Abstract
Background
Heterotopic ossification (HO) is a common extrinsic cause of elbow stiffness after trauma. However, factors associated with the development of HO are incompletely understood.
Questions/purposes
We retrospectively identified (1) patient-related demographic factors, (2) injury-related factors, and (3) treatment-related factors associated with the development of HO severe enough to restrict motion after surgery for elbow trauma. We also determined what percentage of the variation in HO restricting motion was explained by the variables studied.
Methods
Between 2001 and 2007, we performed surgery on 417 adult patients for elbow fractures; of these, 284 (68%) were available for radiographs at a minimum of 4 months and clinical review at a minimum of 6 months after surgery (mean, 7.9 months; range, 6–31 months). HO was classified according to the Hastings and Graham system. Patients with HO restricting motion (defined as a Hastings and Graham Class II or III) were compared with patients without HO restricting motion in terms of demographics, fracture location, elbow dislocation, open wound, mechanism of injury, ipsilateral fracture, head trauma, time from injury to surgery, number of surgeries within 4 weeks, total number of surgeries, bone graft, and infection, using bivariate and multivariable analyses. A total of 96 patients had radiographic HO, and in 27 (10% of those available for followup), it restricted motion.
Results
There were no patient-related demographic factors that predicted the formation of symptomatic HO. Ulnohumeral dislocation in addition to fracture (odds ratio, 2.38; 95% CI, 1.01–5.64; p = 0.048) but not fracture location was associated with HO. Longer time from injury to definitive surgery and number of surgical procedures in the first 4 weeks were also independent predictors of HO (p = 0.01 and 0.004, respectively). These factors explained 20% of the variance in risk for HO restricting motion.
Conclusions
HO restricting motion after operative elbow fracture treatment associates with factors that seem related to injury complexity, in particular, ulnohumeral dislocation, delay, and number of early surgeries; however, a substantial portion of the variation among patients with elbow fracture who develop restrictive HO remains unexplained.
Level of Evidence
Level III, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Heterotopic ossification (HO) is one of the most common extrinsic causes of elbow contracture after elbow trauma, consisting of the pathologic formation of mature lamellar bone that is histologically identical to native bone [14]. The prevalence of HO of the elbow has been reported to range from 3% in simple dislocations to 45% in patients with distal humerus fractures [1, 12, 16]. Part of this wide variation is due to variations in the definition of HO, with some studies including even small amounts of HO in the ligaments.
Proposed risk factors for HO after elbow trauma based on relatively small case series include head trauma [7, 8], spinal cord injury [7], fracture type [1], elbow dislocation [12, 16], time from injury to surgery [3, 4, 6, 12], and severe burn [5, 11, 13]. Although these factors have plausible predictive capacity, it remains uncertain how much of the variation in HO is explained with clinical factors.
We therefore identified (1) patient-related demographic factors, (2) injury-related factors, and (3) treatment-related factors associated with the development of HO severe enough to restrict motion after surgery for elbow trauma. We also determined what percentage of the variation in HO restricting motion was explained by the variables studied.
Patients and Methods
Study Cohort
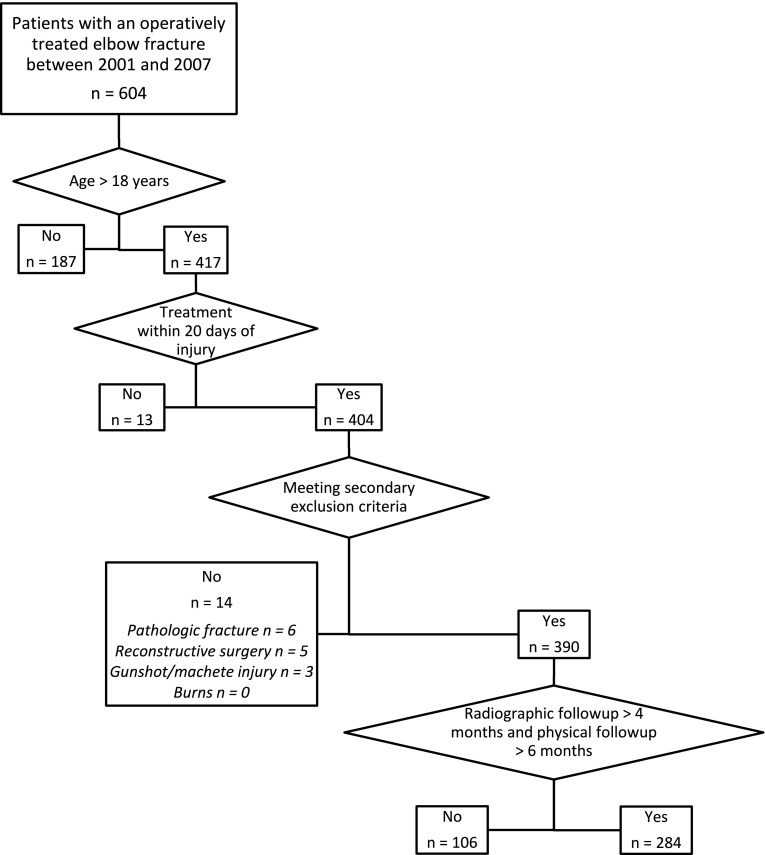
We identified patients who underwent surgery for elbow fractures at our center by reviewing billing records and a trauma database. This study was approved by our Human Research Committee. Between 2001 and 2007, we performed surgery on 604 patients for elbow fractures. A total of 417 patients were adults; of these, 284 (68%) were available for radiographs at a minimum of 4 months and clinical followup at a minimum of 6 months after surgery. General indications for surgery in these patients included displaced intraarticular fractures, fractures related to elbow instability, fractures unlikely to heal without surgery, and fractures with deformity restricting motion; patients medically unfit for upper-extremity surgery generally were managed nonoperatively. Inclusion criteria included fracture of the distal humerus, proximal ulna, or proximal radius; age of 18 years or greater; postoperative radiographs at a minimum of 4 months after injury; and physical examination a minimum of 6 months after injury. Exclusion criteria were time from injury to initial surgery of more than 20 days, pathologic fractures, gunshot and machete fractures, diaphyseal fractures of the radius or ulna with dislocation of the distal or proximal radioulnar joint, patients whose fracture were accompanied by burns, and reconstructive surgeries (consisting of surgeries for stiffness, nonunion, or arthritis). Three hundred twenty patients were excluded (Fig. 1), leaving 284 patients to form the final study cohort.
Fig. 1.
A flowchart shows the process by which patients were excluded and included in our study. A total of 320 patients were excluded. Two hundred eighty-four patients formed the final study cohort.
The senior author (DR) reviewed preoperative radiographs and CT scans when available to assess fracture locations and classify them as involving the distal humerus, radial head, olecranon, or coronoid. In case of combination fractures, locations were separately classified. The injuries included distal humerus fractures (n = 100, 35%), radial head fractures (n = 94, 33%), olecranon fractures (n = 119, 42%), and coronoid fractures (n = 55, 19%); 73 patients (26%) had more than one fracture; 119 patients (42%) had traumatic elbow instability.
No patients received NSAID or radiation prophylaxis. There were 270 definitive open reduction internal fixation procedures in this series (Table 1); we performed a total of 429 procedures on the 284 patients, for a mean of 1.4 procedures per patient (range, one to 14 procedures). The 284 patients in the study were last evaluated an average of 7.9 months after injury (range, 6–31 months). There were no missing data.
Table 1.
Treatment of included patients
| Treatment | Number of patients |
|---|---|
| Open injury: treatment at day of injury | |
| Irrigation and débridement | 20 |
| Irrigation and débridement and fixation | 16 |
| Definitive treatment | |
| Open reduction and internal fixation | 270 |
| Radial head resection | 3 |
| Radial head prosthetic arthroplasty | 43 |
| Total elbow arthroplasty | 2 |
| Subsequent surgeries within 4 weeks of injury | |
| Irrigation and débridement of deep infection | 22 |
| Reduction of recurrent dislocation | 2 |
| Fasciotomy | 2 |
| External fixator removal | 1 |
| Wound closure | 1 |
| Subsequent surgeries 4 weeks or more after injury | |
| Implant removal | 51 |
| Contracture release | 23 |
| Ulnar nerve transposition | 20 |
| Repeat irrigation and débridement of deep infection | 3 |
| Radial head resection | 2 |
| Nonunion repair | 2 |
Variables
Explanatory variables included in the analysis were demographics, fracture location, fracture side, complete ulnohumeral dislocation, open wound, fall from standing height or higher-energy injury (motor vehicle crash, fall from greater height, sports-related injury, etc), ipsilateral injuries (nerve injury, fracture of another part of the extremity, etc), central nervous system trauma, time from injury to definitive surgery, total number of surgeries until HO was diagnosed on radiographs, number of surgeries within 4 weeks of injury, use of a bone graft, and infection requiring operative débridement.
Classification
HO was identified on postoperative radiographs and graded according to the classification of Hastings and Graham [10], based on the ROM that was most recently recorded but at least 6 months after treatment. This classification distinguishes three grades of HO. Patients with Class I have HO that does not cause functional limitation. Patients with Class II HO have a functional limitation that blocks motion: Class IIA represents ulnohumeral limitation of a flexion contracture of 30° or greater and limitation of flexion to less than 130°, Class IIB represents limitation of forearm rotation of less than 50° of pronation or less than 50° of supination, and Class IIC represents heterotopic bone causing limitation in both planes of motion. Patients with Class III HO have ankylosis that prevents ulnohumeral motion (Class IIIA), forearm rotation (Class IIIB), or both (Class IIIC). To distinguish HO from other factors restricting motion, only bone formations that were deemed by the senior author to be highly likely to be the cause of the observed motion restriction were classified as Class II or higher. Patients with restricted motion and HO formations that had an unlikely causal relation were classified as Class I.
There were 27 patients with HO restricting motion (Class II or III, 10% of patients in the series), of whom nine (3.2%) developed ankylosis in at least one plane, and 257 patients without HO restricting motion. Ninety-six patients were diagnosed with HO around the elbow, including 69 patients with Class I, six with Class IIA, four with Class IIB, eight with Class IIC, one with Class IIIA, six with Class IIIB, and two with Class IIIC.
Statistical Analysis
Continuous data are presented as the median and interquartile range because of nonnormal distribution of the data. To identify factors that were associated with HO restricting motion and clinically relevant, patients with HO that did not restrict motion (Hastings and Graham Class I) were pooled with patients who had no HO (Hastings and Graham Class 0). All other patients were pooled as having HO restricting motion (Hastings and Graham Classes II and III).
In bivariate analysis, associations between the response variable HO restricting motion and explanatory variables were analyzed using the Mann-Whitney U test, Fisher’s exact test, and Pearson chi-square test as applicable. We considered p values of less than 0.05 significant. Explanatory variables with p values of less than 0.10 in bivariate analysis were entered into a backward stepwise logistic regression model for multivariable analysis. We used the chi-square p value for the overall model to assess goodness-of-fit and the Nagelkerke R2 to assess the percentage of the variation in HO restricting motion that was explained by the variables studied. Odds ratios (ORs) were calculated with the 95% CIs for significant predictors.
Results
No patient-related or demographic factors that we analyzed were associated with HO restricting motion.
Ulnohumeral dislocation was independently associated with restrictive HO (OR, 2.38; 95% CI, 1.01–5.64; p = 0.048). Coronoid fracture (p = 0.04) and olecranon fracture (p = 0.07) were associated with a higher and lower rate of restrictive HO in bivariate analysis (Table 2), respectively, but in the more definitive multivariate analysis (Table 3), both were found to be nonsignificant predictors of HO restricting motion. There was HO restricting motion in 11 of 100 patients with distal humerus fractures, 12 of 94 patients with radial head fractures, seven of 119 patients with olecranon fractures, and nine of 55 patients with coronoid fractures.
Table 2.
Bivariate analysis: comparison of patients with HO restricting motion versus no HO restricting motion
| Variable | All patients (n = 284) | No HO or HO not restricting motion (n = 257) | HO restricting motion (n = 27) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years)* | 55 (28) | 55 (28) | 54 (24) | 0.75 |
| Sex (number of patients) | 0.11 | |||
| Female | 137 | 128 (50%) | 9 (33%) | |
| Male | 147 | 129 (50%) | 18 (67%) | |
| Injury (number of patients) | ||||
| Type | ||||
| Distal humerus fracture | 100 | 98 (27%) | 11 (28%) | 0.53 |
| Radial head fracture | 94 | 82 (25%) | 12 (31%) | 0.2 |
| Olecranon fracture | 119 | 112 (34%) | 7 (18%) | 0.07 |
| Coronoid fracture | 55 | 46 (14%) | 9 (23%) | 0.04 |
| Mechanism of injury | 0.23 | |||
| Fall from a standing height | 129 | 120 (47%) | 9 (33%) | |
| Higher-energy injury | 155 | 137 (53%) | 18 (67%) | |
| Side | 0.69 | |||
| Left | 146 | 134 (52%) | 12 (44%) | |
| Right | 138 | 123 (48%) | 15 (56%) | |
| Complete ulnohumeral dislocation | 67 | 54 (21%) | 13 (48%) | 0.01 |
| Open fracture | 36 | 33 (13%) | 3 (11%) | 1 |
| Ipsilateral injury | 82 | 72 (28%) | 10 (37%) | 0.37 |
| Central nervous system trauma | 23 | 19 (7%) | 4 (15%) | 0.25 |
| Treatment | ||||
| Time from injury to surgery (days)* | 3 (6) | 2 (5) | 5 (4) | 0.001 |
| Number of surgeries within 4 weeks† | 1.11, 1 (0) | 1.05, 1 (0) | 1.57, 1 (0) | 0.02 |
| Number of total surgeries† | 1.44, 1 (1) | 1.43, 1 (1) | 1.50, 1 (1) | 0.71 |
| Number requiring bone graft | 7 | 6 (2%) | 1 (4%) | 0.52 |
| Infections requiring surgical débridement | 22 | 19 (7%) | 3 (11%) | 0.45 |
* Values are expressed as median, with interquartile range in parentheses; † values are expressed as mean, median, with interquartile range in parentheses; HO = heterotopic ossification.
Table 3.
Multivariable analysis: independent predictors of HO restricting motion
| Variable | p value | Odds ratio (95% CI) |
|---|---|---|
| Coronoid fracture | NS | |
| Olecranon fracture | NS | |
| Complete ulnohumeral dislocation | 0.048 | 2.38 (1.01–5.64) |
| Time from injury to surgery | 0.01 | 1.12 (1.03–1.22) |
| Number of surgeries within 4 weeks of injury | 0.004 | 2.88 (1.39–5.96) |
HO = heterotopic ossification; NS = not significant.
Two treatment-related variables were associated with symptomatic HO: number of surgeries within 4 weeks of trauma and time from injury to surgery. Both variables were retained in the multivariate model as independent predictors of HO restricting motion (number of surgeries within 4 weeks of trauma: OR, 2.88; 95% CI, 1.39–5.96; p = 0.004; time from injury to surgery: OR, 1.12 per day; 95% CI, 1.03–1.22; p = 0.01).
The three factors identified by the multivariable analysis (ulnohumeral dislocation, number of subsequent surgical procedures within 4 weeks, and time from injury to surgery) explained 20% of the variance in risk for HO restricting motion.
Discussion
HO is a common extrinsic cause of elbow stiffness. Recent publications have reported a prevalence in 7% to 20% of patients after surgically treated elbow trauma and assessed clinical predictors of HO restricting motion [1, 3, 6], but there is still uncertainty about the factors associated with development of restrictive HO after elbow injury. We determined whether any demographic, injury, or treatment factors were associated with the development of HO restricting motion after surgery for elbow trauma and how much of the variation in restrictive HO was explained by the variables studied.
This study should be interpreted in light of several shortcomings. First, we relied on medical records, which can be more limited and less accurate than prospective data collection, but we believe that the important elements were easily identified. In addition, many patients (106 of 390, 27%) were excluded from our analysis because they were lost to followup before 4-month radiographic followup or 6-month clinical followup. Because followup in this series was at short term and because not all patients were accounted for, the actual frequency of severe HO is likely higher than was estimated here. Nonetheless, most HO can already be observed on radiographs at 2 weeks postoperatively [1], and it seems unlikely to us that inclusion of the missing patients would have changed the analysis. We also acknowledge the difficulty of demonstrating causation of restricted motion and observed HO on radiographs amid other potential contributors to elbow stiffness. Only restrictive bony formations were included in this analysis. Most of the specific injury types were too rare to allow adequate power for study according to injury pattern, so we studied injury components instead. In addition, we did not have data available for all known clinical predictors. Recently, longer time to mobilization after surgery has been described as a predictor of clinically relevant HO [3], but we suspect this variable would have had substantial colinearity with variables included in our analysis (eg, total number of surgeries). Likewise, the muscle interval used to approach the elbow during the surgical dissection might influence the development of HO; unfortunately, we were not able to include this in the model. However, based on the injury pattern, there is often not much choice about what surgical approach one needs to use, and so this information, even if included in a model, probably would not influence the results to any large degree. Finally, although ulnohumeral dislocation, number of surgeries within 4 weeks of injury, and time from injury to surgery were independent risk factors for HO restricting motion, the CIs for the relevant ORs nearly passed through unity (the threshold for significance), suggesting that their effect size may not be very large. It might be that the identified independent risk factors for HO restricting motion reflect the severity of the overall injury or at least the ligament/soft tissue injury, though fracture location, open wound, and associated ipsilateral nerve injuries or fractures (other potential measure of injury severity) were not associated with HO.
Among patient-related demographic factors, we found that age and sex were not associated with restrictive HO. This finding is consistent with previous literature, as no studies of which we are aware have identified any demographic predictors [1, 12].
Of injury-related factors, the multivariable model identified complete ulnohumeral dislocation as a predictor of HO restricting motion. Previous studies [6, 12, 16], but not all [1, 3], have also shown that dislocation correlates with restrictive HO. Complete ulnohumeral dislocation likely reflects greater soft tissue injury and a more severe injury, which in itself was identified as a predictor by Douglas et al. [4]. Indeed, Foruria et al. [6] recently showed that a prognostic model, which included injury-related factors for open fracture, elbow instability, and chest injury, was able to discriminate between patients with and without restrictive HO. Also, the literature on HO after THA suggests that soft tissue injury such as substantial muscle retraction correlates with an increased risk of HO [2, 15, 17]. Some authors have suggested that fracture type rather than dislocation is a predictor of HO [1]. Our results suggest a variation in the rate of HO restricting motion among different fracture types, ranging from 6% for olecranon fractures to 20% for coronoid fractures. Although this variation seems to reflect the severity of the injury, fracture location was not an independent predictor in the analysis.
We showed in this analysis that there were two treatment-related predictors of restrictive HO: time from injury to surgery and number of surgical procedures within 4 weeks of the injury. The first factor, longer time from injury to surgery, has previously been shown to associate with HO after elbow trauma [3, 4, 6, 12]. We speculate that the delay often relates to referral of more complex fractures through the office or prioritizing in multiply injured patients. Patients with a longer interval between injury and surgery might be considered for prophylaxis against HO formation, although the risk of fracture nonunion must also be considered [9]. Our analysis adds to the current literature that a second treatment-related predictor of restrictive HO is number of surgeries within 4 weeks of injury. Hence, repeat elbow surgery with muscle manipulation and retraction with repeated early surgeries may increase the risk of HO. Alternatively, repeat surgery may reflect more complex injuries that were either staged or experienced adverse events.
The studied clinical variables explained only 20% of the variation in HO restricting motion. No other studies have previously addressed this question, except Foruria et al. [6] who demonstrated that their clinical prognostic model had discriminative power with an area under the curve of 0.78. Validation of that model in larger datasets is needed, and given the low percentage of variation in restrictive HO that was explained in our analysis, additional research is merited on factors other than our studied clinical variables.
We found that 33% of patients who had operative treatment of an elbow fracture and radiographs at least 4 months after injury had radiographic HO, but that HO restricted motion in only 10% overall or just less than 1/3 of patients with HO. Because followup in this series was at short term and because not all patients were accounted for, the actual frequency of severe HO is likely higher than was estimated here. We found that ulnohumeral dislocation, number of subsequent surgical procedures within 4 weeks, and time from injury to surgery were associated with HO that restricted motion after elbow trauma, but these variables accounted for only 20% of the variation in the risk of HO formation at a level severe enough to cause stiffness. This suggests the need for more research on the topic to try to identify what, we hope, will be modifiable risk factors; in our opinion, none of the risk factors we identified is easily modifiable.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Contributor Information
Jimme K. Wiggers, Email: j.k.wiggers@amc.nl.
David Ring, Email: dring@partners.org.
References
- 1.Abrams GD, Bellino MJ, Cheung EV. Risk factors for development of heterotopic ossification of the elbow after fracture fixation. J Shoulder Elbow Surg. 2012;21:1550–1554. doi: 10.1016/j.jse.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 2.Ahrengart L. Periarticular heterotopic ossification after total hip arthroplasty: risk factors and consequences. Clin Orthop Relat Res. 1991;263:49–58. [PubMed] [Google Scholar]
- 3.Bauer AS, Lawson BK, Bliss RL, Dyer GS. Risk factors for posttraumatic heterotopic ossification of the elbow: case-control study. J Hand Surg Am. 2012;37:1422–1429. doi: 10.1016/j.jhsa.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Douglas K, Cannada LK, Archer KR, Dean DB, Lee S, Obremskey W. Incidence and risk factors of heterotopic ossification following major elbow trauma. Orthopedics. 2012;35:e815–e822. doi: 10.3928/01477447-20120525-18. [DOI] [PubMed] [Google Scholar]
- 5.Evans EB, Smith JR. Bone and joint changes following burns; a roentgenographic study; preliminary report. J Bone Joint Surg Am. 1959;41:785–799. [PubMed] [Google Scholar]
- 6.Foruria AM, Augustin S, Morrey BF, Sanchez-Sotelo J. Heterotopic ossification after surgery for fractures and fracture-dislocations involving the proximal aspect of the radius or ulna. J Bone Joint Surg Am. 2013;95:e66. doi: 10.2106/JBJS.K.01533. [DOI] [PubMed] [Google Scholar]
- 7.Garland DE. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop Relat Res. 1988;233:86–101. [PubMed] [Google Scholar]
- 8.Garland DE, O’Hollaren RM. Fractures and dislocations about the elbow in the head-injured adult. Clin Orthop Relat Res. 1982;168:38–41. [PubMed] [Google Scholar]
- 9.Hamid N, Ashraf N, Bosse MJ, Connor PM, Kellam JF, Sims SH, Stull DE, Jeray KJ, Hymes RA, Lowe TJ. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am. 2010;92:2032–2038. doi: 10.2106/JBJS.I.01435. [DOI] [PubMed] [Google Scholar]
- 10.Hastings H, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin. 1994;10:417–437. [PubMed] [Google Scholar]
- 11.Hoffer MM, Brody G, Ferlic F. Excision of heterotopic ossification about elbows in patients with thermal injury. J Trauma. 1978;18:667–670. doi: 10.1097/00005373-197809000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ilahi OA, Strausser DW, Gabel GT. Post-traumatic heterotopic ossification about the elbow. Orthopedics. 1998;21:265–268. doi: 10.3928/0147-7447-19980301-09. [DOI] [PubMed] [Google Scholar]
- 13.Kolar J, Vrabec R. Periarticular soft-tissue changes as a late consequence of burns. J Bone Joint Surg Am. 1959;41:103–111. [PubMed] [Google Scholar]
- 14.Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg Am. 2007;32:1605–1623. doi: 10.1016/j.jhsa.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Morrey BF, Adams RA, Cabanela ME. Comparison of heterotopic bone after anterolateral, transtrochanteric, and posterior approaches for total hip arthroplasty. Clin Orthop Relat Res. 1984;188:160–167. [PubMed] [Google Scholar]
- 16.Thompson HC, III, Garcia A. Myositis ossificans: aftermath of elbow injuries. Clin Orthop Relat Res. 1967;50:129–134. doi: 10.1097/00003086-196701000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Zeckey C, Hildebrand F, Frink M, Krettek C. Heterotopic ossifications following implant surgery—epidemiology, therapeutical approaches and current concepts. Semin Immunopathol. 2011;33:273–286. doi: 10.1007/s00281-011-0240-5. [DOI] [PubMed] [Google Scholar]