Abstract
Purpose
To test the effects of varying vitrification protocols on the cell cycle status and chromosomal integrity in cumulus-enclosed GV stage rat oocytes.
Methods
Vitrified and thawed rat oocytes were labeled with fluorescent markers for chromatin, cell cycle activation, and f-actin and analyzed by conventional and laser scanning confocal microscopy.
Results
In all vitrification groups, significant alterations in cumulus cell connectivity, cell cycle status, and cytoplasmic actin integrity were observed following warming compared to fresh control oocytes. Based on the protein phosphorylation marker MPM-2, it is clear that warmed oocytes rapidly enter M-phase but are unable to maintain chromosome integrity as a result of multiple chromatin fusions. A prominent reduction in f-actin is evident in both the ooplasm and at the cortex of vitrified oocytes. Finally, an irreversible but irregular retraction of TZPs occurs on the majority of oocytes subjected to any of the vitrification protocols.
Conclusions
These findings draw attention to undesirable consequences of immature oocyte vitrification that compromise cell cycle status and chromatin and cytoskeleton integrity that may not be evident until after fertilization.
Keywords: Vitrification, Oocyte cryopreservation, Fertility preservation, F-actin, Immature oocytes, Chromatin patterns, GV stage oocytes, MPM-2, Confocal microscopy
Introduction
Strategies for cryostorage of human gametes, embryos, and gonadal tissues have found widespread adoption and utility in the field of human ARTs. In addition, the particular case of fertility preservation has added both impetus and direction for expanding the capabilities of cryostorage especially for patients undergoing gametotoxic procedures associated with cancer treatments or bone marrow transplantation. Aggressive cancer chemotherapy frequently causes premature ovarian failure and infertility in premenopausal women due to depletion of follicular reserves [1]. Infertility and iatrogenic menopause were both previously viewed by physicians and patients as an acceptable cost of curative chemotherapy and radiation regimens. However, as more women survive their cancer treatments at earlier ages, presenting options to patients that would ensure fecundity has become a major goal in the field of fertility preservation.
Whereas embryo cryopreservation remains a well-established clinical strategy to preserve fertility in females, certain limitations preclude clinical application since it requires sperm from a spouse or a donor and may impose delays in cancer treatment for more than 2 weeks. This has prompted recent efforts in the application of cryobiological strategies in order to achieve long term storage of oocytes or ovarian tissue for women lacking a male partner or uncomfortable with the practice of embryo storage. For young women, immature oocyte cryopreservation offers an attractive and much-needed alternative.
While there is a growing need to develop oocyte cryopreservation protocols that could be performed on oocytes at different stages of development, the use of mature metaphase-2 (MII) oocytes has largely dominated the clinical landscape. Mature oocyte cryopreservation using either slow freeze or vitrification technologies has been successfully applied. In the case of vitrification there is a growing indication that both the efficiency and safety are superior to slow freeze methods [2]. Vitrification of mature oocytes is very successful and no longer considered experimental [3]. However, this approach imposes additional risks, especially for patients bearing hormone dependent malignancies, as it necessitates controlled ovarian hyperstimulation (COS) and follicle aspiration resulting in delayed cancer treatment [1].
An alternative strategy under current investigation is the cryopreservation of immature germinal vesicle (GV) stage oocytes to circumvent the need for ovarian stimulation. Despite recognition of the problems imposed by cryopreservation on mature MII oocytes [4, 5], there is much less known about the effects of immature oocyte cryopreservation on subsequent maturation and developmental competencies. Studies indicating diminished maturation and defective developmental potential for frozen-thawed immature oocytes have appeared [6]. More recently, Wu et al. have shown that GV oocyte vulnerability to cryoinjury parallels mature MII oocytes with respect to fertilization capacity [7]. Concerns regarding the feasibility of cryopreserving GV oocytes were raised by the initial studies from Van Blerkom and colleagues [8]. Among the adverse effects observed were premature chromosome condensation, externalization of chromatin fragments into the cytoplasm, defects in microtubule and mitochondria organization, and alterations in protein synthesis [8].
With increases in successful utilization of mature oocytes and recognition of difficulties of cryopreservation of immature oocytes, the interest in research for cryopreservation of oocytes at GV stage waned. When considering successful achievements of mature oocyte cryopreservation in recent years, especially with vitrification, the study of immature oocyte cryopreservation should not be discouraged. To determine the value of clinical utility of this approach, systematic studies controlling for protocol variations for vitrification must be undertaken to achieve immature oocyte cryopreservation.
Thus, the aim of this study was to analyze the integrity of GV stage rat oocytes with respect to cytoskeletal and cell cycle integrity that have not been previously explored. We also compared the effects of vitrification by three different approaches including a novel four-step modification.
Materials and methods
-
Collection and vitrification of rat GV-stage cumulus-enclosed oocytes
Oocytes were obtained from 4 to 6 week old Sprague–Dawley rats by follicle puncture of ovaries 48 h after priming with pregnant mare serum gonadotropin (Sigma Chemical Co., PMSG 10 IU, ip). Animal handling protocols were reviewed and approved by the IACUC at Kansas University Medical Center. Forty-eight hours after PMSG injection, ovaries were removed, and antral follicles were punctured with a 19 g needle in Hepes-buffered Leibowitz L15 medium maintained at 37 °C on a heated stage of a Nikon stereomicroscope. GV oocyte-cumulus-complexes were pooled from 12 ovaries and maintained in the same medium at 37 °C until vitrification.
The study design is aimed at comparing 3 vitrification protocols with freshly prepared controls that were maintained at 37 °C in a standard culture (37 °C/5 %CO2 in KSOM) medium lacking meiosis arresting agents. Thus, for each experiment, pooled oocytes were divided into four groups: (a) fresh control, (b) VP1; vitrification in 20 % ethylene glycol (EG) + 20 % dimethyl sulfoxide (DMSO) + 0.5 M sucrose + 10 % human serum albumin (HSA) after 4 step preparation, (c) VP2; vitrification in 40 % EG + 0.5 M sucrose + 10 % HSA after 2 step preparation, (d) VP3; vitrification in 20 % EG + 20 % DMSO + 0.5 M sucrose + 10 % HSA after 2 step preparation. The experiment was repeated three times with media and vitrification solutions freshly prepared the day before the experiment.
-
Vitrification
GV-stage cumulus-enclosed oocytes were vitrified on copper EM grids (E.F. Fullam, Inc.), under the following conditions (Table 1):-
(i)VP1 (4-step equilibration):Cumulus enclosed GV oocytes were immersed in a solution containing 1) 2.5 % EG + 2.5 % DMSO + 10 % HSA for 5 min, 2) then transferred to a solution of 7.5 % EG + 7.5 % DMSO + 10 % HSA for 2 min, 3) followed by to a solution of 15 % EG + 15 % DMSO + 10 % HSA for 20 s, 4) and equilibrated in a vitrification solution containing 20 % EG + 20 % DMSO + 10 % HSA + 0.5 M sucrose for 20 s before plunge into liquid nitrogen in a 1.8 ml cryovial.
-
(ii)VP2 (2-step equilibration)Pre-equilibrated in a solution of 15 % EG and 10 % HSA for 5 min and transferred to a vitrification solution containing 40 % EG + 10 % HSA + 0.5 M sucrose for equilibration for 20 s.
-
(iii)VP3 (2 step equilibration)Pre-equilibrated in a solution containing 7.5 % EG + 7.5 % DMSO + 10 % HSA for 5 min followed by equilibration in a vitrification solution of 20 % EG + 20 % DMSO + 10 % HSA + 0.5 M sucrose for 20 s
-
(i)
-
Warming and washing steps
Vitrified cumulus oocyte complexes (COCs) were warmed at 37 °C, and washed using our 4 step warming protocol at room temperature in the following fashion. Cryovials were removed from liquid nitrogen, the cap was opened at room temperature for 20 s, and vials were filled with the first warming solution (WS1) which had been incubated at 37 °C. After 3 min in WS1, oocytes on the EM grids were transferred to the WS2, WS3, and WS4 solution for 3 min each. Then oocytes were washed two more times in L15 medium for 2 min each (WS1: 2.5 % EG + 1.0 M sucrose + 10 % HSA + 1 % Supercool X1000 in L15 medium, WS2: 1.0 M sucrose + 10 % HSA, WS3: 0.5 M sucrose + 10 % HSA, WS4: 0.25 M sucrose + 10 % HSA).
Table 1.
Vitrification parameters
| Vitrification protocol | Number of steps | CPA | |||
|---|---|---|---|---|---|
| Step | EG (%) | DMSO (%) | Exposure | ||
| VPI | 4 | 1 | 2.5 | 2.5 | 5 min |
| 2 | 7.5 | 7.5 | 2 min | ||
| 3 | 15 | 15 | 20 s | ||
| 4 | 20 | 20 | 20 s | ||
| VPII | 2 | 1 | 15 | – | 5 min |
| 2 | 40 | – | 20 s | ||
| VPIII | 2 | 1 | 7.5 | 7.5 | 5 min |
| 2 | 20 | 20 | 20 s | ||
Assessment of oocyte viability
Warmed oocytes (170 in each study group) were examined under the microscope and non-viable oocytes were identified by morphology (dark, pycnotic or irregular shape). Vital staining with calcein and propidium iodide was performed to confirm viability. In addition, cumulus intact oocytes were enumerated The COCs were classified into three categories; complete (when more than 75 % cumulus cells were maintained), partial (less than 75 %) and no cumulus.
-
4)
Assessment of chromatin configuration, phosphoproteins, and cytoskeletons in oocytes and granulosa cells after vitrification and warming
Viable fresh and vitrified-warmed immature oocytes were incubated for 1 h at 37 °C/5 % CO2 in KSOM prior to fixation. Oocytes were fixed at 37 °C for 5 min in 3 % paraformaldehyde then 30 min at 37 °C in a microtubule stabilizing buffer extraction fixative (MTSB XF) (100 mM PIPES, 5 mM MgCl2, 2.5 mM EGTA, 2 % formaldehyde, 0.1 % Triton-X-100, 1 mM taxol, 10 U/mL aprotinin, 50 % deuterium oxide) with phosphatase inhibitors (40 uM phenylarsine oxide, 100 uM NaVO4, and 50 ng/ml Calyculin A) [9]. All COCs were labeled with M-phase specific antibody (MPM-2) to establish meiosis re-entry kinetics for thawed COCs based on the transition from nuclear to cytoplasmic protein phosphorylation, and counterstained with Hoechst 33258 (1 ug/ml). All COCs were labeled with 1 U/ml Alexafluor 568 phalloidin (Invitrogen) to monitor alterations in f-actin and triple labeled samples were imaged as described previously [9]. 3D data sets were collected on a Zeiss LSM 510 confocal microscope using excitation and emission settings such that chromatin configuration (Hoechst 33258) could be directly compared to cell cycle status (MPM-2) and filamentous-actin (F-actin, Alexafluor 548-phalloidin) of individual oocytes.
Conventional terminology has been used to classify the various chromatin configurations observed in this study as related to SN (surrounded) or NSN (non-surrounded) GVs, initial states of chromosome condensation typical of diakinesis to prometaphase of meiosis-1 (CHR), or a central chromatin mass (CCM) as illustrated in Fig. 1.
MPM-2 labeling was evaluated by determining the percentage of oocytes in each experimental group that displayed either cytoplasmic or nuclear labeling as the transition from a GV or G2 cell cycle state to that of M-phase of the cell cycle has been shown to be indicative of CDK1 activation [10, 11]
-
5)
Statistical analysis
Fisher’s Exact Test was used to analyze the differences in survival, cumulus intact oocytes, and MPM2 labeling between groups (fresh control, VP1, VP2, VP3). Proportions of chromatin patterns between each group were compared with a Chi-square analysis. Differences were considered significant if p < 0.05.
Fig. 1.
Hoechst 33258 labeling chromatin patterns in immature rat oocytes. Full projections of z-stacks from representative oocytes in each category are represented depicting NSN1 (a), SN2 (b), CHR3 (c), and CCM4 (d). All images magnified 100× from projections that reveal the total chromatin content of control samples. 1NSN—Non-Surrounded Nucleolus, 2SN—Surrounded Nucleolus, 3CHR—Chromosomes, 4CCM—Central Chromatin Mass
Results
-
Evaluation of survival rates and cumulus integrity in thawed GV oocytes:
Survival rates were slightly higher in the VP1 (85 %) group after warming in comparison to VP2 (73.8 %) and VP3 (65.3 %) with a modest statistical significance of p < 0.05 indicating that overall viability was similarly compromised relative to fresh control samples (p < 0.001). In control groups incubated for the same duration as treated COCs, greater than 88 % of COCs retained a complete or partial cumulus complex. In contrast, each of the vitrified groups displayed a loss of cumulus integrity (VP1 23 %, VP2 32 %, and VP3 24 %). These results show that while the majority of vitrified samples retain viability, each of the treatment groups negatively influenced cumulus integrity.
-
Assessment of chromatin integrity:
Variations in the patterns of chromatin organization are shown in Fig. 1. When oocytes were evaluated for the pattern of chromosome organization, more than 74 % of control oocytes (105/142) had retained GVs displaying either SN or NSN configurations (Table 2). Vitrified oocytes, on the other hand, generally retained GVs in 54 % (VP1), 38 % (VP2), and 44 % (VP3) and showed a corresponding increase in CCM and CHR (46 % VP1, 62 % VP2, 56 % VP3) categories relative to controls (26 %) (Table 2). It is important to note that the majority of oocytes in the vitrified samples showed a marked tendency to undergo abnormal condensation into CCM rather than chromosome formation as would be expected if cell cycle entry followed a normal pathway. This finding is consistent with a G2 to M cell cycle transition but suggests that conditions of vitrification impair the normal progression of nuclear maturation.
-
Assessment of meiotic resumption by MPM-2 labeling
The transition of G2 to M of the cell cycle is known to involve the emergence of phosphoproteins in the cytoplasm as an indication of CDK1 activation [11]. Using MPM-2 staining as an indicator of this transition confirmed that the majority of control oocytes (76 %) retained low levels of labeling within the nucleus even after the additional 1 h of incubation at 37 °C prior to sample fixation (Table 3). In contrast, there was a 2–3 fold increase in the percentage of vitrified oocytes that displayed clear signs of cytoplasmic phosphorylation, indicating that these oocytes had initiated a cell cycle transition from G2 to M phases. Interestingly, the VP2 group showed the greatest degree of cell cycle activation relative to the other groups consistent with the higher degree of chromatin changes in the CCM category. Together these results indicate that, while vitrified oocytes all initiate precocious cell activation relative to non-vitrified controls under the same conditions, the VP2 group is particularly influenced.
-
Confocal analysis of cytoskeleton.
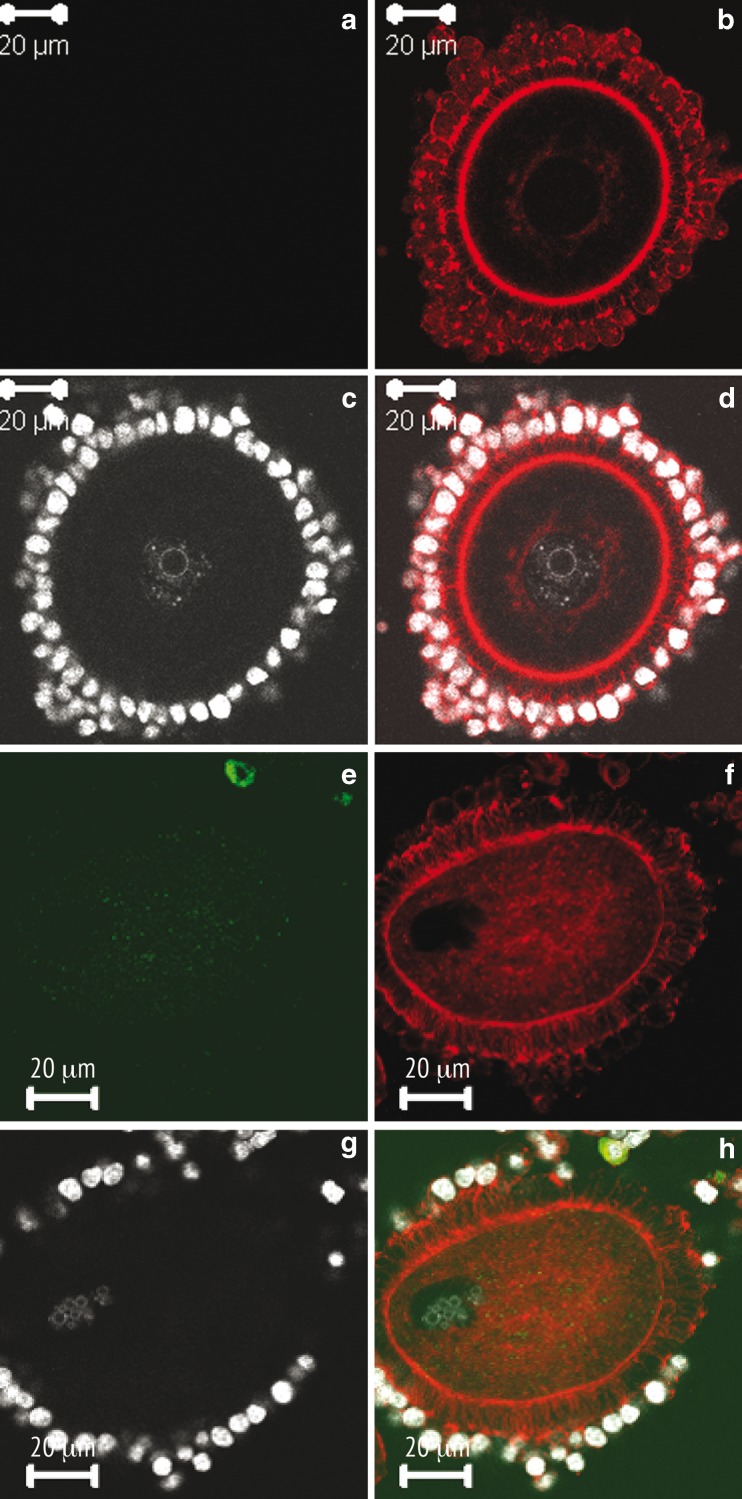
We next explored the impact of vitrification on the actin cytoskeleton of immature rat oocytes using Alexafluor 568 phalloidin as a probe for filamentous actin. The results of our analysis confirm previous studies in identifying f-actin as a major constituent of the oocyte cortex as well as the transzonal projections that connect the cumulus cells to the oolemma (Fig. 2b and d). What was novel, however, was the presence of a distinct perinuclear ring of f-actin in >90 % of the GV stage oocytes that we examined by confocal microscopy. While our ability to detect this network of actin surrounding the GV was to some extent aided by the optical sectioning capability of laser scanning microscopy, we feel that the conditions of fixation, specifically as they apply to the use of phosphatase inhibitors, may have rendered a greater degree of stability to this component of the cytoskeleton. For this reason, it is apparent that vitrification exerts dramatic effects on the organization of f-actin (Fig. 2e–h).
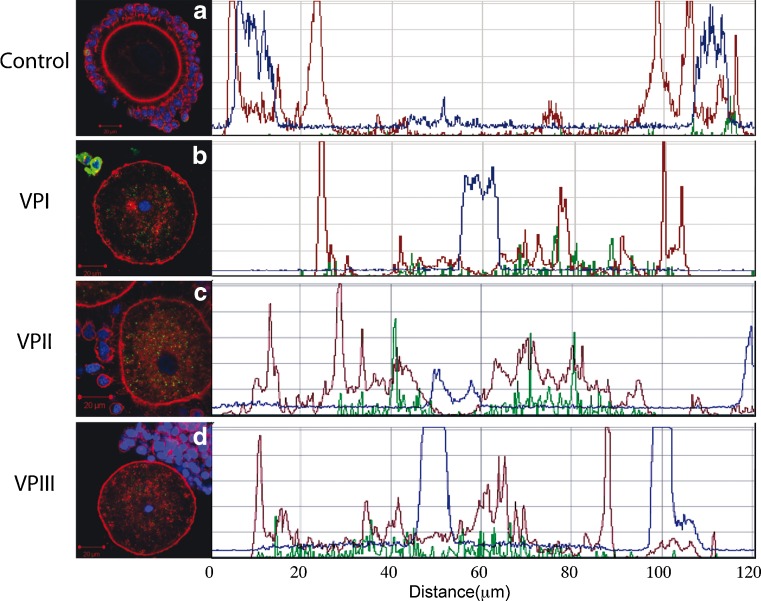
Specifically, the majority of GV stage oocytes that were subjected to vitrification exhibited three main differences relative to control oocytes. First, the perinuclear ring of f-actin disappeared. This is shown quantitatively by measurements that were performed on single confocal images of control and VP1, VP2, or VP3 oocytes as shown in Fig. 3. A reduction of the f-actin signal at the surface of the GV was noticed in all vitrified oocytes examined . Second, while low cytoplasmic signals are observed in control oocytes in which the predominant signal is confined to the outermost cortex (Figs. 2b and 3a), a substantial increase in cytoplasmic signal is typically apparent in oocytes subjected to vitrification (Figs. 2f and 3b–d). Interestingly, in vitrified oocytes there appears to be a corresponding reduction in the cortical signal. The final effect observed is on the cumulus cells and their TZPs. In controls, cumulus cells display a prominent focal pattern of staining that represents the numerous cell junctions that exist between cumulus cells and the oocyte (Fig. 2b). Numerous actin-filled TZPs are also evident throughout the zona pellucida encircling the oocyte with prominent focal adhesions evident at the surface of the zona. While somewhat variable in the vitrified samples, there was a general decrease in the density of TZPs in all groups examined and some oocytes retained clusters of TZPs (Fig. 3c) or exhibited almost total loss of TZPs (Fig. 3b). The reasons for this variability in the integrity of the cumulus are not clear and not simply assignable to a given vitrification protocol. However, it is noteworthy that in all vitrified samples there was a clear loss of f-actin within the cumulus cells showing a direct and consistent effect of cytoskeletal stability in the somatic cells of the rat COC.
Table 2.
Effect of vitrification on chromatin configuration
| Fresh | VP1 | VP2 | VP3 | |||||
|---|---|---|---|---|---|---|---|---|
| SN | 58 | (41 %)a | 53 | (34 %)b,c | 42 | (30 %)b,d | 42 | (32 %)b,c |
| NSN | 47 | (33 %)a | 32 | (20 %)b.c | 11 | (8 %)b,d | 15 | (12 %)b,c |
| Chromosomes | 14 | (10 %)a | 15 | (10 %)b,c | 23 | (16 %)b,d | 23 | (18 %)b,d |
| CCM | 23 | (16 %)a | 57 | (36 %)b,c | 64 | (46 %)b,d | 50 | (38 %)b,c |
| Total | 142 | (100 %) | 157 | (100 %) | 140 | (100 %) | 130 | (100 %) |
*different letters represent significant differences between groups (p < 0.05)
a,b(between fresh and treatment groups)
c, d(between VP1 and other treatment groups)
Table 3.
Effect of vitrification on cell cycle phosphorylation status
| MPM2 | Fresh | VP1 | VP2 | VP3 | ||||
|---|---|---|---|---|---|---|---|---|
| Cytoplasmic | 43 | (24 %)a | 69 | (48 %)b,c | 84 | (65 %)b,d,e | 52 | (50 %)b,c,f |
| Nuclear | 139 | (76 %)a | 74 | (52 %)b,c | 45 | (35 %)b,d,e | 52 | (50 %)b,c,f |
| Total | 187 | (100 %) | 143 | (100 %) | 129 | (100 %) | 104 | (100 %) |
*different letters represent significant differences between groups (p < 0.05)
a,b(between fresh and treatment groups)
c,d(between VP1 and other treatment groups)
e,f(between VP2 and VP3)
Fig. 2.
MPM-2 (a, e), f-actin (b, f), chromatin (c, g), and combined (d, h) representative images from control (a–d), and VP1 (e–h) groups. Control oocytes exhibit little to no MPM-2 signal in this example of an SN oocyte (a, c); note prominent perinuclear and cortical disposition of f-actin in the oocyte and punctate appearance of phalloidin staining within the cumulus cells (b, d). Vitrified oocytes display cytoplasmic MPM-2 staining (e and h) with aggregating chromatin (g); f-actin is disorganized in cytoplasm and cortex of affected oocytes and phalloidin labeling of cumulus cells is markedly diminished (compare F to B). Transzonal projections that are typically displayed uniformly in control oocytes (b) are in heterogeneously distributed throughout the zona after vitrification (f and h). Scale bar equals 20 μm for all images
Fig. 3.
Triple fluorescence images of a single plane for f-actin (red), chromatin (blue), and MPM-2 (green) are shown for representative control (a), VP1 (b), VP2 (c), and VP3 (d) oocytes. In each case, a line was drawn across the entire oocyte that transected the chromatin and the graphs to the right depict the optical density profiles for each signal in their respective colors. NSN (a) and CCM (b–d) chromatin signals are evident in the center of the plot (b–d). MPM-2 shows no detectable signal in control and cytoplasmic signal in each of the vitrified oocytes shown here (b–d, green). The prominent f-actin signal in the cortex of control oocytes (a, red) is notably diminished in vitrified samples that also show an increase in total cytoplasmic phalloidin labeling (b–d, red)
Discussion
Immature oocyte cryopreservation followed by in vitro maturation can be a powerful tool in the field of assisted reproductive technologies. There are several advantages of cryopreservation of immature oocytes over mature MII oocytes. Most of all, immature oocyte cryopreservation does not require full ovarian stimulation, which would be a significant benefit for cancer patients who cannot delay cancer treatment or for women with hormone dependent malignancy. It would also be indicated for patients who are at high risk of ovarian hyper-stimulation syndrome (OHSS) such as PCOS patients. Furthermore, retrieval of immature oocytes for cryopreservation and subsequent IVM would be more cost effective for the poor responder patient population.
Mature human oocytes are known to be extremely sensitive to temperature changes (even chilling above 0 °C) and have limited capacity for repairing cytoplasmic damage [4, 5]. Cryoprotective agents (CPAs) and/or ice crystal formation during a freeze-thaw procedure leads to de-polymerization of the meiotic spindle and, consequently, contributes to the high incidence of aneuploidy observed in human oocytes and embryos [12]. Previous studies have shown that changes in the spindle morphology are partially irreversible [13], which contributed to increased digyny after fertilization [14]. Moreover, zona hardening also occurs as a result of premature release of cortical granules when oocytes are chilled or exposed to certain types of CPA [15].
Although it has been assumed that GV oocytes tolerate cryoinjury better than MII stage oocytes, earlier studies drew attention to the fact that GV oocytes are as vulnerable to cryoinjury as mature oocytes and compromised during subsequent meiotic maturation and fertilization [16]. In fact, some years ago Van Blerkom demonstrated lesions at the ultrastructural level in chromatin, mitochondria, and the nuclear envelope as a result of CPA-induced chemical and osmotic toxicity [8].
In recent years, the choice of cryotechnology has been shifting from slow freeze protocols to vitrification for mature human oocytes, as more reports have shown equivalent or higher pregnancy rates with vitrification [17–19] compared to slow freeze technology. There remain to be few detailed studies examining the effects of vitrification on GV stage mammalian oocytes. In addition, the present lack of standardized vitrification methods makes comparisons of the safety and efficacy difficult to assess for clinical utility. In principle, a higher rate of survival would be expected if the toxicity of cryoprotectants is minimized and devitrification avoided. Critical examination of these factors prompted the present investigation.
We applied our newly developed four step equilibration protocol to minimize osmotic toxicity, as immature oocytes are more sensitive to osmotic changes due to unstable cytoplasmic structures such as the cytoskeleton [20]. In addition we applied synthetic polymers (Super cool X-1000) as ice blocking agent which mimics action of AFP in warming step since devitrification can be more damaging to cells. In nature, many arctic animals and plants produce AFP to survive cold climates. The main cryoprotective mechanism of AFP is to prevent intracellular ice crystal formation and growth. It appears that AFP has beneficial effects when vitrifying immature mouse oocytes [21].
Our results showed that oocyte survival was superior with the four-step equilibration protocol when compared to two different two-step equilibration protocols. However, with respect to precocious cell cycle entry, chromatin configurations, and disruption of the actin cytoskeleton, only minor differences in the extent of these alterations were found between the three vitrification protocols that were investigated. Interestingly, the majority of vitrified oocytes from groups VP1-3 showed an abnormal pattern of chromatin condensation (over 50 %) raising questions regarding the vulnerability of immature oocyte chromatin to cryoinjury and whether or not the cell cycle and cytoskeletal modifications observed are linked in any way to the effect on chromatin.
Our findings from the expression of MPM-2 epitopes are consistent with a loss of coordination between nuclear and cytoplasmic maturation as a result of vitrification as first suggested by Van Blerkom [8]. Premature initiation of meiosis in the cytoplasm can disturb the synchronous resumption of meiosis, which can affect fertilization and development of embryos. Given the well-known partitioning of CDK1 activating components between the cytoplasm and nucleus of immature rodent oocytes, it is perhaps not surprising that damage incurred at the nuclear-cytoplasmic boundary would lead to premature activation of CDK 1 and precocious entry into M-phase of the cell cycle [11]. In this context, the apparent fusion of meiotic chromatin evidenced as a CCM configuration in this work may be a result of inappropriate intermixing of factors required to regulate the normal progression of chromosome condensation. It is interesting in this regard that a similar perturbation in meiotic chromatin has been reported in rat [22] and mouse [23] oocytes subjected to drugs that cause the depolymerization of microtubules. Whether this is a direct consequence of temperature induced changes in microtubule stability during cooling or an indirect effect of CPAs remains to be determined, as does the relationship to chromatin integrity. However, it should be emphasized that many microtubule–dependent processes take place during meiotic progression that would likely be impaired with subsequent efforts to mature vitrified immature oocytes. Among these are important alterations in the positioning of mitochondria [23], endoplasmic reticulum [24], and acidic organelles [22].
Besides the well-studied role of microtubules in oocyte maturation, there is mounting evidence to suggest that the actin cytoskeleton is a major contributor to the motility dynamics exhibited during meiotic spindle assembly and polar body extrusion [25, 26]. It is noteworthy then, that our studies have revealed a dramatic effect of vitrification on the stability of the actin cytoskeleton resulting in a loss of f-actin in the cortex and perinuclear region of oocytes and a severe disruption of TZPs at the interface of the oocyte and surrounding cumulus cells. When coupled with studies showing a positive effect of actin disrupting drugs on oocyte recovery after freezing [27], the prospect that conventional cryopreservation indeed can differentially affect components of the cytoskeleton, as noted previously [13, 14], must be reinterpreted for future attempts to optimize this technology. Specifically, drugs that disassemble the actin cytoskeleton may protect subunits and filaments from the adverse effects of temperature and CPAs allowing for the dynamic components to be reassembled following thawing and re-equilibration. Finally, we would propose that the sensitivity of the actin cytoskeleton demonstrated in this work infers that the actin targets are manifold and may have the overall effect of disengaging filament interactions at the oocyte cortex and chromatin as has been shown recently [28].
Moreover, to achieve a successful GV oocyte cryopreservation, it is crucial to synchronize nuclear and cytoplasmic maturation as well as to minimize cytoplasmic damage to maintain normal protein synthesis activities during maturation. Our study showed premature cytoplasmic maturation with vitrification. Perhaps, treatment with inhibitors such as isobutyl methylxanthine (IBMX) can prevent asynchrony in nuclear and cytoplasmic maturation and improve normal oocyte development and fertilization.
We observed that many GV oocytes lost surrounding cumulus cells after vitrification and warming. Communication between the oocyte and cumulus cells is important for the post-thaw survival, maturation and fertilization. Gap junctional coupling between oocytes and cumulus cells is also known to have an important role in the maturation process [29, 30]. The loss or impairment of cell contact between the oocyte and cumulus is likely to impact oocyte quality in at least two ways. The first as mentioned above is the important role that TZPs play in the maintenance of cell communication through junctions or paracrine interactions [31–33]. A second problem, especially relevant to the cryopreservation of intact COCs, is the effect of disengagement via TZPs on the biophysical properties of the oocyte cortex and the ability of the cortex to store and immobilize factors important for later development [34].
The present study draws attention to the undesirable consequences of immature oocyte vitrification that compromise cell cycle status, somatic cell interactions, and actin integrity, which may not be evident until after fertilization. Optimizing methods to restore or protect against such changes will be an imperative for future studies. It is also desirable to study these changes using other cryopreservation techniques such as slow freezing with updated protocols. Ultimately, future studies should focus on demonstration of functionality by verifying development al competence of the cryopreserved oocytes.
Acknowledgments
We would like to extend our sincerest thanks to Kimball Pomeroy for statistical analysis and Darlene Limback for her continued technical support. Most of all, our special thanks and acknowledgment go to Sara Brown and Rebekah Lang without whom this study would not be possible. This project was supported by grants from the Eshe Fund (DFA), the National Institute of General Medical Sciences from the National Institutes of Health (P20 GM103318), and the National Institutes of Health (HD 20068 DFA).
Conflict of interest
The authors declare no conflicts of interest regarding this manuscript.
Footnotes
Capsule
A comparison of vitrification protocol variations on cumulus-enclosed rat GV oocytes has revealed dramatic effects on cell cycle status, somatic cell-oocyte interactions, and the stability of the actin cytoskeleton.
References
- 1.Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85(1):1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 2.Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT., Jr Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012;98(3):644–649. doi: 10.1016/j.fertnstert.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committees of American Society for Reproductive M, Society for Assisted Reproductive T Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Coticchio G, Bromfield JJ, Sciajno R, Gambardella A, Scaravelli G, Borini A, et al. Vitrification may increase the rate of chromosome misalignment in the metaphase II spindle of human mature oocytes. Reprod Biomed Online. 2009;19(Suppl 3):29–34. doi: 10.1016/S1472-6483(10)60281-7. [DOI] [PubMed] [Google Scholar]
- 5.Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod. 2009;24(9):2114–2123. doi: 10.1093/humrep/dep182. [DOI] [PubMed] [Google Scholar]
- 6.Isachenko EF, Nayudu PL. Vitrification of mouse germinal vesicle oocytes: effect of treatment temperature and egg yolk on chromatin and spindle normality and cumulus integrity. Hum Reprod. 1999;14(2):400–408. doi: 10.1093/humrep/14.2.400. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73(11):1454–1462. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]
- 8.Van Blerkom J. Maturation at high frequency of germinal-vesicle-stage mouse oocytes after cryopreservation: alterations in cytoplasmic, nuclear, nucleolar and chromosomal structure and organization associated with vitrification. Hum Reprod. 1989;4(8):883–898. doi: 10.1093/oxfordjournals.humrep.a137006. [DOI] [PubMed] [Google Scholar]
- 9.McGinnis LK, Albertini DF. Dynamics of protein phosphorylation during meiotic maturation. J Assist Reprod Genet. 2010;27(4):169–182. doi: 10.1007/s10815-010-9391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickramasinghe D, Albertini DF. Centrosome phosphorylation and the developmental expression of meiotic competence in mouse oocytes. Dev Biol. 1992;152(1):62–74. doi: 10.1016/0012-1606(92)90156-B. [DOI] [PubMed] [Google Scholar]
- 11.Wickramasinghe D, Albertini DF. Cell cycle control during mammalian oogenesis. Curr Top Dev Biol. 1993;28:125–153. doi: 10.1016/S0070-2153(08)60211-2. [DOI] [PubMed] [Google Scholar]
- 12.Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54(1):102–108. doi: 10.1016/s0015-0282(16)53644-9. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu A, Toth TL, Toner M. Alterations of the cytoskeleton and polyploidy induced by cryopreservation of metaphase II mouse oocytes. Fertil Steril. 1998;69(5):944–957. doi: 10.1016/S0015-0282(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 14.Eroglu A, Toner M, Leykin L, Toth TL. Cytoskeleton and polyploidy after maturation and fertilization of cryopreserved germinal vesicle-stage mouse oocytes. J Assist Reprod Genet. 1998;15(7):447–454. doi: 10.1007/BF02744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickering SJ, Braude PR, Johnson MH. Cryoprotection of human oocytes: inappropriate exposure to DMSO reduces fertilization rates. Hum Reprod. 1991;6(1):142–143. doi: 10.1093/oxfordjournals.humrep.a137248. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Racowsky C, Combelles CM. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology. 2012;65(2):79–87. doi: 10.1016/j.cryobiol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 18.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–396. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–776. doi: 10.1016/S1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 20.Bagchi A, Woods EJ, Critser JK. Cryopreservation and vitrification: recent advances in fertility preservation technologies. Expert Rev Med Devices. 2008;5(3):359–370. doi: 10.1586/17434440.5.3.359. [DOI] [PubMed] [Google Scholar]
- 21.Jo JW, Jee BC, Suh CS, Kim SH. The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoSOne. 2012;7:e37043. doi: 10.1371/journal.pone.0037043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertini DF. Cytoplasmic reorganization during the resumption of meiosis in cultured preovulatory rat oocytes. Dev Biol. 1987;120(1):121–131. doi: 10.1016/0012-1606(87)90110-2. [DOI] [PubMed] [Google Scholar]
- 23.Van Blerkom J. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci U S A. 1991;88(11):5031–5035. doi: 10.1073/pnas.88.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305(1):133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14(3):141–152. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 26.Azoury J, Lee KW, Georget V, Hikal P, Verlhac MH. Symmetry breaking in mouse oocytes requires transient F-actin meshwork destabilization. Development. 2011;138(14):2903–2908. doi: 10.1242/dev.060269. [DOI] [PubMed] [Google Scholar]
- 27.Hosu BG, Mullen SF, Critser JK, Forgacs G. Reversible disassembly of the actin cytoskeleton improves the survival rate and developmental competence of cryopreserved mouse oocytes. PLoS One. 2008;3(7):e2787. doi: 10.1371/journal.pone.0002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett SL, Albertini DF. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet. 2010;27(1):29–39. doi: 10.1007/s10815-009-9376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod. 2004;70(3):548–556. doi: 10.1095/biolreprod.103.021204. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Costa P, Correia SC, Gouveia-Oliveira A, Negreiro F, Jorge S, Cidadao AJ, et al. Effects of mouse ovarian tissue cryopreservation on granulosa cell-oocyte interaction. Hum Reprod. 2005;20:1607–1614. doi: 10.1093/humrep/deh787. [DOI] [PubMed] [Google Scholar]
- 31.Godard NM, Pukazhenthi BS, Wildt DE, Comizzoli P. Paracrine factors from cumulus-enclosed oocytes ensure the successful maturation and fertilization in vitro of denuded oocytes in the cat model. Fertil Steril. 2009;91(5 Suppl):2051–2060. doi: 10.1016/j.fertnstert.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 33.Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- 34.Antczak M, Van Blerkom J. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod. 1997;3(12):1067–1086. doi: 10.1093/molehr/3.12.1067. [DOI] [PubMed] [Google Scholar]