Abstract
Purpose
To compare proteomic profiles of spermatozoa from patients with varicocele and poor sperm quality before and after varicocelectomy.
Methods
This work was designed as a prospective and observational study. The study was based on 20 men with varicocele grade 3 and poor sperm quality undergoing varicocelectomy at the Fertility Unit of Royan institute in 2009. Two semen samples were collected, one before varicocelectomy and the other after surgery. Protein separation was done by two-dimensional protein electrophoresis, and analyzed by gel densitometry and mass spectrometry. Differential sperm protein expression levels were measured by gel densitometry.
Results
Comparison of the sperm parameters showed that sperm motility and concentration were increased after varicocelectomy. At the level of protein, a total of 3 protein spots were identified whose expression was significantly lower in sperm samples before varicocelectomy compared with after surgery including heat shock protein A5 (HSPA5), superoxide dismutase 1 (SOD1) and δ-subunit of the catalytic core of mitochondrial adenosine triphosphate synthase (ATP5D).
Conclusions
High grade varicocoele affects sperm protein expression presumably because of increasing testicular temperature. These proteins play essential roles in sperm production, DNA integrity protection, and sperm motility. This novel study demonstrates that varicocelectomy can improve both sperm quality and proteins expression.
Keywords: Varicocelectomy, Sperm, Protein, Two dimensional gel electrophoresis
Introduction
Varicocele is the most common abnormal physical finding in male infertility, with a prevalence of 19–41 % of men with primary infertility and 45–81 % of men with secondary infertility [1]. Varicocele is characterized by dilation of veins of the pampiniform plexus and impairs the countercurrent heat exchange mechanism. Theoretically, this loss of testicular thermoregulation causes an increase in stage-specific apoptosis of germ cells at most susceptible stages of spermatogenesis, chronic hypoxia, and excessive production of reactive oxygen species. Excessive reactive oxygen species production that is not counterbalanced by antioxidant systems results in a number of deleterious effects to the sperm, including increased levels of sperm membrane lipid peroxidation, reduced motility, decreased mitochondrial activity, increased DNA fragmentation and apoptosis [2].
It is widely believed that clinical varicocele in adult men should be surgically corrected when changes in the results of semen analysis or functional tests are confirmed [3, 4]. Although varicocele repair is widely used as a treatment for male infertility, its efficacy has been a subject of intense debate for nearly 50 years. Although the ultimate goal of treating male factor infertility is to increase the pregnancy rate, varicocelectomy also seeks to maximize a couple’s fertility potential by improving sperm quality or avoiding a decline in testicular function [1].
There is a need for more objective criteria for the indication of varicocele repair in adolescents. Semen analysis is not reliable for evaluating varicocele in adolescents, and current clinical criteria for the indication of surgical treatment are unsatisfactory.
The study of the sperm proteome has a double interest at present: it is important to understand fundamental aspects of reproduction, but it is also very relevant towards the identification of many yet unknown causes of human infertility [5]. A reasonable hypothesis is that changes in proteomic composition might be responsible for the functional differences associated with an important proportion of cases of male infertility [6].
Although some studies have been published sperm proteome differences in men with and without varicocele [6, 7], there is no study about the comparison of the sperm protein profile between men with varicocele before and after varicocelectomy. Therefore, our main objective was to detect differences in protein expression of sperm before and after surgery.
Materials and methods
Patients and semen samples
This work designed as an observational study. Patients were recruited from the Infertility Unit of Royan Institute in 2009. We included 20 patients with grade 3 varicocele and poor sperm quality from our previous study [6]. The men with varicocele were infertile and in the present research, underwent varicocelectomy. The patients were evaluated by a single male infertility specialist and varicocele was diagnosed by physical examination and Doppler ultrasonography. Varicocelectomy performed at our institution were accomplished using Ivanissevich technique. Average of patients’ age was 30.15 ± 0.68 years. They did not have history of any systemic illnesses, cryptorchidism, orchitis, epididymitis, urethritis, testicular atrophy or sexually transmitted diseases such as human immunodeficiency virus. All the patients were nonsmoker and had normal body mass index. None of them had been exposed to environmental stressors, including radiation or chemicals. Men with azoospermia and those with sperm concentration <10 million sperm/mL were excluded from study. The ethics committee approved the study, and the patients provided informed consent.
Semen samples before varicocelectomy and 6 months after surgery were collected in specific sterile containers after 3 days of sexual abstinence and allowed to liquefy. After liquefaction of the semen, the sperm parameters (eg, volume, sperm concentration, percentage of motility) were evaluated according to the published recommendations [8] using a computer-assisted semen analyzer [6]. Sperm morphology was evaluated using strict Kruger criteria [9], and ≥100 cells were examined per slide. None of the semen samples had significant numbers of round cells or leukocytospermia according to the World Health Organization guidelines [8].
Sample preparation
Sperm purification was done by the AllGrad (Life Global, Canada) gradient technique, as previously mentioned [6]. AllGrad fractions of 80, 60, and 40 % in a Falcon tube were prepared (80 % fraction under the Falcon, then the 60 % fraction, and finally the 40 % fraction, 1 mL of each) and overlaid with semen (1 mL). After centrifugation (800 g, 20 min), the pellet of the 80 % fraction, 60 % fraction (the layer between the 60 and 80 % fraction), and the 40 % fraction (the layer between the 40 and 60 % fraction) was aspirated, washed, and resuspended in phosphate-buffered saline. Although gradient technique was used to remove the non-sperm cell, inevitably some non-motile sperm were removed by this method. So, the final suspension was included motile and non-motile sperm. The sperm concentration was determined using an improved Neubauer hemocytometer. After AllGrad purification, there were no non-sperm cells in the sperm suspensions.
Isoelectric focusing (IEF), SDS-PAGE and staining of the 2-D gels
Solubilized protein from AllGrad-prepared cells (150 μg proteins for analytic gels and 0.5 mg proteins for preparative gels) were placed in the rehydration tray with a 18 cm IPG linear strips (pH 4–7); and rehydrated for at least 12 h. IEF and SDS-PAGE were performed as previously described [6]. The analytic and preparative 2-dimensional gel electrophoresis gels were stained with silver nitrate and Coomassie Brilliant Blue (CBB) G250 [10], respectively. The analytical experiment was performed in triplicate.
Scanning and computer analysis of the 2-D
The silver-stained gels were scanned in transmission scan mode using a high-resolution GS-800 calibrated scanner (BioRad). The protein spots were characterized by their Mr and pI using internal 2-DE standards (BioRad). Matching of the same spots in the different gels was performed with the aid of Melanie 3 software (GeneBio, Geneva, Switzerland). When all the spots were matched, the values were normalized following the ‘total quantity in valid spots’ method [6]. In the present study, we were interested in the absence of spots in 1 gel compared with the other or a marked difference in spot intensity. Statistically significant differences (P < 0.01) that were consistently present in all 3 replications were considered for additional analysis. Also, the marked difference in spot intensity was arbitrarily set as a > 1.5 fold difference according to the advice from the software manufacturers.
Protein identification had been done by MS in the previous study [6], and in the current research, we compared the preceding identified proteins expression on the gels before and after varicocelectomy. If more than one protein was identified per spot; all peptides with a score above the significance threshold were considered to be positively identified. A score was calculated for each peptide based on the number of fragments and length of the peptide in the fixed and variable modifications. Spot intensity was analyzed both qualitatively and quantitatively. The final identification of proteins was done by the UniProt website (a database contains protein information that has been extracted from the literature and computational analyses).
Statistical analysis
The SPSS, version 16, statistical package was used. Differences between sperm parameters and the expression of the sperm proteins before and after varicocelectomy were analyzed using the paired t test. P < 0.01 was considered statistically significant.
Results
Mean of sperm parameters in men with varicocele before and after varicocelectomy have been shown in Table 1. There were no differences in volume and normal morphology in these patients before and after varicocelectomy. Sperm motility and concentration significantly increased after varicocelectomy (Table 1).
Table 1.
Sperm parameters before and after varicocelectomy (mean ± SE)
| Variables | Before | After | p value |
|---|---|---|---|
| Volume (ml) | 3.12 ± 0.08 | 3.36 ± 0.08 | > 0.05 |
| Concentration (×106sperm/ml) | 12.25 ± 0.28 | 47.45 ± 1.99 | < 0.001 |
| Total motility (%) | 40.3 ± 1.27 | 60.46 ± 0.94 | < 0.001 |
| Normal morphology (%) | 6.00 ± 0.50 | 6.55 ± 0.39 | > 0.05 |
In the previous study, we found 15 differences in protein expression between men with and without varicocele [6]. We followed the patients with grade 3 varicocele and abnormal sperm quality 6 months after varicocelectomy. Results of 2-DE revealed unique sperm total protein patterns in patients with varicocele before and after varicocelectomy. Three proteins showed differential expression levels after normalization between the groups (Table 2). Spots of interested proteins had been identified by tandem mass spectrometry and then using the Mascot search algorithm in our previous study, including heat shock protein A5 (HSPA5), superoxide dismutase 1 (SOD1) and δ-subunit of the catalytic core of mitochondrial adenosine triphosphate synthase (ATP5D). Expression of these proteins was significantly lower in sperm samples before varicocelectomy compared with after surgery (Fig. 1).
Table 2.
Protein expression in the sperm samples of patients with varicocele before and after varicocelctomy
| Spot name | Protein name | Accession no.a | MOWSE scoreb | Masses match (%)c | Protein covered (%)d | M r/pI determinede | M r/pI expectedf | Function | Expression before varicocelectomy |
|---|---|---|---|---|---|---|---|---|---|
| SOD1 | Superoxide dismutase | IPI00789078 | 315 | 3 | 33 | 18/5.62 | 14/5.68 | Antioxidant | Low |
| ATP5D | ATP synthase subunit delta, mitochondrial | IPI00024920 | 93 | 1 | 8 | 14/5.00 | 17/5.38 | Mitochondrial ATP synthase | Low |
| HSPA5 | 78 kDa glucose regulated Protein precursor | IPI00003362 | 588 | 7 | 11 | 72/5.43 | 72/5.07 | Assembly of multimeric protein complexes | Low |
aAccession number in Swiss-Prot (a high quality annotated and non-redundant protein sequence database)
bMascot score
cMasses matched percentages
dSequence percentage coverage
eExperimental isoelectric point and molecular weight
fTheoretical isoelectric point and molecular weight
gProtein expression levels in patients with varicocele before varicocelectomy
Fig. 1.
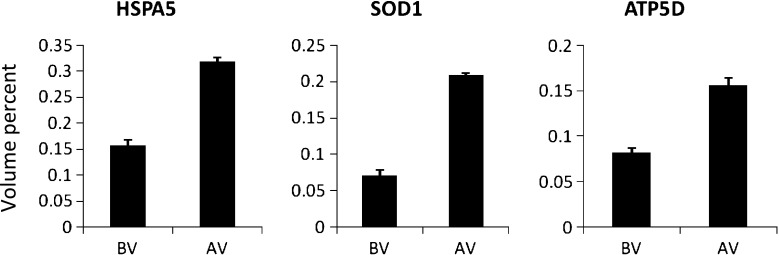
The abundance (percent volume) of 3 identified proteins in men with varicocele grade 3 before (BV) and after varicocelectomy (AV)
Discussion
Recently, there have been several studies about differences between protein profile in sperm or seminal plasma in men with and without varicocele [6, 7, 11]. The first report was ours so that we detected 15 significant differences in sperm protein expression [6]. In the present study, we followed men with varicocele and abnormal sperm quality 6 months after varicocelectomy and observed alterations in expression of 3 of 15 previously identified proteins (HSPA5, ATP5D and SOD1).
The present study demonstrated a significantly lower expression of HSPA5 in patients with varicocele and poor sperm quality before varicocelectomy compared with after it. HSPA5 is a member of the HSP70 family. Some studies have demonstrated relation between HSPs family and varicocele [12, 13] but only one study has reported low expression of HSPA5 in men with varicocele [6]. Yeşilli et al. (2005) showed that sperm HSPA2 activity is lower in infertile men with varicocele, and varicocelectomy increases HspA2 activity in these patients. Lima et al. (2006) reported that HSPA2 expression was down-regulated in adolescents with varicocele and oligozoospermia compared to controls. The expression level of the proteins is correlated with sperm maturity, function and fertility, and a dysfunctional expression of such genes result in abnormal spermatogenesis.
SOD1 is responsible for destroying free superoxide radicals in the body [14]. Our data suggested that down-regulation of SOD1 in patients with varicocele and abnormal spermogram can elevate DNA fragmentation and apoptosis, but after varicocelectomy, SOD1 activity increases and it can be resulted in improving DNA damage. Several studies have demonstrated that the total seminal plasma antioxidant levels in patients with varicocele are decreased and DNA damage in these patients was increased [12–18]. Moreover, some studies have shown that varicocelectomy can improve DNA fragmentation and total antioxidant capacity [19, 20]. Besides, our results in another study showed that after varicocelectomy, DFI significantly decreased compared with before surgery (Data not shown).
The ATP5D gene encodes the δ-subunit of the catalytic core of mitochondrial adenosine triphosphate synthase. Also, the energy involved in sperm motility is obtained by the catalytic reaction. Therefore, Down-regulation of either subunits of the complex results in decreased sperm motility [6]. In our study, patients had low sperm motility before surgery which could be due to low expression of ATP5D. It was presumabe that as the protein expression was elevated after varicocelectomy; mean of sperm motility was increased. Some reports have indicated that defects in mitochondrial proteins play important roles in varicocele [21].
Also, we compared results of protein expression between patients with varicocele after varicocelectomy with normal control group in previous study. It was shown that there was no significant difference between them (Data not shown).
It should be considered that confounding factors, e.g. age and weight, have been omitted from this study. Therefore, the alteration in these proteins may be due to varicocele repair.
There are limitations with the 2DE approach. The wide range immobilized pH gradients do not produce enough resolution to separate all of the proteins, especially in some overloaded regions of the gel. This might be overcome by running multiple gels either with different levels of protein loading or by using multiple overlapping ‘zoom gels’ which have small pI ranges. The latter approach might also improve detection of the more minor proteins that may be expressed differently. In addition, the focusing strips used will fail to resolve the more acidic and basic proteins. Finally, some proteins, particularly hydrophobic membrane proteins, may not be solubilized during sample preparation [22].
Conclusions
It can be concluded that varicocele with high grade can affect sperm proteome presumably because of increasing testicular temperature. The affected proteins play essential roles in sperm production, DNA integrity protection, and sperm motility. Moreover, our data showed that varicocelectomy can improve both sperm quality and proteins expression. Although this study is the first proteomic analysis in patients with grade 3 varicocele before and after varicoceletomy, there is a need to confirm these data using other molecular methods and to detect new protein spots in other pH ranges.
Acknowledgments
We thank those patients who have participated in this study.
Conflict of interest
There is no conflict of interest.
Footnotes
Capsule In this study, it was demonstrated different expression in three proteins before and after varicocelectomy by 2-DE. These proteins play essential roles in sperm production, DNA integrity protection, and sperm motility.
References
- 1.Cocuzza M, Cocuzza MA, Bragais FM, Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics (Sao Paulo) 2008;63(3):395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setchell BP. The Parkes lecture. Heat and the testis. J Reprod Fertil. 1998;114(2):179–194. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- 3.Blumer CG, Restelli AE, Giudice PT, Soler TB, Fraietta R, Nichi M, et al. Effect of varicocele on sperm function and semen oxidative stress. BJU Int. 2012;109(2):259–265. doi: 10.1111/j.1464-410X.2011.10240.x. [DOI] [PubMed] [Google Scholar]
- 4.Lacerda JI, Del Giudice PT, da Silva BF, Nichi M, Fariello RM, Fraietta R, et al. Adolescent varicocele: improved sperm function after varicocelectomy. Fertil Steril. 2011;95(3):994–999. doi: 10.1016/j.fertnstert.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 5.de Mateo S, Martínez-Heredia J, Estanyol JM, Domínguez-Fandos D, Vidal-Taboada JM, Ballescà JL, et al. Marked correlations in protein expression identified by proteomic analysis of human spermatozoa. Proteomics. 2007;7:4264–4277. doi: 10.1002/pmic.200700521. [DOI] [PubMed] [Google Scholar]
- 6.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, Sabbaghian M, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81(2):293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Chan CC, Sun GH, Shui HA, Wu GJ. Differential spermatozoal protein expression profiles in men with varicocele compared to control subjects: upregulation of heat shock proteins 70 and 90 in varicocele. Urology. 2013 doi: 10.1016/j.urology.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 8.WHO laboratory manual for the examination and processing of human semen. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 9.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, et al. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987;30(3):248–251. doi: 10.1016/0090-4295(87)90246-9. [DOI] [PubMed] [Google Scholar]
- 10.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 11.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, Souza GH, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99(1):92–98. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Yesilli C, Mungan G, Seckiner I, Akduman B, Acikgoz S, Altan K, et al. Effect of varicocelectomy on sperm creatine kinase, HspA2 chaperone protein (creatine kinase-M type), LDH, LDH-X, and lipid peroxidation product levels in infertile men with varicocele. Urology. 2005;66(3):610–615. doi: 10.1016/j.urology.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 13.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, Cedenho AP. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86(6):1659–1663. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox KC, Zhou L, Jordon JK, Huang Y, Yu Y, Redler RL, et al. Modifications of superoxide dismutase (SOD1) in human erythrocytes: a possible role in amyotrophic lateral sclerosis. Journal of Biol Chem. 2009;284(20):13940–13947. doi: 10.1074/jbc.M809687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, Srougi M. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85(3):625–628. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Giulini S, Sblendorio V, Xella S, La Marca A, Palmieri B, Volpe A. Seminal plasma total antioxidant capacity and semen parameters in patients with varicocele. Reprod Biomed Online. 2009;18(5):617–621. doi: 10.1016/S1472-6483(10)60004-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod. 2006;21(4):986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 18.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96(6):1283–1287. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Mancini A, Meucci E, Milardi D, Giacchi E, Bianchi A, Pantano AL, et al. Seminal antioxidant capacity in pre- and postoperative varicocele. J Androl. 2004;25(1):44–49. doi: 10.1002/j.1939-4640.2004.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 20.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189(1 Suppl):S146–S150. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, Cedenho AP. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90(5):1716–1722. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Pixton KL, Deeks ED, Flesch FM, Moseley FL, Bjorndahl L, Ashton PR, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19(6):1438–1447. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]


