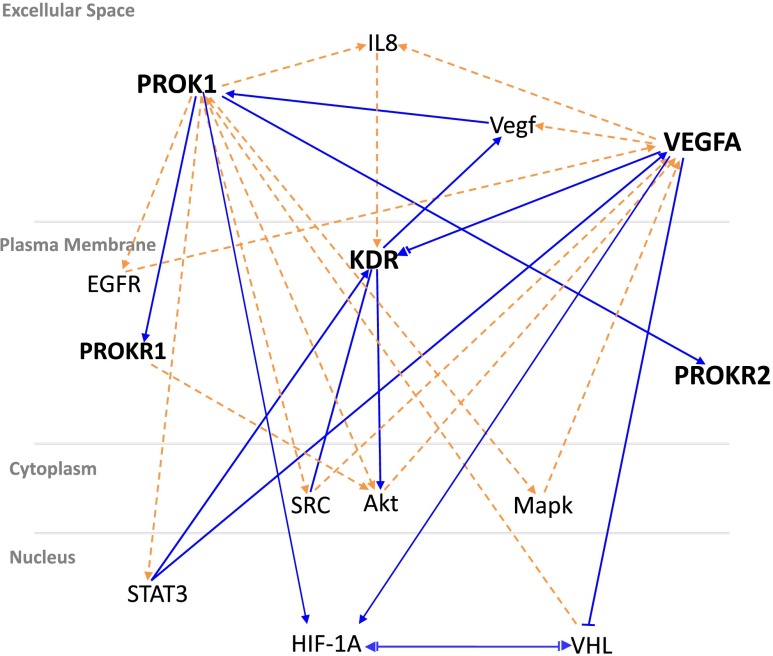
IPA analysis showed EG-VEGF and VEGFA systems shared several enriched pathways, and gene-gene interactions were generated with functional themes, such as Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3 (Fig. 1). Most IPA-identified “hubs” were canonical signaling pathways that directly or indirectly regulated cell growth, proliferation, apoptosis, migration, cell adhesion, and immune and inflammation responses in both the VEGFA and EG-VEGF systems.
Fig. 1.
VEGFA and prokineticin systems theme analysis. Genes/proteins are illustrated as nodes and molecular relationships as connecting lines between 2 nodes (direct relationships as normal lines; indirect relationships as dashed lines). Molecular relationships are supported by at least 1 literature reference, or by canonical information stored in the Ingenuity Pathways Knowledge Base (IPKB). Black-bold nodes represent genes of interest (VEGFA and EG-VEGF systems), while black non-bold nodes represent hubs that were added by the IPA algorithm to connect a set of genes of interest. Each gene/protein was categorized in the network according to its subcellular localization
Discussion
In the present study, we investigated the genetic association between ligands and receptor gene polymorphisms of EG-VEGF and VEGFA systems and the occurrence of idiopathic recurrent pregnancy loss (RPL). We demonstrated that PROKR2 (V331M) and KDR (Q472H) were significantly associated with idiopathic RPL, and KDR (Q472H) was the best locus to confer susceptibility in the study. Although PROKR2 (V331M) and KDR (Q472H) failed to show gene-gene interactions by statistical analysis, IPA revealed several shared signaling pathways (Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3) existed among 5 genes in EG-VEGF and VEGFA systems, suggesting their functional interactions may occur through these canonical hubs and pathways.
Although EG-VEGF is not structurally related to the VEGF family, the biological activities of the 2 molecules are indistinguishable. These 2 major angiogenesis systems were both regulated by oxygen tension with a hypoxia-inducible factor-1alpha (HIF-1α)-dependent mechanism [12, 24], and were also similarly hormonally regulated by estrogen, progesterone and human chorionic gonadotropin [25]. Both VEGFA/VEGFR and PROK1/PROKRs were expressed in the human placenta, but their expression patterns were temporally and spatially different in the feto-maternal interface [16, 26]. The EG-VEGF expression increased gradually in early pregnancy, reaching peaks at 8–10 weeks of gestation, but there was no significant change in the VEGFA level during the first trimester [16]. In human full-term gestation, VEGFA/VEGFR expressions were highest in the maternal side of the interface, and PROK1/PROKRs were highest in the fetal side of the placenta [26]. It was therefore suspected that the 2 systems play complementary roles during the first trimester; whereas in later gestation, the PROK1/PROKR system specifically mediates angiogenesis in the fetus, and the VEGFA/VEGFR system predominantly mediates maternal angiogenesis.
All 5 genetic variants investigated in the present study are biologically functional SNPs, and 4 of them are common polymorphisms in the general population. The common functional polymorphism of VEGFA (−1154G > A) in the promoter region have been reported to affect VEGF activity and expression [27], and was associated with an increased risk of RPL and recurrent implantation failure [28, 29]. KDR exhibits a strong tyrosine kinase activity towards pro-angiogenic signals, and the variant at residue 472H > Q was demonstrated to decrease VEGF binding efficiency and was believed to alter their downstream signaling pathways [30]. Several KDR polymorphisms are reported to be associated with RPL, but genetic susceptibility varies in different ethnicity [20, 31]. We speculated that these variations may result in changing the efficiency of KDR on VEGF binding and interfere with pregnancy establishment/maintenance. The biological interaction of EG-VEGF and PROKR1/2 is a ligand-receptor relationship. The effects of amino acid changes in EG-VEGF and its receptors are not quite clear, and their genetic polymorphisms are not reported except our preceding studies [21, 32]. In our previous study, tag SNPs of PROKR1 and PROKR2 were associated with RPL, and EG-VEGF was a modifier gene [32]. We further demonstrated 2 specific variants of receptor genes, PROKR1 (I379V) and PROKR2 (V331M), may play as protective genotypes of human early pregnancy through altering trophoblast calcium influx and facilitate cell invasive ability [21]. We also found the gene expressions of V67I in RNA and protein levels are significantly lower compared with its wild-type (data not shown). Since all these 5 genetic variants in 2 major angiogenesis systems have functional effects on gene expression or/and cell behaviors, the biological impact of gene-gene interaction is difficult to investigate either in vitro or in vivo studies. Therefore, to evaluate the clinical data using in silico method is a good solution to answer the question of genetic interaction in complex disease.
RPL is a complex disease, and a woman carries different genetic polymorphisms will confer variable susceptibility to recurrent abortions. To characterize the genetic background of each individual may help to understand the etiology of RPL and to design diagnostic and treatment program. IPA is a powerful tool to explore gene-gene/protein interactions, and it provides comprehensive pathways and network analysis of complex biological data by integrating web-based information from a variety of experimental platforms. In the study, we demonstrated 8 molecules or genes (Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3) that directly or indirectly linked the EG-VEGF/PROKRs and VEGFA/KDR systems. Among these genes or molecules, the AKT, EGFR and MAPK pathways were common canonical signaling pathways in both the EG-VEGF and VEGFA systems, and were involved in regulating cell growth, proliferation, apoptosis, migration and cell adhesion in a variety of pathophysiological conditions, including tumor angiogenesis, polycystic ovarian syndrome (PCOS), and preeclampsia [18, 33, 34]. IL-8 and SRC provided evidence of the role of EG-VEGF and VEGFA in the inflammatory and immune response in the decidua and placenta, and that EG-VEGF and VEGFA may be involved in embryo implantation, term or preterm labor and preeclampsia [17, 18, 35, 36]. SRC and STAT3 play a role as transcriptional factors or complexes that mediate gene transcription and activation, and were related to tumor growth, tumor angiogenesis, embryo implantation and antiphospholipid syndrome [17, 36–39]. HIF-1α activated transcription of VEGFA and EG-VEGF gene expression in a variety of developmental and physiological processes. VHL (von Hippel-Lindau) is a tumor suppressor gene, and is acquired for HIF-1alpha ubiquitination and degradation under normoxic conditions [37, 39, 40].
There are some limitations in this study. First, the sample size was relatively small, and thereby attenuated the power of the statistical significance. The MDR method was therefore introduced to identify gene–gene interactions that are associated with idiopathic RPL. The MDR method reduces high-dimensional genetic data into a single dimension, thus permitting gene-gene or gene-environmental interactions to be detected in relatively small sample sizes. Second, the IPA-identified hubs shared by the 2 angiogenesis systems were not validated in this study. Although these shared molecules were identified from evidence of complex biological data, the exact interactions between the 2 systems are not clear and warrant further study.
The individual functional relevance of the EG-VEGF and VEGFA systems in human early pregnancy is already known. The co-existent expressions of the 2 angiogenesis systems in reproductive tissues (placenta, endometrium, myometrium and embryo) further suggest their functional interactions. In the present study, although gene-gene interactions were not shown to be significant between the 2 major angiogenesis systems using statistical methods, their possible functional interactions were demonstrated by a literature search updated with the IPA database. Eight molecules or genes (Akt, IL-8, EGFR, MAPK, SRC, VHL, HIF-1A and STAT3) were shown to directly or indirectly link the EG-VEGF/PROKRs and VEGFA/KDR systems in the shortest paths. In conclusion, KDR (Q472H) and PROKR2 (V331M) gene polymorphisms are associated with idiopathic RPL; VEGFA and EG-VEGF gene systems may jointly contribute to idiopathic RPL through several shared canonical pathways. Our study supports the roles of the VEGFA/KDR and EG-VEGF/PROKR systems in human early pregnancy and may provide genetic information of RPL for future personalized medicine.
Acknowledgement
We thank Miss Shang-Chi Lee, Department of Research Center of Clinical Medicine, National Cheng-Kung University Hospital for her statistics support.
Financial disclosures
This study was supported by grants from the National Science Council of the Republic of China (NSC-98-2314-B-006-040, NSC-101-2314-B-006-039-MY3) and an intramural grant of National Cheng-Kung University Hospital (NCKH-9803017).
Authors’ roles
Study design, execution and manuscript drafting: Mei-Tsz Su
Statistical analysis: Sheng-Hsiang Lin and Yi-Chi Chen
Patient collection: Mei-Tsz Su and Pao-Lin Kuo
Critical discussion and correspondence: Pao-Lin Kuo
Footnotes
Capsule Gene polymorphisms of VEGFA and EG-VEGF systems are associated with idiopathic recurrent pregnancy loss and they may jointly contribute to miscarriages through several shared canonical pathways.
References
- 1.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, et al. Uteroplacental vascular development and placental function: an update. Int J Dev Biol. 2010;54:355–366. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- 2.Schiessl B, Innes BA, Bulmer JN, Otun HA, Chadwick TJ, Robson SC, et al. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta. 2009;30:79–87. doi: 10.1016/j.placenta.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. 2010;36:309–320. doi: 10.1055/s-0030-1253453. [DOI] [PubMed] [Google Scholar]
- 4.Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:803–812. doi: 10.1093/humupd/dmr027. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Zygmuni M, Herr P, Munstedt K, Lang U, Liung OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110:S10–S18. doi: 10.1016/S0301-2115(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 7.Jackson MR, Carney BW, Lye SJ, Ritchie JW. Localization of two angiogenic growth factors (PDECGF and VEGF) in human placenta throughout gestation. Placenta. 1994;15:341–353. doi: 10.1016/0143-4004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 9.Hiratsuka S, Kataoka Y, Nakao K, Nakamura K, Morikawa S, Tanaka S, et al. VEGF-A is involved in guidance of VEGF-receptor positive cells to the anterior portion of early embryos. Mol Cell Biol. 2005;25:355–363. doi: 10.1128/MCB.25.1.355-363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuorela P, Carpen O, Tulppala M, Halmesmaki E. VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol Hum Reprod. 2000;6:276–282. doi: 10.1093/molehr/6.3.276. [DOI] [PubMed] [Google Scholar]
- 11.Semczuk M, Borczynska A, Bialas M, Rozwadowska N, Semczuk-Sikora A, Malcher A, et al. Expression of genes coding for proangiogenic factors and their receptors in human placenta complicated by preeclampsia and intrauterine growth restriction. Reprod Biol Endocrinol. 2013;13:133–138. doi: 10.1016/j.repbio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature Reviews Cancer. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 13.LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, et al. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci U S A. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquali D, Rossi V, Staibano S, De Rosa G, Chieffi P, Prezioso D, et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology. 2006;147:4245–4251. doi: 10.1210/en.2006-0614. [DOI] [PubMed] [Google Scholar]
- 15.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, et al. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 17.Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorowiec MR, Catalano RD, Norman JE, Denison FC, Jabbour HN. Prokineticin 1 induces inflammatory response in human myometrium: a potential role in initiating term and preterm parturition. Am J Pathol. 2011;179:2709–2719. doi: 10.1016/j.ajpath.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouillet S, Hoffmann P, Feige JJ, Alfaidy N. EG-VEGF: a key endocrine factor in placental development. Trends Endocrinol Metab. 2012;23:501–508. doi: 10.1016/j.tem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Su MT, Lin SH, Lee IW, Chen YC, Kuo PL. Association of polymorphisms/haplotypes of the genes encoding vascular endothelial growth factor and its KDR receptor with recurrent pregnancy loss. Hum Reprod. 2011;26:758–764. doi: 10.1093/humrep/deq401. [DOI] [PubMed] [Google Scholar]
- 21.Su MT, Lin SH, Chen YH, Wu LW, Kuo PL. Prokineticin Receptor Variants (PKR1-I379V and PKR2-V331M) Are Protective Genotypes in Human Early Pregnancy. Reproduction. 2013;146:63–73. doi: 10.1530/REP-13-0043. [DOI] [PubMed] [Google Scholar]
- 22.Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- 23.Prassas I, Karagiannis GS, Batruch I, Dimitromanolakis A, Datti A, Diamandis EP. Digitoxin- induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol Cancer Ther. 2011;10:2083–2093. doi: 10.1158/1535-7163.MCT-11-0421. [DOI] [PubMed] [Google Scholar]
- 24.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature Reviews Cancer. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado-Pérez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matjila M, Millar R, van der Spuy Z, Katz A. The differential expression of Kiss1, MMP9 and angiogenic regulators across the feto-maternal interface of healthy human pregnancies: implications for trophoblast invasion and vessel development. PLoS One. 2013;8:e63574. doi: 10.1371/journal.pone.0063574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi M, Bazrafshani MR, Day PJ, Ollier WE. Vascular endothelial growth factor production is regulated by gene polymorphisms. Iran J Immunol. 2009;6:119–129. [PubMed] [Google Scholar]
- 28.Goodman C, Jeyendran RS, Coulam CB. Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod Biomed Online. 2008;16:720–723. doi: 10.1016/S1472-6483(10)60487-7. [DOI] [PubMed] [Google Scholar]
- 29.Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med. 2009;22:371–378. doi: 10.1080/14767050802645035. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rah H, Jeon YJ, Lee BE, Choi DH, Yoon TK, Lee WS, et al. Association of kinase insert domain-containing receptor (KDR) gene polymorphisms with idiopathic recurrent spontaneous abortion in Korean women. Fertil Steril. 2013;9:753–760.e8. doi: 10.1016/j.fertnstert.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Su MT, Lin SH, Lee IW, Chen YC, Hsu CC, Pan HA, et al. Polymorphisms of endocrine gland-derived vascular endothelial growth factor gene and its receptor genes are associated with recurrent pregnancy loss. Hum Reprod. 2010;25:2923–2930. doi: 10.1093/humrep/deq256. [DOI] [PubMed] [Google Scholar]
- 33.Ngan ES, Tam PK. Prokineticin-signaling pathway. Int J Biochem Cell Biol. 2008;40:1679–1684. doi: 10.1016/j.biocel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Denison FC, Battersby S, King AE, Szuber M, Jabbour HN. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology. 2008;149:3470–3477. doi: 10.1210/en.2007-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai A, Sado T, Naruse K, Noguchi T, Haruta S, Yoshida S, et al. Antiangiogenic-induced hypertension: the molecular basis of signaling network. Gynecol Obstet Invest. 2012;73:89–98. doi: 10.1159/000334458. [DOI] [PubMed] [Google Scholar]
- 36.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 37.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 38.Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6:e25799. doi: 10.1371/journal.pone.0025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Ippolito S, Marana R, Di Nicuolo F, Castellani R, Veglia M, Stinson J, et al. Effect of Low Molecular Weight Heparins (LMWHs) on antiphospholipid Antibodies (aPL)-mediated inhibition of endometrial angiogenesis. PLoS One. 2012;7:e29660. doi: 10.1371/journal.pone.0029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu X, Zhuang G, Yu L, Meng G, Ferrara N. Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b + Gr1+ cells: key role of Stat3 signaling. J Biol Chem. 2012;287:19574–19584. doi: 10.1074/jbc.M111.326801. [DOI] [PMC free article] [PubMed] [Google Scholar]