Abstract
Background
Primary reconstruction of the lateral collateral ligament complex (LCLC) using graft tissue restores elbow stability in many, but not all, elbows with acute or chronic posterolateral rotatory instability (PLRI). Revision reconstruction using a tendon allograft is occasionally considered for persistent PLRI, but the outcome of revision ligament reconstruction in this setting is largely unknown.
Questions/purposes
We determined whether revision allograft ligament reconstruction can (1) restore the stability and (2) result in improved elbow scores for patients with persistent PLRI of the elbow after a previous failed primary reconstructive attempt and in the context of the diverse pathology being addressed.
Methods
Between 2001 and 2011, 160 surgical elbow procedures were performed at our institution for the LCLC reconstruction using allograft tissue. Only patients undergoing revision allograft reconstruction of the LCLC for persistent PLRI with a previous failed primary reconstructive attempt using graft tissue and at least I year of followup were included in the study. Eleven patients (11 elbows) fulfilled our inclusion criteria and formed our study cohort. The cohort consisted of six female patients and five male patients. The mean age at the time of revision surgery was 36 years (range, 14–59 years). The revision allograft reconstruction was carried out after a mean of 3 years (range, 2.5 months to 9 years) from a failed attempted reconstruction of the LCLC. Osseous deficiency to some extent was identified in the preoperative radiographs of eight elbows. Mean followup was 5 years (range, 1–12 years).
Results
Revision allograft reconstruction of the LCLC restored elbow stability in eight of the 11 elbows; two of the three elbows with persistent instability were operated on a third time (at 6 and 7 months after allograft revision reconstruction). For elbows with no persistent instability, the mean Mayo Elbow Performance Score at most recent followup was 83 points (range, 60–100 points), and six elbows were rated with a good or excellent result. All patients with persistent instability had some degree of preoperative bone loss.
Conclusions
Revision allograft reconstruction of the LCLC is an option for treating recurrent PLRI, although this is a complex and resistant problem, and nearly ½ of the patients in this cohort either had persistent instability and/or had a fair or poor elbow score.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Insufficiency of the lateral collateral ligament complex (LCLC) of the elbow may result in recurrent posterolateral rotatory instability (PLRI) [8]. Acute LCLC tears are oftentimes surgically repaired, whereas chronic insufficiency is best addressed with ligament reconstruction using autograft or allograft. Several authors have reported successful restoration of elbow stability in many patients undergoing ligament reconstruction; however, reconstruction does fail in some patients [2, 4, 5, 7, 9, 11]. Currently, allograft reconstruction of the LCLC is our treatment of choice for patients with PLRI.
Patients presenting with persistent PLRI after a previous failed LCLC reconstruction may represent a substantial challenge. Most will present with associated pathology, such as osseous deficiency or posttraumatic arthritis. A careful evaluation is required to identify all elements contributing to persistent stability, exclude occult infection, and understand associated factors that may have contributed to failure of the previous surgery (incorrect surgical technique, lack of compliance, underlying collagen disorders, and others). The bone stock on the lateral epicondyle may be compromised with osteopenia and bone loss. In our surgical practice, we have attempted LCLC reconstruction a second time in a small number of patients presenting with persistent PLRI after a previous reconstruction performed at our institution or elsewhere. The results of this approach, to our knowledge, have not been reported in the literature.
We therefore determined whether revision allograft ligament reconstruction can (1) restore the stability and (2) result in improved elbow scores for patients with persistent PLRI of the elbow after a previous failed primary reconstructive attempt of the LCLC.
Patients and Methods
This study was approved by our institutional review board. Our Departmental Bone and Tissue Bank Database collects information on all patients undergoing surgery with the use of any kind of allograft tissue. We queried this database to identify all patients who underwent reconstruction of the LCLC using a tendon allograft and reviewed the medical records for these patients to identify those who underwent revision surgery. Between 2001 and 2011, 160 surgical elbow procedures were performed at our institution for the LCLC reconstruction using allograft tissue. Only patients undergoing revision allograft reconstruction of the LCLC for persistent PLRI with a previous failed primary reconstructive attempt using graft tissue and at least I year of followup were included in this study. Patients who underwent primary allograft reconstruction or had allograft reconstruction of the LCLC in the setting of medial collateral ligament reconstruction, interposition arthroplasty, lateral column of distal humerus reconstruction, or common extensors reconstruction were excluded. A total of 11 patients (11 elbows) operated on during the study period fulfilled our inclusion and exclusion criteria and formed the basis of this study. There were six females and five males, with a mean age at the time of the index revision ligament reconstruction of 36 years (range, 14–59 years) (Table 1). The right elbow was affected in six patients and the left in five patients; the dominant arm was involved in four patients. Two patients had a clear traumatic event substantial enough to explain failure of their initial ligament reconstruction. All patients had at least one previous elbow surgery, including their previous failed ligament reconstruction (Table 1); five patients had only undergone previous elbow surgery once. The mean time between their initial injury and their initial reconstructive attempt was 4 years (range, 1 month to 26 years). Eight elbows showed various degrees of skeletal pathology; of these, two had irreducible subluxation or dislocation on radiographs at presentation.
Table 1.
Patient demographics and preoperative assessment data
| Patient | Sex | Age (years) | Side | Primary LCLC reconstruction (graft type and/or technique), if known | Other procedures, if any | Imaging | Underlying conditions and/or skeletal lesions*, if any | Time from primary reconstruction to revision surgery (years) | Pain | Extension to flexion (°) | Pivot shift test† | Drawer test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 16 | Left | Palmaris longus autograft | Plain radiographs | Traumatic event | 1 | Moderate | 0–145 | Yes | Yes | |
| 2 | Female | 42 | Left | Gracilis allograft, docking technique | Joint debridement | Plain radiographs | Underlying collagen disorder with general hyperlaxity | 1 | None | 0–150 | Yes | NR |
| 3 | Male | 44 | Right§ | Allograft (unknown source and technique) | Capsulorrhaphy, ulnar nerve transposition | Plain radiographs, 3-D CT scan | 2 | Severe | 0–150 | Yes | Yes | |
| 4 | Female | 26 | Left§ | Palmaris longus autograft | Plain radiographs | Capitellum, trochlea | 9 | Mild | 0–150 | Yes | NR | |
| 5 | Male | 32 | Right | Plantaris allograft, docking technique | LCLC repair, joint debridement | Plain radiographs | Underlying collagen disorder with general hyperlaxity, capitellum‡ | 2 | None | 30–145 | Grossly unstable | Grossly unstable |
| 6 | Female | 54 | Right§ | Plantaris allograft, yoke configuration technique | Plain radiographs, 3-D CT scan | Radial head, capitellum, coronoid | 7 | Mild | 0–140 | No | No | |
| 7 | Male | 14 | Right | Palmaris longus autograft | Plain radiographs | Traumatic event, coronoid, capitellum | 3 | Moderate | 5–150 | Yes | Yes | |
| 8 | Female | 59 | Right§ | Allograft (unknown source and technique) | Radial head replacement | Plain radiographs, 3-D CT scan | Radial head (replaced), capitellum | 0.21 | Severe | 0–150 | Yes | NR |
| 9 | Male | 46 | Right | Plantaris allograft, interference screw | ORIF for radial head, joint debridement, radial head replacement, contracture release, LCLC repair | Plain radiographs | Radial head (replaced), capitellum, coronoid | 0.34 | None | 38–130 | Yes | Yes |
| 10 | Female | 20 | Left | Palmaris longus autograft | Plain radiographs, 3-D CT scan | Coronoid‡ | 4 | Mild | 0–155 | Yes | Yes | |
| 11 | Female | 38 | Left | Plantaris allograft, interference screw | Release and debridement for lateral epicondylitis, LCLC repair | Plain radiographs | Underlying lateral epicondylitis, capitellum | 1 | Moderate | 0–135 | Yes | NR |
* Skeletal lesions included any degree of bone loss and/or varus angulation of the elbow, if present; §dominant arm is involved; †clinical posterolateral rotatory pivot shift test with apprehension and/or showing subluxation and relocation; ‡elbows with irreducible subluxation or dislocation on radiographs at presentation; LCLC = lateral collateral ligament complex; ORIF = open reduction and internal fixation; 3-D = three-dimensional; NR = not recorded.
The mean time elapsed between the original reconstructive attempt and the index revision ligament reconstructive surgery was 3 years (range, 2.5 months to 9 years). Four surgeons (BFM, SWOD, JSS, SPS) from the same institution performed all of the revision surgeries. The techniques used for graft passage and fixation evolved over time and were surgeon dependent. In our series, the technique and the type of allograft used were based on the specific nature of the pathology and surgeon judgment and experience in performing the LCLC reconstruction and other complex elbow reconstruction. The reconstruction was carried out using a so-called yoke configuration in six elbows, using a docking technique in four elbows, and using interference screws in one patient. Allograft tissue used included plantaris allograft (six elbows), semitendinous allograft (four elbows), and achilles tendon allograft (one elbow) (Table 2). Early after the revision surgery, the elbow was immobilized in 70° to 90° flexion and pronation, protecting the allograft reconstruction for 2 to 3 weeks. Motion was then started and gradually progressed as tolerated until restoring the range of motion (ROM), usually between 6 to 8 weeks after the revision surgery. Protective bracing was continued for 3 months. In our series, two patients required a dynamic external fixator for the elbow. Patients were allowed to return to full activities at 6 months according to their needs and activity type [11].
Table 2.
Operative details, followup assessment outcomes and complications
| Patient | Revision LCLC reconstruction (graft type and technique) | Additional procedures, if any | Type of followup | Followup (years)* | Most recent pain | MEPS (points) | MEPS grade | Quick-DASH score (points) | Complications, if any | Additional operations, if any |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Semitendinosus, yoke configuration | Removal of loose bodies | Questionnaire | 12 | None | 100 | Excellent | 0 | ||
| 2 | Semitendinosus, interference screw | Questionnaire | 3 | Mild | 85 | Good | 9 | |||
| 3 | Plantaris, yoke configuration | Submuscular ulnar nerve transposition | Questionnaire | 5 | Moderate | 65 | Fair | 59 | Surgical wound complication | Irrigation and debridement |
| 4 | Plantaris, yoke configuration | Capsule plication | Questionnaire | 6 | None | 100 | Excellent | 0 | ||
| 5 | Achilles, docking technique | MCL plication, ulnar nerve decompression, prosthesis replacement of the capitellum, external fixator was applied | Clinic visit | 1 | Mild | 85 | Good | 18 | Nonunion/malunion fracture of the ulna (traumatic) | Open reduction and corrective osteotomy of the ulna |
| 6 | Plantaris, docking technique | Joint debridement | Clinic visit | 1 | Mild | 85 | Good | 11 | ||
| 7 | Plantaris, yoke configuration | Coronoid reconstruction using bone autograft | Questionnaire | 10 | Mild | 85 | Good | 18 | ||
| 8 | Plantaris, yoke configuration | MCL plication, external fixator was applied | Questionnaire | 2 | Moderate | 60 | Fair | 45 | ||
| 9 | Semitendinosus, docking technique | Questionnaire | 9 | Moderate | 65 | Fair | 59 | Persistent instability, arthrosis, ulnar neuropathy | ||
| 10 | Plantaris, yoke configuration | Coronoid reconstruction using bone autograft, capsule plication | Clinic visit | 0.50 | Moderate | 55 | Poor | 75 | Persistent instability, inadequate medial coronoid trochlear articulation, trochlear erosion | Coronoid reconstruction using osteochondral autograft, LCLC revision using plantaris allograft, capsule plication, ulnar nerve transposition |
| 11 | Semitendinosus, docking technique | Ulnar nerve decompression | Clinic visit | 0.58 | Severe | 40 | Poor | 77 | Persistent instability | Repair of the LCLC allograft construct and the annular ligament |
* Until the most recent clinical evaluation or until the clinic visit before reoperation for persistent posterolateral rotatory instability; LCLC = lateral collateral ligament complex; MEPS = Mayo Elbow Performance Score; MCL = medial collateral ligament.
We reviewed the medical records of the 11 patients to collect patient demographics, details regarding their clinical presentation, physical examination maneuvers (ROM, posterolateral rotatory pivot shift test, posterolateral rotatory drawer test), surgical reports, complications, and reoperations. All patients had been followed in person during the clinic visit after their revision surgery (mean, 2 years; range, 1 month to 7 years). Additional effort was made to obtain followup assessment through elbow evaluation questionnaire for all patients. Patients were not requested to return for examination by the treating surgeons at our institution. Thus, at most recent followup, four elbows were assessed by the treating orthopaedic surgeon during an office visit and the remaining seven elbows were assessed through questionnaires. Outcomes were measured using the Mayo Elbow Performance Score (MEPS) [6] and the Quick-DASH score [1, 3]. The possible range of the MEPS is 0 to 100 points, with higher scores indicating better results. Scores of more than 90 points represent excellent results, 75 to 89 points good results, 60 to 74 points fair results, and less than 60 points poor results. The possible range of the Quick-DASH score is 0 to 100 points, with lower scores indicating better results. Radiographs obtained before surgery and at most recent followup were also reviewed for all elbows. The followup duration was calculated until the most recent clinical evaluation. Mean followup was 5 years (range, 1–12 years).
Unless otherwise specified, the data are expressed as means with ranges for continuous variables and numbers for discrete variables. All descriptive statistical analyses were performed using JMP® software (Version 10.0.0; SAS Institute Inc, Cary, NC, USA).
Results
During the followup duration, revision reconstruction of the LCLC using a tendon allograft restored elbow stability in eight of the 11 elbows (Table 2). Of these eight elbows with restored stability, six had absent or mild pain based on MEPS and Quick-DASH scores. For the eight elbows with no persistent instability, the mean MEPS was 83 points (range, 60–100 points), and six elbows were graded as a good or excellent result. The most recent mean QuickDASH score was 20 points (range, 0–59 points) for these elbows. For the three elbows with persistent instability, the most recent mean MEPS score was 53 points (range, 40–65 points) and the mean Quick-DASH score was 70 points (range, 59–77 points) (Fig. 1).
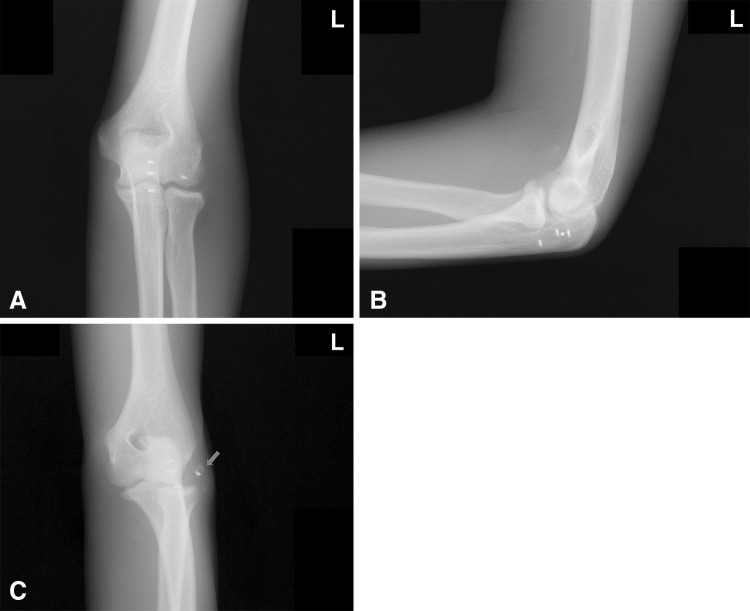
Fig. 1A–C.
A 16-year-old male patient had a fall with recurrent PLRI of the left elbow. He had previously undergone ligament reconstruction using suture anchors. (A) AP, (B) true lateral, and (C) medial oblique views of the left elbow after the new injury demonstrate a detached suture anchor from the humerus with some bony flakes (arrow). He underwent revision reconstruction of the LCLC.
Of the seven patients who had been followed through questionnaires, the mean MEPS was 80 points (range, 60–100 points) and four elbows were graded as a good or excellent result. Their mean Quick-DASH score was 27 points (range, 0–59 points). One of these seven patients had persistent instability but did not undergo reoperation. Of the four patients who had been evaluated during the clinic visit, the mean MEPS was 66 points (range, 40–85 points) and two elbows were graded as a good result. Their mean Quick-DASH was 45 points (range, 11–77 points). Two of these four patients had persistent instability and required reoperation.
Of the three elbows with persistent instability, two underwent reoperation for the LCLC; all three elbows had underlying skeletal pathology. One elbow with coronoid deficiency required a third-time LCLC reconstruction using plantaris allograft and a coronoid reconstruction using bone autograft (6 months after index) and one elbow with capitellar deficiency required a repair procedure for both LCLC allograft construct and annular ligament (7 months after index).
Discussion
Reconstruction of the LCLC of the elbow using autograft or allograft is a well-accepted treatment for patients with insufficiency of this ligamentous complex leading to PLRI [2, 4, 5, 7, 9–11]. However, a small number of patients experience failure of their reconstruction and may present with persistent instability bothersome enough to justify further surgical intervention. Persistent instability has been reported in 7% to 25% of elbows after LCLC repair or reconstruction [2, 4, 5, 7, 9, 11]. Revision reconstruction of the LCLC would seem the natural next step in these circumstances, but to our knowledge, there is no published information about the outcome of this procedure. In our surgical practice, we have attempted LCLC reconstruction a second time in a small number of patients presenting with persistent instability after a previous reconstruction performed at our institution or elsewhere. We recognize this is a very complex issue. The goal of this study was to focus on and to document the efficacy of the allograft reconstruction in the context of the diverse pathology being addressed. Therefore, we determined whether this approach (1) restored the stability and (2) improved the elbow scores in patients with persistent PLRI of the elbow after a previous failed primary reconstructive attempt of the LCLC.
Our study has several limitations. First, this is a retrospective study of limited numbers of patients with marked heterogeneity in terms of patient presentation, symptom chronicity, associated skeletal pathology, reconstructive techniques, and types of graft used. Because of the small numbers, statistical analyses were not a robust approach for evaluating risk factors for repeat surgery in these complex and difficult reconstructions. Second, not all patients were examined in person at the most recent followup, so we may have missed subtle degrees of persistent PLRI detectable on examination. In our cohort, two of the four patients examined in person during their latest evaluation had persistent instability and required additional reconstruction. Third, these results represent a selected population of patients as patients undergoing more complex reconstruction for the elbow were not included during the review. Lastly, the followup duration is relatively short in some patients compared to others in the study cohort. So, we might encounter more failures if we follow them longer, as the allograft tissue might stretch out over time. Despite these limitations, our report is the only report to date in the literature documenting the possibility of success when revision ligament reconstruction of the LCLC is attempted.
Revision allograft reconstruction of the LCLC is a treatment of choice in selected patients presenting with persistent PLRI after a failed reconstruction of the LCLC. However, it remains a multifactorial and complex dilemma for the patients and surgeons, as five of the 11 patients treated in this cohort had persistent instability and/or had a fair or poor elbow score. Although we were not able to run a further investigation about risk factors for failure in these complex and difficult reconstructions due to the small numbers in our series, we have encountered in our practice two situations in which revision ligament reconstruction may represent a technical challenge. The first group of patients include those with severe osteolysis and bony deficiencies in the lateral column of the humerus secondary to very large bone tunnels or bone erosion by the previous graft or interference screws; when plain radiographs look concerning for bone loss, a CT scan may be required to understand the possible need for alternative reconstructive strategies, such as use of a graft with a bone plug or fixation of the allograft tendon across the distal humerus over the medial side of the joint. The second group of patients includes those with severe atrophy of lateral tissues, including common extensor and skin over the joint after multiple failed surgeries; isolated reconstruction of the LCLC may be insufficient, and consideration may be given to reconstruction using an allograft, including the lateral epicondyle, lateral collateral ligament, and common extensor group. These extreme reconstructions have been performed selectively in our institution but are not part of the current report.
In summary, revision allograft reconstruction of the LCLC represents a possible approach for the treatment of persistent PLRI in patients with a previous failed reconstructive attempt. Care must be taken to evaluate these patients carefully to understand their expectations, identify factors that may have contributed to failure, and address bone loss and marked soft tissue deficiencies accordingly. The presence of osseous deficiency is a challenging issue that makes these revisions more difficult. This might be proven to be associated with a poor elbow score in a larger cohort or different study design. Restoring the bone stock with bone graft or performing more complex compartmental prosthetic replacement remains our current surgical approach in managing these patients with marked bone deficiency.
Footnotes
The institution of the authors has received, during the study period, funding from Stryker Orthopaedics (Mahwah, NJ, USA), Zimmer, Inc (Warsaw, IN, USA), and DePuy Orthopaedics, Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87:1038–1046. doi: 10.2106/JBJS.D.02060. [DOI] [PubMed] [Google Scholar]
- 2.Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75:516–523. doi: 10.1080/00016470410001367-1. [DOI] [PubMed] [Google Scholar]
- 3.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Jones KJ, Dodson CC, Osbahr DC, Parisien RL, Weiland AJ, Altchek DW, Allen AA. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21:389–395. doi: 10.1016/j.jse.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43:1657–1661. doi: 10.1016/j.injury.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Morrey BF, An KN. Functional evaluation of the elbow. In: Morrey BF, Sanchez-Sotelo J, editors. The Elbow and its Disorders. Philadelphia, PA: Elsevier Inc; 2009. pp. 80–91. [Google Scholar]
- 7.Nestor BJ, O’Driscoll SW, Morrey BF. Ligamentous reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1992;74:1235–1241. [PubMed] [Google Scholar]
- 8.O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73:440–446. [PubMed] [Google Scholar]
- 9.Olsen BS, Sojbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85:342–346. doi: 10.1302/0301-620X.85B3.13669. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Sotelo J. Lateral collateral ligament insufficiency. In: Morrey BF, Sanchez-Sotelo J, editors. The Elbow and its Disorders. Philadelphia, PA: Elsevier Inc; 2009. pp. 669–679. [Google Scholar]
- 11.Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87:54–61. [PubMed] [Google Scholar]