Abstract
Background
Periprosthetic joint infection is a leading cause of failure after two-stage reimplantation. One cause of relapse may be persistent subclinical infection. Difficulty exists in detecting biofilm-forming infections. Sonication disrupts biofilm and has led to higher rates of positive intraoperative cultures.
Questions/purposes
Our aims in this study were to determine (1) if sonication results were predictive of failure, including reinfection, at 2-year followup; and (2) whether sonication of antibiotic spacers at the time of reimplantation improves sensitivity of intraoperative cultures.
Methods
We prospectively followed 36 consecutive patients undergoing two-stage reimplantation for periprosthetic hip or knee infection. Minimum followup was 19 months (mean, 29.9 months; range, 19–38 months). Results of intraoperative cultures and sonicated antibiotic spacers were analyzed.
Results
Positive sonication results were predictive of failure as defined by reinfection at 2-year followup. Among the 18 patients who had positive sonication results, reinfection developed in nine patients (50%) compared with two of 18 patients (11%) with negative sonication results (odds ratio, 8.0; 95% CI, 1.2–69.0). Sonication of antibiotic spacers improved the sensitivity of intraoperative cultures from 36% to 82%.
Conclusions
Sonication of antibiotic spacers appears to be useful in predicting failure attributable to recurrent infection after two-stage reimplantation. For patients with positive sonication cultures during reimplantation, more aggressive antimicrobial treatment may be indicated after reimplantation.
Level of Evidence
Level III, diagnostic study. See the Instructions for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3571-4) contains supplementary material, which is available to authorized users.
Introduction
In the United States, more than 600,000 primary TKAs and THAs are performed each year [13]. This number has been projected to reach four million major joint replacements annually by 2030 [13]. A prosthetic joint infection constitutes a leading cause of failure, imparting substantial economic effect on healthcare systems and society and pronounced morbidity on patients [14, 19, and 22]. In light of a maturing American populace [3], increasing number of joint replacement procedures [13], and risk of periprosthetic joint infection magnified with resident time of the implant in the body [22], the problem of prosthetic joint infection is likely to increase [1].
For patients with a chronically infected TKA or THA, the gold standard treatment is two-stage exchange arthroplasty. Two-stage prosthesis exchange with intervening placement of an antibiotic-impregnated polymethylmethacrylate (PMMA) spacer enables local delivery of high concentrations of antibiotic, preserves joint space and joint motion, and maintains patient mobility [5, 12]. However, it is not uniformly successful. Despite best current practices, investigations have documented reinfection rates as much as 33% [8, 12, and 20]. Recurrence of infection may be more frequent in the setting of resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis or in cases requiring multiple prior open procedures [8, 11, 12, 15].
After a two-stage revision for infection, a prosthetic joint infection may recur or persist, often with the same organism as the index infection [6–8, 12]. Recurrence may be seen even with negative synovial fluid and intraoperative per prosthetic tissue cultures [2, 21]. Such clinical scenarios suggest an inadequacy of our current tests to identify a persistent subclinical infection. Given that microorganisms associated with a periprosthetic joint infection may adhere to a prosthesis surface through biofilm formation, an improved detection strategy may be to collect microbiologic specimen from the implant [4]. Sonication of explanted prosthetic devices is a novel, validated technology that has shown greater culture sensitivity in detecting infections of metallic hip and knee prostheses [21]. Antibiotic-impregnated cement spacers, despite initial high local antimicrobial release, also may act as a scaffold on which biofilm formation may occur [9].
We performed this study to determine whether subclinical, biofilm-forming infections are a cause of otherwise unexplained failures of two-stage exchange arthroplasty. Our aims were to determine (1) if sonication results were predictive of failure, including reinfection at 2-year followup; and (2) whether sonication of antibiotic spacers at the time of reimplantation improves sensitivity of intraoperative cultures.
Patients and Methods
We retrospectively and prospectively evaluated 36 consecutive patients undergoing sonication of an antibiotic cement spacer at Geisinger Medical Center from November 1, 2010 through November 30, 2011. Patients were divided into two cohorts, those with positive cultures after cement spacer sonication and those with negative cultures after cement spacer sonication. The study was approved by our Institutional Review Board, and permission was granted to evaluate retrospective data from November 1, 2010 and follow subsequent patients prospectively. Demographic data were collected, including patient age, sex, BMI, history of tobacco use, diagnosis of inflammatory arthropathy, and presence of pharmacologic immunosuppression. When data were available, date of primary arthroplasty and number of prior open surgical procedures to the affected joint were recorded. Patients were followed postoperatively and monitored for failure, reinfection, or reoperation.
Sample-size calculation was difficult as at the time of initiation of the study, there were no published reports evaluating sonication results for antibiotic cement spacers An investigation from the Mayo Clinic reported an 18% difference in culture sensitivity between explants that were sonicated versus standard intraoperative cultures (79% versus 61%, respectively) [21]. Assuming a group difference of the same magnitude, and an equal number in each group (positive sonication cultures versus negative sonication cultures), nine patients per group would be necessary for 80% power at alpha less than 0.05. We conservatively enrolled 36 subjects to ensure a detectible difference between groups.
Our study population was comprised of 36 patients (29 knees and seven hips). Of patients with periprosthetic joint infection of the knee, 22 had an articulating cement spacer placed (StageOne®; Biomet, Warsaw, IN, USA) (Fig. 1); seven knees underwent placement of a nonarticulating, hand-molded cement spacer. Patients with infected THAs received an articulating spacer (Prostalac®; DePuy, Warsaw, IN, USA). Average duration of systemic intravenous antibiotic treatment before reimplantation was 6.6 weeks. The average antibiotic-free period before reimplantation was 6.9 weeks. The most common infecting organisms were methicillin-sensitive S aureus, Staphylococcus species not S aureus, and MRSA, respectively.
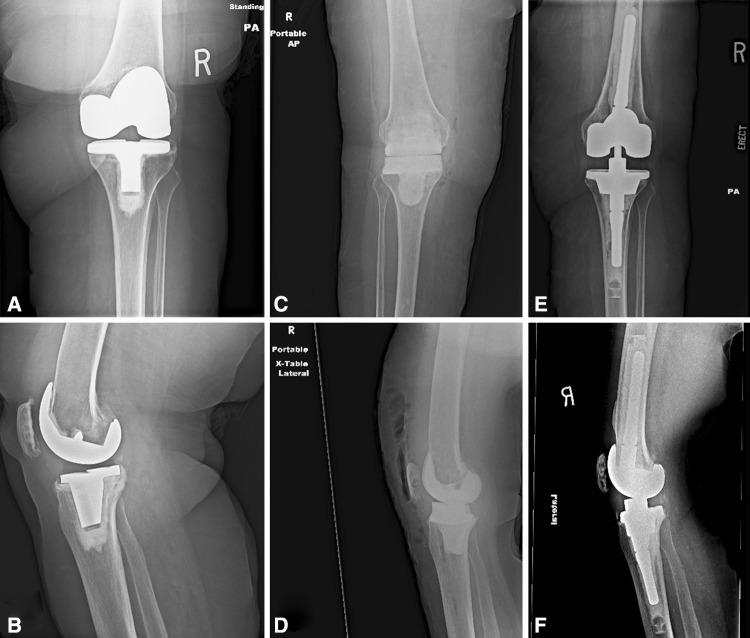
Fig. 1A–F.
Preoperative (A) AP and (B) lateral radiographs show an infected TKA before prosthesis removal. (C) AP and (D) lateral radiographs show the knee of the same patient after removal of the prosthesis and placement of an antibiotic impregnated articulating spacer. (E) AP and (F) lateral radiographs after removal of the antibiotic spacer prosthesis and successful two-stage revision TKA with a stemmed revision knee implant are shown.
Subjects were an average of 68 years old. The study population was 53% male (19 men and 17 women). Diabetes mellitus was present in 33% (12 total) of patients. Thirty-one percent (11 patients total) of the patients used tobacco. Average BMI was 36 kg/m2. Two patients (6%) had inflammatory arthropathy, and two patients (6%) were pharmacologically immunosuppressed. Patients underwent an average of 2.3 prior open surgical procedures involving the ipsilateral knee (Table 1).
Table 1.
Demographic data (N = 36 patients)
| Demographic | Number (%) |
|---|---|
| Age (years) | 68; SD = 13; range, 20–86 |
| Sex | 19 (53) men; 17 (47) women |
| BMI (kg/m2) | 36; SD = 10 |
| Diabetes mellitus | 12 (33) |
| Tobacco use | 11 (31) |
| Inflammatory arthropathy | 2 (6) |
| Immunosuppression | 2 (6) |
| Prior open surgical procedures | 2.3; range, 1–5 |
After determination of periprosthetic joint infection, component explantation was performed in association with a thorough débridement. Implanted antibiotic spacers were impregnated with high concentration antibiotic agent(s) selected empirically or to cover the established prosthesis infecting microorganism (Table 2. Supplemental material is available with the online version of CORR®). Systemic antibiotics were given for a minimum of 6 weeks through a peripherally inserted central catheter line and tailored to the organism isolated by preoperative joint aspiration and intraoperative cultures by the infectious disease department. By protocol, this was followed by a 6-week antibiotic-free period. When clinical presentation and laboratory data including erythrocyte sedimentation rate and C-reactive protein suggested clearance of infection, patients proceeded with second-stage component reimplantation.
For each case, a standard protocol included sending at least three conventional periprosthetic tissue cultures to the microbiology laboratory for evaluation. Tissue selected for sampling was that which showed the most obvious inflammatory changes by visual inspection of the surgeon. Analysis included aerobic culture and anaerobic culture with customary handling, incubation, and interpretation protocols (plates held 5 days, broth 7 days). For the purposes of this study, any reference to periprosthetic joint infection may be understood to mean the definition of per prosthetic joint infection as described by Parvizi et al. [17].
Collected laboratory data points included erythrocyte sedimentation rate, C-reactive protein, and anaerobic and aerobic culture results from joint aspiration; these indices were obtained at the times of initial periprosthetic joint infection diagnosis, just before reimplantation, and if reinfection was suspected. Cell count and cell differential at the time of initial periprosthetic joint infection diagnosis were recorded. Antibiotic spacer sonication culture results were obtained at the time of spacer removal and component reimplantation.
Clinical outcomes noted included recurrent or persistent infection, reoperation for any reason, need for chronic antibiotic suppression, amputation, and death. Type of antibiotics impregnated in the spacer and site (hip or knee) of the infected joint were recorded. Type of intravenous antibiotic dispensed, duration of intravenous antibiosis, and length of antibiotic-free interval before reimplantation also were recorded.
Technical aspects of prosthesis sonication, specimen incubation, and results interpretation and reporting were based on protocols developed at the Mayo Clinic [21]. Explanted antibiotic spacers were collected using wide-mouthed 1-L polypropylene bottles (Nalgene®, Rochester, NY, USA). Bottles were sterilized by autoclave at 250° F and 17 psi for a 30-minute sterilization and a 30-minute dry time. Using a sterile technique, antibiotic spacers were transferred directly from the surgical site to the bottle. Bottles then were sealed firmly with a screw top and transported to the microbiology laboratory, where processing was begun within 1 hour. Four hundred milliliters of Ringer’s solution was poured in each container. This admixture then was vortexed on high speed (3000 rpm) for 30 seconds using a Vortex Mixer® (Model 02215365; Fisher Scientific, Pittsburgh, PA, USA). The sonication device (Ultrasonic Cleaner®, Model 5510; Branson Ultrasonics Corporation, Danbury, CT, USA) then was prepared by filling the tank with deionized water and running for 5 minutes to remove all gas from the added water. The sealed bottle was placed in a water-filled basin with weights overlying to keep it well situated. Sonication then was performed for 5 minutes (40 kHz, 185 W). After an additional 30 seconds of high-speed vortexing, the bottle was unsealed so that two 50-mL vials could be filled with sonicate fluid using a sterile pipettor (Pipet-Aid®; Drummond Scientific Company, Broomall, PA, USA). The vials were spun by centrifuge at 4000 rpm for 5 minutes (Allegra® X-22 Centrifuge; Beckman Coulter, Brea, CA, USA). Ultrafiltrate was poured off until only 0.5 mL remained with each bullet. Each remaining admixture then was vortexed again on high speed for 30 seconds before combining to form a final 1-mL sample to be used for culture. A sterile pipette finally was used to inoculate 0.1 mL of remaining fluid down the center of each culture medium plate with customary lawn streaking. The culture media included one each of chocolate agar, blood agar, MacConkey agar, and CDC-blood agar (anaerobic).
Aerobic plates were incubated for 4 days, and anaerobic plates were incubated for a total of 14 days. If there was no growth after this period, the final culture result was declared negative. For positive workup, colonies on the plate were counted and reported as less than 20 colony-forming units (CFU)/10 mL, 20 to 50 CFU/10 mL, 50 to 100 CFU/10 mL, or greater than 100 CFU/10 mL (0.1 mL of inoculum equals 10 mL of the original, nonconcentrated sonicate sample). For growth less than 20 CFU/10 mL, common bacterial species were identified with no susceptibility testing. If the organism was coagulase-negative Staphylococcus, Bacillus species, Gram-positive Bacillus resembling Corynebacterium species, Streptococcusviridans group, Micrococcus species, Propionibacterium, or nonfermenting Gram-negative Bacilli other than Pseudomonasaeruginosa, it was identified as such in the final report. For the other CFU categories described, speciation data and sensitivity testing results were reported.
After reimplantation of total joint components, 11 patients ultimately required treatment for persistent or recurrent infection. All 11 required at least one additional procedure, including two patients treated with amputation and two patients treated with resection arthroplasty. Eight patients ultimately required chronic suppressive antibiotic therapy and one patient died. The minimum followup was 19 months (mean, 30 months; range, 19–38 months).
Statistical analysis was performed using an OR calculator to evaluate the likelihood of reinfection among patients with negative results compared with positive sonication results with the alpha error level set at less than 0.05.
Results
Positive sonication results were predictive of reinfection after two-stage reimplantation for periprosthetic infection. Eighteen of 36 patients (50%) had positive sonication results. Eight showed significant bacterial growth (> 50 CFU), one showed intermediate growth (20–50 CFU), and nine showed subtle growth (< 20 CFU). Among the eight patients with significant growth by sonication, six (75%) had reinfections develop. The one patient with intermediate sonication growth had a recurrent infection. Therefore, of the 18 patients with positive sonication cultures, nine had reinfections (Table 2. Supplemental material is available with the online version of CORR). Among the nine patients with subtle sonication growth, two had reinfections, and of the 18 with negative sonication cultures, only two had reinfections. The OR for reinfection in patients with a positive sonicate result was 8.0 (95% CI, 1.41–45.40; p = 0.0189).
Sonication of spacer improved the sensitivity of intraoperative cultures. In our study population of 36 patients undergoing two-stage revision arthroplasty, standard periprosthetic tissue cultures obtained at the time of reimplantation yielded sensitivity of 36%% and specificity of 63% for reinfection. Sonication cultures performed at the time of second-stage revision had a sensitivity of 82% and specificity of 50%. If we considered only significant and intermediate growth sonication cultures as positive, the sensitivity would decrease to 63% and the specificity would increase to 78%, respectively. Standard intraoperative cultures were able to predict only four of the nine reinfections with positive sonication results and none of the two reinfections with negative sonication results.
Discussion
Periprosthetic joint infection with biofilm-forming organisms is a leading cause of failure and reinfection after two-stage reimplantation. Difficulty often exists in detecting such infections, particularly in patients who have received antibiotic treatment. The National Institutes of Health Consensus Development Conference statement on THA decries the inaccuracy of diagnostic tests for periprosthetic joint infection [16]. Improved diagnostic tools for periprosthetic infection would be of great value, particularly tests that not only confirm infection, but also allow identification of an organism to which antimicrobial treatment can be directed. The introduction of sonication protocols for diagnosis of periprosthetic joint infections has shown great promise. The process of implant sonication is relatively simple, can be performed in less than 10 minutes at most microbiology laboratories, and procures viable microorganisms for growth on culture media [21]. A couple studies have documented an increased sensitivity in detection of periprosthetic joint infection compared with customary intraoperative periprosthetic tissue cultures [20, 21]. Sonication techniques have been shown to increase the culture sensitivities for patients who have received antibiotic treatment during the previous 2 weeks [21]. To date, nearly all systematic investigations on sonication for detection of periprosthetic joint infection have harvested metallic implants for analysis. Less well established is the use of sonication of antibiotic spacers. PMMA, even embedded with antibiotics, still comprises a foreign material surface on which biofilm potentially may form. Laboratory and in vivo studies have shown that microorganisms may grow on and adhere to antibiotic-laden cement [9, 10]. The aims of our study were to determine (1) if sonication results were predictive of failure, including reinfection, at 2-year followup; and (2) whether sonication of antibiotic spacers at the time of reimplantation improves sensitivity of intraoperative cultures. Our results confirmed a statistically significant association between positive sonication growth at any colony count and failure including reinfection. In addition, sonication of antibiotic spacers was shown to improve the sensitivity of intraoperative cultures.
Our study has several limitations. Given the relatively small sample size of our study population, statistical power is relatively low. Nevertheless, the association between positive sonication results and development of reinfection was highly statistically significant. Members of the orthopaedics and infectious disease teams were not blinded to spacer sonication or tissue culture results. By treatment protocol, however, no antibiotic treatment or other alteration in care was given to patients with positive sonicate results after reimplantation unless failure and reinfection already had been confirmed. Even though a minimum followup of 19 months was available for all patients, it is possible that some patients, whether their sonication results were positive or negative, may have subclinical infections which have not yet been detected or cannot be confirmed using Musculoskeletal Infection Society criteria [17]. However, the lack of definite reinfection or cure is a potential criticism of every study evaluating periprosthetic infection, and we did use the criteria established as the gold standard for confirming the presence or absence of periprosthetic joint infection. Some reinfections occurred with organisms that differed from the original infection or organism detected after sonication. This is a common finding among many studies evaluating outcomes after management of periprosthetic infections [5, 7, 8, 11, 12, 15]. It is possible that some reinfections were the result of a new infection rather than a persistent infection not detected by standard intraoperative cultures. Complex prolonged surgery in patients undergoing multiple prior knee procedures is associated with higher infection rates than primary procedures. Host factors also may play a role in some patients having reinfections develop despite appropriate surgical and antimicrobial management.
Positive sonication cultures were associated with a significantly higher failure and reinfection rate after two-stage reimplantation in our study. Our results are consistent with those reported by Sorli et al. [20] who described antibiotic spacer sonication results for a group of 55 patients who underwent two-stage revision surgery for deep infection of hip, knee, or shoulder hardware. Patients in that study were followed for a mean of only 1 year. Their sonication results were binary; a positive sonication value was considered 5 CFU or greater and negative sonication value was less than 5 CFU. Sorli et al. determined that sonication of antibiotic spacers was a sensitive means of assessing otherwise subclinical infection and observed a statistically significant association of positive sonication result with the likelihood of clinical failure. The sonication technique we used was based on methods developed at the Mayo Clinic [18, 21]. As in those two studies, our sonication technique involved a concentration step and yielded results by quantified CFU categories. One clinically interesting question that arises from these data is what value can reasonably be considered positive or clinically significant. Some have suggested that 20 CFU can be considered such a threshold value [18]. Our results suggest that a cutoff value of 20 CFU may lead to false negatives if applied to sonication of antibiotic spacers. Categorical CFU data for sonication of antibiotic spacers before reimplantation have not been published to our knowledge. It is possible that the number of CFU correlates with the likelihood of infection recurrence. It is notable from our study that among patients with positive sonication culture results, seven of nine patients with greater than 20 CFU had reinfections versus only two of nine patients with less than 20 CFU. Our study did not include sufficient numbers to answer this question. Future study on this topic is needed. Noting that the average time to reinfection after two-stage revision of THA and TKA has been calculated at a mean of 468 days in a previous study [12], we chose to follow patients undergoing two-stage exchange arthroplasty for an average of greater than 2 years.
Strengths of our study are that this is the first report of antibiotic spacer sonication results stratified by CFU growth. Moreover, reinfection was diagnosed using the new agreed-on criteria for periprosthetic joint infection and the mean followup was 30 months (range, 19–38 months).
Sonication of spacers at the time of two-stage reimplantation also was shown to increase the sensitivity of detection of persistent periprosthetic joint infection. Among the 11 patients (31%) who had reinfections develop after two-stage reimplantation, standard intraoperative cultures were able to detect persistent infection in only four. Using failure via reinfection as a marker for persistent infection, the sensitivity of standard intraoperative cultures was 36%. However, antimicrobial cultures after sonication were able to detect persistent infection in nine of the 11 patients who had reinfections develop, for a sensitivity of 82%.
Our study showed that sonication of explanted antibiotic spacers improves sensitivity for detection of persistent infection over customary perioperative tissue cultures. We noted a statistically significant association between positive sonication result and failure of two-stage reimplantation. Based on these results, we believe additional study is indicated to evaluate the utility of sonication on antibiotic spacers in predicting reinfection after two-stage reimplantation. We recommend that centers where sonication equipment is available routinely sonicate their spacers at the time of two-stage reimplantation and prospectively follow patients for reinfection. If sonication cultures are positive, we recommend either close followup of the patient for reinfection or antibiotic treatment to potentially decrease the likelihood of reinfection. Further study is necessary to determine if temporary intravenous antibiotics and/or long-term suppressive antimicrobial treatment will lead to lower rates of failure or reinfection in the case of positive sonication results. Future research also may be helpful regarding stratifying the risk of reinfection related to the quantity of bacterial growth after sonication of antibiotic spacers.
Electronic supplementary material
Acknowledgments
We thank Kaan Irgit MD, Department of Orthopaedics, Geisinger Medical Center (now at Cankaya Hospital, Ankara, Turkey) who assisted with the initial data gathering; Kent Strohecker MS (Department of Orthopaedic Surgery, Geisinger Medical Center) for assistance in preparation of the institutional review board documents; and Annamarie Horan PhD (Department of Orthopaedic Surgery, University of Pennsylvania, Philadelphia, PA) for review of statistical analysis.
Footnotes
One of the authors (CLN) certifies that he has or may receive payments or benefits during the study period from Zimmer, Inc (Warsaw, IN, USA) for consulting activities in an amount of USD (USD 10,000–100,000).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Geisinger Medical Center, Danville, PA, USA.
References
- 1.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Heckman JD, Petrie DP. AOA An AOA critical issue: aging of the North American population: new challenges for orthopaedics. J Bone Joint Surg Am. 2003;85:748–758. [PubMed] [Google Scholar]
- 4.Donlan RM. New approaches for the characterization of prosthetic joint biofilms. Clin Orthop Relat Res. 2005;437:12–19. doi: 10.1097/01.blo.0000175120.66051.29. [DOI] [PubMed] [Google Scholar]
- 5.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis: The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 8.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 9.Kendall RW, Duncan CP, Beauchamp CP. Bacterial growth on antibiotic-loaded acrylic cement: a prospective in vivo retrieval study. J Arthroplasty. 1995;10:817–822. doi: 10.1016/S0883-5403(05)80081-6. [DOI] [PubMed] [Google Scholar]
- 10.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH. Persistence of bacteria on antibiotic loaded acrylic depots: a reason for caution. Clin Orthop Relat Res. 1996;329:273–280. doi: 10.1097/00003086-199608000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124. doi: 10.1097/00003086-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. doi: 10.1007/s11999-010-1296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 15.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health. NIH Consensus Statement:Total hip replacement. 1994;12:1–31. [PubMed]
- 17.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wong worawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rand JA, Ilstrup DM. Survivorship analysis of total knee arthroplasty: cumulative rates of survival of 9200 total knee arthroplasties. J Bone Joint Surg Am. 1991;73:397–409. [PubMed] [Google Scholar]
- 20.Sorli L, Puig L, Torres-Claramunt R, Gonzalez A, Alier A, Knobel H, Salvado M, Horcajada JP. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. J Bone Joint Surg Br. 2012;94:249–253. doi: 10.1302/0301-620X.94B2.27779. [DOI] [PubMed] [Google Scholar]
- 21.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.