Abstract
Age-related decline in dopamine receptor levels has been observed in regional studies of animal and human brains; however, identifying specific cellular substrates and/or alterations in distinct neuronal populations remains elusive. To evaluate whether age-related decreases in dopamine receptor subtypes are associated with specific cell populations in the hippocampus and entorhinal cortex, antisense RNA amplification was combined with cDNA array analysis to examine effects of aging on D1–D5 dopamine receptor mRNA expression levels in hippocampal CA1 pyramidal neurons and entorhinal cortex layer II stellate cells from post-mortem human brains (19–92 years). In CA1 pyramidal neurons, significant age-related decline was observed for dopamine receptor mRNAs (D1–D4, P < 0.001; D5, P < 0.05) but not for the cytoskeletal elements β-actin, three-repeat (3R) tau, and four-repeat (4R) tau. In contrast, no significant changes were observed in stellate cells across the same cohort. Thus, senescence may be a factor responsible for cell-specific decrements in dopamine receptor gene expression in one population of neurons within a circuit that is critical for learning and memory. Furthermore, these results support the hypothesis that alterations in dopaminergic function may also be related to behavioral abnormalities, such as psychosis, that occur with aging.
Indexing terms: aging, hippocampus, entorhinal cortex, gene expression, microarray
Dopaminergic neurotransmission in the CNS is involved in a number of functions, most notably cognition, affect, and motor control. Increased life expectancy, resulting from recent medical advances, has led to a need for furthering understanding of neurobiology of aging and causes of age-related disabilities in order to improve quality of life (Drachman, 1997). Region-specific changes in the functional integrity of the dopaminergic system have been associated with impairment of motor and cognitive function in humans resulting from age. For example, unbiased estimation studies demonstrate age-related losses in sub-stantia nigra pars compacta (SNpc) neurons, a major source of forebrain dopaminergic projections (Fearnley and Lees, 1991; Ma et al., 1999b). Furthermore, immunohistochemistry and gene expression studies reveal down-regulation of the dopamine (DA) transporter in the SNpc of aged humans and monkeys (Bannon et al., 1992; Bannon and Whitty, 1997; Emborg et al., 1998; Ma et al., 1999a). A loss of DA itself has been demonstrated in normal aging as well (Goldman-Rakic and Brown, 1981; Fearnley and Lees, 1991). Moreover, age-related decreases in D1 and D2 DA receptor subtypes have been demonstrated within the aged human forebrain, including the striatum and hippocampus, by using in vivo imaging techniques combined with DA-selective ligands (Rinne et al., 1993; Volkow et al., 1996; Kaasinen et al., 2000) and in post-mortem studies (Severson et al., 1982; Rinne et al., 1993; Joyce et al., 1998). Thus, pre- and postsynaptic alterations in nigrostriatal dopaminergic circuitry exist in the aging brain and may account for alterations in motor function (Fearnley and Lees, 1991; Emborg et al., 1998).
The hippocampal formation is another brain region that receives dopaminergic projections from the mesencephalon, specifically the vental tegmentum (Scatton et al., 1980; Verney et al., 1985), and is also vulnerable to aging. Previous studies in rats have demonstrated significant age-related decreases in DA levels (Godefroy et al., 1989; Miguez et al., 1999) and DA receptors (Amenta et al., 2001) in the hippocampus of aged rats. Parallel alterations in humans have been demonstrated in brain regions associated with cognition during aging, including the hippocampus and temporal neocortex (Rinne, 1987; Seeman et al., 1987; Camps et al., 1989; Cortes et al., 1989; Rinne et al., 1990; Kaasinen et al., 2000; Inoue et al., 2001). At present, few primary data exist regarding the cellular specificity of age-related decline of DA receptor expression within the human temporal lobe. Primary difficulties in evaluating DA receptor subtypes in temporal lobe and other cortical regions include a moderate DA receptor subtype density and the paucity of high-affinity/receptor-selective ligands for D1–D5 DA receptors. Alternatively, use of gene expression technologies provides the means to evaluate selectively all DA receptor subtypes in discrete brain regions. For example, Meador-Woodruff and colleagues (Meador-Woodruff, 1994; Meador-Woodruff et al., 1996) have demonstrated the presence of mRNAs encoding the five known DA receptors in the human hippocampal formation by using in situ hybridization. These studies suggest a heterogeneous distribution within subfields of the hippocampal formation and laminae of the temporal lobe. However, the relative abundance of DA receptor mRNAs in specific neuronal populations making up these subregions remains elusive. In situ hybridization allows the analysis of a given mRNA in a single neuron, yet the sensitivity may not allow the analysis of low-abundance mRNAs or the means to evaluate numerous transcripts within the same tissue section. Moreover, reliance on regional assessment of gene expression emphasizes transcripts contained in the majority of neuronal and glial populations and/or transcripts in highest abundance in the region, which may not adequately reflect alterations in gene expression in target neuronal populations. Single-cell molecular biological procedures allow precise localization of changes in gene expression within brain regions (Eberwine et al., 1992; Surmeier et al., 1996; Ginsberg et al., 1999, 2000; Hemby et al., 2002). In the present study, single-cell gene expression procedures were used to assess the relative abundance of DA receptor mRNA levels in CA1 pyramidal neurons and entorhinal cortex (EC) layer II stellate neurons from human post-mortem brain tissue, permitting precise dissection and detailed molecular characterization of specific neuronal populations that are the principal neuronal conduits of information to the hippocampal-entorhinal circuit.
MATERIALS AND METHODS
Subjects
Post-mortem human brain specimens were obtained at autopsy from 18 neurologically normal individuals from the established brain collection of the Center for Neurodegenerative Disease Research, University of Pennsylvania School of Medicine, and all of the tissue samples were harvested using the same methods and procedures (Table 1). Ages ranged from 19 to 92 years; 10 subjects were male, and eight were female. Gross and microscopic diagnostic neuropathologic examinations, which included examination of multiple cortical and subcortical regions, were performed in all cases, and no neuropathological abnormalities relevant to mental status were found. All examined patients were without history of neurological or major psychiatric illness.
TABLE 1.
Clinical Characteristics of Subjects1
| Subject | Gender | Age (years) | PMI (hours) | Brain wt (g) | Cause of death | Final diagnosis | SP | NFT | LB | Braak stage | Other pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | F | 89 | 7 | 1,000 | Cardiac arrest | Hypertensive heart disease | Rare hip, ctx | Rare amyg, hip, sub | n.d. | I–II | None |
| C2 | F | 81 | 8 | 1,020 | Pulmonary artery embolism | Mild CHF | n.d. | Rare | n.d. | I–II | None |
| C3 | M | 92 | 9 | 1,090 | Postop (hip replace surgery) CHF; renal failure | Diabetes; hypertension | n.d. | Rare amyg, EC, hip | n.d. | I–II | Small Hemorrhagic infarct-RT fr ctx |
| C4 | M | 70 | 19 | 1,490 | CHF | CHF; diabetes; end- stage alcoholic cirrhosis | rare | n.d. | n.d. | 0 | None |
| C5 | M | 64 | 15 | 1,600 | Postop pacemaker failure | Aortic aneurism; hypertension | n.d. | Rare | n.d. | I–II | None |
| C6 | M | 49 | 5 | 1,300 | Pneumonia postlung transplant; ARDS | Pulmonary fibrosis | n.d. | n.d. | n.d. | 0 | None |
| C7 | M | 59 | 14 | 1,340 | Pulmonary embolis | Obesity; diabetes | n.d. | Rare | n.d. | I–II | None |
| C8 | F | 36 | 6 | 1,280 | Arrythmia; myocarditis | Obesity; diabetes | n.d. | n.d. | n.d. | 0 | None |
| C9 | F | 38 | 3.5 | NA | Multiorgan failure; ARDS | Postop sepsis; hysterectomy | n.d. | n.d. | n.d. | 0 | None |
| C10 | M | 19 | 16.5 | 1,500 | NA | NA | n.d. | n.d. | n.d. | 0 | None |
| C11 | F | 74 | 6 | 1,250 | Infection; pneumonia; renal failure | Hypertension; renal insufficiency; peripheral vascular disease | n.d. | Rare | n.d. | I–II | Microscopic cortical infarcts |
| C12 | F | 83 | 22 | 1,100 | Ischemia; myocardial infarction | Peripheral vascular disease; renal insufficiency | n.d. | Rare; amyg, EC, hip | n.d. | I–II | None |
| C13 | M | 71 | 13 | 1,300 | Renal failure | Metastatic adenocarcinoma | Rare | Rare | n.d. | I–II | None |
| C14 | M | 65 | 26 | 1,350 | Cardiac arrest; ischemia; ventricular arrythmia | Chronic ischemia; CHF; diabetes; hypertension | n.d. | Rare | n.d. | I–II | None |
| C15 | F | 90 | 8 | 1,260 | NA | NA | Rare | Amyg, EC, hip | n.d. | III–IV | None |
| C16 | M | 62 | 5 | 1,360 | Hypotension; septic fungemia | Chronic myelogenous leukemia | n.d. | Rare | n.d. | I–II | None |
| C17 | F | 74 | 3.5 | 1,000 | Complications of metastatic colon cancer | Metastatic colon cancer | n.d. | Rare; amyg, EC, hip | n.d. | I–II | None |
| C18 | M | 75 | 17 | 1,390 | Ischemic event; acute renal failure | CHF; coronary artery disease | Rare | Rare | n.d. | I–II | None |
ARDS, adult respiratory distress syndrome; amyg, amygdala; CHF, congestive heart failure; ctx, cortex; EC, entorhinal cortex; final dx, final diagnosis; hip, hippocampus; LB, Lewy bodies; NA, not available; n.d., none detected; NFT, neurofibrillary tangles; SP, senile plaques; sub, subiculum.
Immunocytochemistry
Tissue blocks, which included the middle portion of the EC (subfield EI), were dissected from the temporal lobe at autopsy, fixed in ethanol (70%/150 mM NaCl), embedded in paraffin, and cut into 6 μm sections, as described previously (Ginsberg et al., 1999, 2000; Hemby et al., 2002). One section from each individual was stained with acridine orange to verify the presence of nucleic acids in the tissue (Ginsberg et al., 1997, 1998; Hemby et al., 2002). To identify individual neurons for subsequent single-cell analysis, immunocytochemistry was performed on sections using a monoclonal antibody to nonphosphorylated neurofilaments (RmdO20; Ginsberg et al., 1999, 2000; Hemby et al., 2002). The antibody was labeled with the avidin-biotin method (ABC Vectastain; Vector Laboratories) and visualized with 3,3′-diaminobenzidine.
Single-cell gene expression
After immunolabeling, a 66-base-pair oligo(dT)-T7 primer/promoter [AAACGACGGCCAGTGAATTGTAAT-ACGACTCACTATAGGCGC(T)24] was hybridized to poly(A+) mRNA overnight in 50% formamide/5× SSC at 25°C in a humidifying chamber. cDNA was synthesized directly on the tissue sections (in situ transcription) using avian myeloblastosis virus reverse transcriptase (AMVRT; 0.5 U/μl; Seigagaku America) in Tris buffer (pH 8.3) containing 6 mM MgCl2; 120 mM KCl; 7 mM dithio-threitol (DTT); 250 μM each of dATP, dCTP, dGTP, and TTP; and 0.12 U/μl of RNAsin (Tecott et al., 1988). Tissue sections were incubated in the aforementioned solution at 37°C for 90 minutes, washed twice in 2× SSC at 25°C for 5 minutes, and stored at 4°C in 0.5× SSC for up to 72 hours. After in situ transcription, layer II/II stellate neurons and CA1 pyramidal neurons were dissected using a micropipette attached to a micromanipulator under high-power objective field (40×). Although there was minimal disruption of the surrounding neuropil, the possibility exists that mRNA of other cells within the vicinity of the dissection may have been included in the analysis. Contents were collected in the pipette and emptied into 1.5 ml microcentrifuge tubes for second-strand cDNA synthesis and subsequent aRNA amplification. The amplification and reamplification procedures have been described in detail elsewhere (Eberwine et al., 1992, 1998; Ginsberg et al., 2000; Hemby et al., 2002). During the second round of amplification, 33P-UTP was incorporated for the RNA probes from each subject destined for custom-designed cDNA array hybridization. Under optimal conditions, the first round of aRNA amplification results in approximately a 1,000-fold yield, and two rounds of amplification result in approximately a 106-fold increase over the original amount of each poly(A+) mRNA. The aRNA procedure is a linear amplification process with minimal change in the relative abundance of the mRNA population in the native state of the neuron. mRNA can be reliably amplified from small amounts of fixed tissue including individual neurons (Ginsberg et al., 1999, 2000; Hemby et al., 2002).
Custom-designed cDNA array analysis
Custom-designed cDNA arrays were synthesized using 1 μg of linearized plasmid cDNAs/ESTs cross-linked to nylon membranes (Hybond XL; Amersham Biosciences, Arlington Heights, IL). Arrays contained D1–D5 DA receptor subtypes (Table 2). Clones for the DA receptor subytpes contained sequences specific for the seven-transmembrane-spanning region of the respective receptor cDNAs. Comparisons of the cDNA sequences used for this study revealed no homology among the receptor subtypes. In addition to the DA receptor cDNAs, approximately 220 cDNAs encoding transcripts for classes of genes, including cytoskeletal elements, neurotrophins, glutamate receptors, protein phosphatases/kinases, and synaptic markers (Ginsberg et al., 1999, 2000; Hemby et al., 2002) were included on the array. Each cDNA/EST on the custom-designed cDNA arrays was verified by restriction digestion and sequence analysis.
TABLE 2.
Dopamine Receptor Subtype cDNAs
| cDNA | Product (bp) | Accession No./position |
|---|---|---|
| D1 | 166 | M35077.1/710–875 |
| D2 | 226 | M36831.1/841–1,066 |
| D3 | 278 | NM 017140.1/745–1,022 |
| D41 | 365 | NM 012944.1/1,045–1,409 |
| D52 | 764 | M69118.1/690–1,454 |
Courtesy of Karen O’Malley, Washington University.
Courtesy of Marc Caron, Duke University.
Custom-designed cDNA arrays were hybridized for 24 hours at 44°C in a rotisserie hybridization oven (Hybaid) with the following solution: 50% formamide, 6× SPPE, 5× Denhardt’s solution, 0.1% sodium dodecyl sulfate (SDS), 200 ng/ml sheared salmon sperm, and 1.0 mM sodium pyrophosphate. After hybridization, membranes were washed sequentially with 2× SSC/0.1% SDS, 0.5× SSC/0.1% SDS, and 0.1× SSC/0.1% SDS for 20 minutes each at 44°C. Labeled hybridized products were detected using phosphoimager cassettes, and hybridization signal intensities were analyzed using ImageQuant software (Amersham Biosciences).
Data analysis
Densitometry values (hybridization intensities) were obtained for each clone and subtracted from the value obtained for vector only (pBluescriptII) as well as background (nonspecific) hybridization on the array. The net densitometry value was divided by the summed values for all of the clones on the array (global normalization) to yield a normalized value for each clone on the array, thereby minimizing variations resulting from differences in the specific activity of the probe and the absolute quantity of probe present (Ginsberg et al., 2000; Hemby et al., 2002). Data were analyzed using multiple linear regression analysis with age as the dependent variable and gene expression as the independent variable. The null hypothesis was rejected at P < 0.05.
RESULTS
Examination of neurofilament-immunoreactive tissue sections of the human temporal lobe revealed a distinct laminar pattern of immunoreactivity confined to the somatodendritic region of neurons in layers II/III and V of the EC and Ammon’s horn subfields of the hippocampal formation. No apparent differences in the staining intensity or reaction product distribution were observed between the cell groups. Acridine orange staining did not reveal any significant differences in the presence of nucleic acid content between the two cell groups, which is similar to previous observations by our group (Ginsberg et al., 1997, 1998).
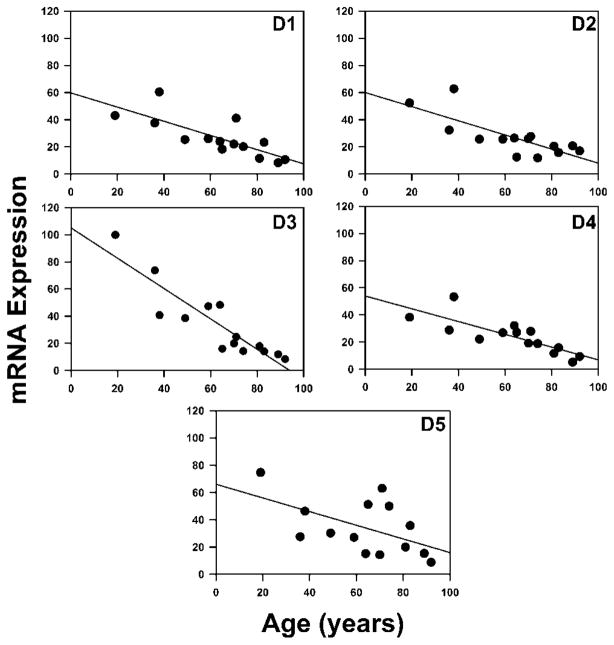
mRNAs encoding all five of the DA receptors were detected in all CA1 pyramidal and layer II/III EC stellate neurons in the present study. Relative abundance of the DA receptor subtype mRNAs varied slightly between CA1 pyramidal neurons (D3 > D5 > D1 > D2 > D4) and layer II stellate cells (D3 > D5 > D2 > D1, D4). In contrast, no difference in relative expression was observed between the two cell populations for the cytoskeletal elements β-actin and tau. A significant age-related decline was found in CA1 pyramidal neurons for D1 (y = −0.51 · age + 58.92, P < 0.001, R = 0.764), D2 (y = −0.52 · age + 60.24, P < 0.001, R = 0.775), D3 (y = −1.11 · age + 103.69, P < 0.01, R = 0.895), D4 (y = −0.48 · age + 53.24, P < 0.001, R = 0.789), and D5 (y = −0.54 · age + 65.36, P < 0.028; R = 0.533) DA receptor mRNAs (Fig. 1). The percentage declines per decade for each receptor subtype were 5.2%, 5.0%, 11.2%, 4.7%, and 5.0%, respectively. In contrast to CA1 pyramidal neurons, no significant age-related changes were observed in EC layer II stellate neurons for D1 (y = 0.02 · age + 9.48, P = 0.86, R = 0.06), D2 (y = −0.11 · age + 25.88, P = 0.31, R = 0.31), D3 (y = −0.32 · age + 54.79, P = 0.31, R = 0.31), D4 (y = 0.07 · age + 11.58, P = 0.61, R = 0.16), or D5 (y = −0.12 · age + 34.13, P = 0.56, R = 0.12) subtypes (Fig. 2).
Fig. 1.
Age-dependent assessment of DA receptor mRNA levels in hippocampal CA1 pyramidal neurons. mRNA expression values correspond to hybridization signal intensity for individual transcripts divided by the total blot hybridization signal intensity · 100. Symbols represent relative abundance of mRNAs for the transcripts for each subject.
Fig. 2.
Age-dependent assessment of DA receptor mRNA levels in EC layer II stellate cells. mRNA expression values correspond to hybridization intensity for individual transcripts divided by the total blot hybridization intensity · 100. Symbols represent relative abundance of mRNAs for the respective transcripts for each subject. In contrast to the case for CA1 pyramidal neurons, no significant age-related decrease in DA receptor expression was found.
β-Actin and tau mRNA abundances were also assessed in these neuronal populations in order to evaluate the specificity of transcript regulation. No significant age-related differences were observed for β-actin in CA1 neurons (y = 0.03 · age + 17.17, P = 0.802, R = 0.07) or EC layer II stellate cells (y = 0.07 · age + 10.66, P = 0.496, R = 0.21). Similarly, there were no significant differences in the abundance of tau in CA1 neurons (4Rtau: y = 0.007 · age + 23.21, P = 0.962, R = 0.01; 3Rtau: y = 0.03 · age + 17.99, P = 0.578, R = 0.16) or layer II stellate cells (4Rtau: y = 0.05 · age + 8.23, P = 0.653, R = 0.14; 3Rtau: y = −0.18 · age + 32.11, P = 0.16, R = 0.45). These observations suggest a selective age-related decline in DA receptor subtypes rather than a global down-regulation of several classes of transcripts (Fig. 3).
Fig. 3.
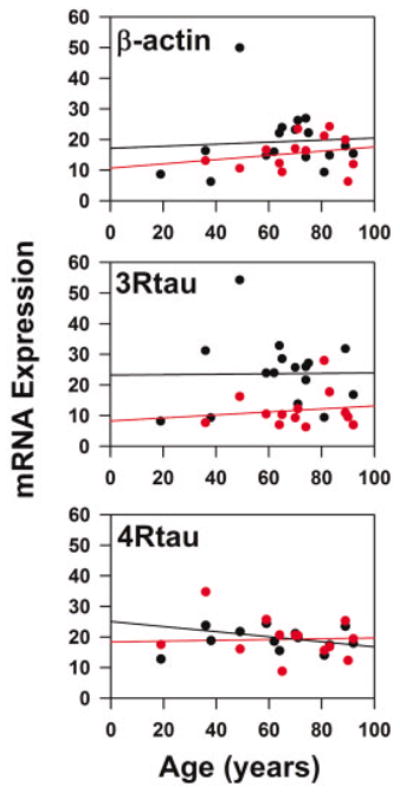
Relationship of β-actin, 4Rtau, and 3Rtau mRNA expression in CA1 and stellate cells from EC layers II/III to age. Symbols represent relative abundance of mRNAs for the transcripts for each subject for CA1 (red dots) and EC layer II stellate cells (black dots).
DISCUSSION
The present study demonstrates a significant age-related decline in DA receptor subtype mRNAs in CA1 pyramidal neurons but not EC layer II stellate cells in clinically normal human post-mortem brain tissues. The localization of DA receptor mRNAs to human hippocampal and EC neurons is consistent with observations using alternative molecular-based techniques (e.g., in situ hybridization histochemistry) in human brain (Meador-Woodruff, 1994; Meador-Woodruff et al., 1996) and rodent brain (Ciliax et al., 1994; Meador-Woodruff et al., 1994). All five of the cloned DA receptor subtype mRNAs were coexpressed in both CA1 pyramidal neurons and EC layer II stellate neurons. Slight differences in the relative abundance of DA receptor subtype mRNAs were observed between CA1 pyramidal neurons (D3 > D5 > D2, D1 > D4) and layer II stellate cells (D3 > D5 > D2 > D1, D4), variations that likely reflect intrinsic differences between the individual cell types, in that many classes of transcripts (e.g., synaptic markers, neurotrophin receptors) have been shown to differ across individual cell types as well as within discrete subregions (Ginsberg et al., 1999, 2000; Hemby et al., 2002). Importantly, in addition to providing expression profile-based evidence of the presence of the five DA receptor mRNAs in hippocampal neurons, this report demonstrates a selective, cell-type-specific, direct association between age and decreased DA receptor mRNA levels in individual neurons obtained post-mortem from cognitively normal human subjects.
Age-related impairments of motor and cognitive function in humans have been associated with regional-specific changes in DA function, primarily in the nigrostriatal pathway (Kane et al., 1982; Odenheimer et al., 1994; Volkow et al., 1996). Age-related loss of SNpc neurons and decreased striatal DA neurotransmitter levels have also been observed (Goldman-Rakic and Brown, 1981; Fearnley and Lees, 1991; Ma et al., 1999a). Furthermore, gene expression studies have revealed decreased DA transporter mRNA in the SNpc of aged humans (Bannon et al., 1992; Bannon and Whitty, 1997) and complementary decreases in DA transporter and tyrosine hydroxylase immunoreactivity in humans (Ma et al., 1999a) and nonhuman primates (Emborg et al., 1998). However, others report no significant loss of midbrain DA neurons with age (Muthane et al., 1998; Kubis et al., 2000). Nevertheless, alterations in the SNpc are paralleled by numerous studies reporting age-related declines in striatal D1-like and D2-like receptor binding sites shown with in vivo imaging techniques (Rinne et al., 1990, 1993; Antonini and Leenders, 1993; Iyo and Yamasaki, 1993; Antonini et al., 1995; Volkow et al., 1996, 1998, 2000; Wong et al., 1997; Ichise et al., 1998; Wang et al., 1998) and from post-mortem studies (Severson et al., 1982; Rinne, 1987; Rinne et al., 1990).
As noted previously, alterations in hippocampal DA function may be related to memory formation and related cognitive functions (Squire and Zola-Morgan, 1991; Zola-Morgan and Squire, 1993; Zola-Morgan et al., 1994; Leonard et al., 1995). Disruption of the functional integrity of DA neurotransmission in these regions has been correlated with the pathophysiology of schizophrenia (Akil et al., 2000; Bigliani et al., 2000; Xiberas et al., 2001) and Alzheimer’s disease (AD; Ryoo and Joyce, 1994; Joyce et al., 1998; Volkow et al., 1998; Ginsberg et al., 2000) and may contribute to the cognitive decline associated with normal aging (Volkow et al., 1998). Previous studies have demonstrated significant age-related decreases in DA transmission (Godefroy et al., 1989; Miguez et al., 1999; Amenta et al., 2001) in the hippocampus of aged rats, and simlar alterations have been demonstrated in the hippocampus and temporal neocortex of humans (Rinne, 1987; Seeman et al., 1987; Rinne et al., 1990; Kaasinen et al., 2000; Inoue et al., 2001). Recently, two studies have reported age-related decreases in D2/D3 receptor density in hippocampus (10–12% decline per decade) and temporal cortex (9–12% decline per decade) by using positron emission tomography and [11C]FLB 457 (Kaasinen et al., 2000; Inoue et al., 2001). These studies provide important information on age-related changes in DA receptor density in the human hippocampal formation. However, a comprehensive evaluation of DA receptor regulation in humans is limited by the specificity of available ligands for D1–D5 receptors and the ability to discern changes in specific neuronal populations within these regions. The present study extends previous observations at the regional/binding site level to include cell-specific localization of DA receptors. Down-regulation of D1, D2, and D3 receptors is consistent with imaging and binding studies; however, age-related decreases in D4 and D5 receptor mRNAs are a novel finding because of the ability to discriminate DA receptor subtypes based on sequence composition as well as cellular morphology and localization of discrete cell types within the hippocampal formation. Future studies are warranted to determine the localization of D4 and D5 receptor subtypes in the hippocampal formation and the potential quantitative and qualitative alterations in these receptors during ageing and disease, provided that subtype-selective and/or -specific ligands become available.
The reliability of mRNA levels as an indicator of protein levels and the functional role of proteins in cellular function is variable. Translational accessibility, posttranslational modifications, and phosphorylation states of proteins can all disrupt the balance between mRNA and functional protein. The absence of age-related decreases in layer II stellate neurons does not imply a lack of functional alterations or that involvement in a protein is not occurring, or vice versa that a differentially expressed mRNA is involved. Though not assessing protein levels, gene expression strategies provide the specificity to evaluate each of the DA receptor subtypes in defined brain regions. Moreover, single-cell RNA amplification procedures combined with microarrays allows the evaluation of coordinate changes in the expression of multiple transcripts from discrete neuronal populations within regions (Ginsberg et al., 1999, 2000; Hemby et al., 2002). The present results extend previous imaging studies by demonstrating neuron-specific age-related decreases in all of the DA receptor subtype mRNAs. The demonstration of cell-type-specific, age-related decreases in DA receptor subtypes is particularly important when attempting to compare relative expression levels between cognitively normal control subjects and patients with neurodegenerative or neuropsychiatric disorders. Specifically, extra care must be taken to age match subjects appropriately or, at a minimum, have no significant differences in age across the groups. Otherwise, age-related decline in DA receptor subtype mRNAs may obscure data analysis through increased variation in the control and/or disease groups. Importantly, age-related decline in DA receptors (observed in normal and/or diseased subjects) may not be involved with mechanisms underlying the pathophysiology of a disease and may reflect changes specific to senescence. Notwithstanding this caveat, our group has demonstrated a clear down-regulation of the five DA receptor subtypes in neurofibrillary-tangle-bearing CA1 pyramidal neurons in AD brains vs. nontangle-bearing CA1 neurons in age-matched controls (Ginsberg et al., 2000).
The results of the present study identify a selective, age-related alteration in receptor subtypes of a single neurotransmitter system that may affect the functional integrity of hippocampal circuitry. For example, decreased expression of DA receptor mRNAs in CA1 pyramidal neurons may result in decreased receptor protein levels in the CA1 region and/or CA1 projection areas, subiculum, and to a lesser extent EC. Future studies should include a detailed immunohistochemical assessment of CA1 perikarya and terminal fields to determine the degree of correlation between D1–D3 DA receptor subtype mRNA and protein levels. As mentioned previously, similar assessments for D4 and D5 DA receptor subtypes is dependent on the future availability of selective and specific antibodies and ligands. Evidence indicates decreased densities of D1 and D2 receptors in the hippocampus that are age related (Kaasinen et al., 2000, 2002; Inoue et al., 2001); however, the resolution of these techniques obviates the assessment of DA receptor subtypes at the subregional level. By contrast, lack of age-related decreases in DA receptor mRNAs in entorhinal stellate cells does not indicate that this neuronal population was unaffected by the aging process. On the contrary, there were significant alterations in synaptic proteins and glutamate receptors in a single-cell survey of stellate cells in aged schizophrenics (Hemby et al., 2002). Future studies are warranted to determine whether these or other transcripts may be preferentially dysregulated in this cell population with aging.
In summary, the present study provides direct evidence of age-related decreases in DA receptor mRNAs that are cell type specific within the hippocampal formation. Understanding alterations in the functional integrity of dopaminergic neurotransmission within the hippocampal formation and other forebrain regions is a critical step in the development and/or refinement of pharmacotherapies that may delay the onset and/or prevent some of the psychopathology associated with senescence. Future characterization of altered expression patterns for thousands of genes via molecular fingerprinting techniques in homogeneous cell populations within the human brain, as well as the brains of appropriate animal models, will provide a panoramic view of the potential molecular underpinnings of aging.
Acknowledgments
Grant sponsor: National of Institute of Health; Grant number: DA013772; Grant number: NS043939; Grant number: AG10668; Grant number: AG09215; Grant number: AG10124; Grant sponsor: Stanley Foundation; Grant sponsor: Alliance for Autism Research; Grant sponsor: Alzheimer’s Association; Grant number: NIRG-00-2250.
We thank Mr. J. Le and Dr. S. Che for technical assistance and the staff of the Center for Neurodegenerative Disease Research and the Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, for assistance in case accrual and evaluation. We also express our appreciation to the families of the patients studied, who made this research possible. Disclosures: J.Q.T. is a Founding Scientist and consultant for Layton Biosciences, which has licensed the aRNA amplification and in situ transcription methodologies from the University of Pennsylvania, where J.Q.T. is a faculty member.
LITERATURE CITED
- Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry. 2000;47:361–370. doi: 10.1016/s0006-3223(99)00282-6. [DOI] [PubMed] [Google Scholar]
- Amenta F, Mignini F, Ricci A, Sabbatini M, Tomassoni D, Tayebati SK. Age-related changes of dopamine receptors in the rat hippocampus: a light microscope autoradiography study. Mech Ageing Dev. 2001;122:2071–2083. doi: 10.1016/s0047-6374(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders KL. Dopamine D2 receptors in normal human brain: effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Ann N Y Acad Sci. 1993;695:81–85. doi: 10.1111/j.1749-6632.1993.tb23033.x. [DOI] [PubMed] [Google Scholar]
- Antonini A, Vontobel P, Psylla M, Gunther I, Maguire PR, Missimer J, Leenders KL. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Arch Neurol. 1995;52:1183–1190. doi: 10.1001/archneur.1995.00540360061017. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48:969–977. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci USA. 1992;89:7095–7099. doi: 10.1073/pnas.89.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigliani V, Mulligan RS, Acton PD, Ohlsen RI, Pike VW, Ell PJ, Gacinovic S, Kerwin RW, Pilowsky LS. Striatal and temporal cortical D2/D3 receptor occupancy by olanzapine and sertindole in vivo: a [123I]epidepride single photon emission tomography (SPET) study. Psychopharmacology. 2000;150:132–140. doi: 10.1007/s002130000435. [DOI] [PubMed] [Google Scholar]
- Camps M, Cortes R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989;28:275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Hersch SM, Levey AI. Immunocytochemical localization of D1 and D2 receptors in rat brain. In: Niznik HB, editor. Dopamine receptors and transporters. New York: Marcel Dekker; 1994. pp. 383–400. [Google Scholar]
- Cortes R, Gueye B, Pazos A, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D1 sites. Neuroscience. 1989;28:263–273. doi: 10.1016/0306-4522(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Aging and the brain: a new frontier. Ann Neurol. 1997;42:819– 828. doi: 10.1002/ana.410420602. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwine J, Crino P, Arnold S, Trojanowski J, Hemby S. Molecular analysis of the single cell: importance in the study of psychiatric disorders. In: Watson SJ, editor. Psychopharmacology: fifth generation of progress [CD-ROM] New York: Lippincott-Raven Press; 1998. [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Crino PB, Lee VM, Eberwine JH, Trojanowski JQ. Sequestration of RNA in Alzheimer’s disease neurofibrillary tangles and senile plaques. Ann Neurol. 1997;41:200–209. doi: 10.1002/ana.410410211. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Galvin JE, Chiu TS, Lee VM, Masliah E, Trojanowski JQ. RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol. 1998;96:487–494. doi: 10.1007/s004010050923. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Crino PB, Hemby SE, Weingarten JA, Lee VM, Eberwine JH, Trojanowski JQ. Predominance of neuronal mRNAs in individual Alzheimer’s disease senile plaques. Ann Neurol. 1999;45:174–181. [PubMed] [Google Scholar]
- Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Ann Neurol. 2000;48:77–87. [PubMed] [Google Scholar]
- Godefroy F, Bassant MH, Weil-Fugazza J, Lamour Y. Age-related changes in dopaminergic and serotonergic indices in the rat forebrain. Neurobiol Aging. 1989;10:187–190. doi: 10.1016/0197-4580(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch Gen Psychiatry. 2002;59:631–640. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Ichise M, Ballinger JR, Tanaka F, Moscovitch M, St George-Hyslop PH, Raphael D, Freedman M. Age-related changes in D2 receptor binding with iodine-123-iodobenzofuran SPECT. J Nucl Med. 1998;39:1511–1518. [PubMed] [Google Scholar]
- Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T, Yoshikawa K, Tanada S. Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci. 2001;69:1079–1084. doi: 10.1016/s0024-3205(01)01205-x. [DOI] [PubMed] [Google Scholar]
- Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415–421. doi: 10.1016/0278-5846(93)90075-4. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Myers AJ, Gurevich E. Dopamine D2 receptor bands in normal human temporal cortex are absent in Alzheimer’s disease. Brain Res. 1998;784:7–17. doi: 10.1016/s0006-8993(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Kemppainen N, Nagren K, Helenius H, Kurki T, Rinne JO. Age-related loss of extrastriatal dopamine D(2)-like receptors in women. J Neurochem. 2002;81:1005–1010. doi: 10.1046/j.1471-4159.2002.00895.x. [DOI] [PubMed] [Google Scholar]
- Kane JM, Weinhold P, Kinon B, Wegner J, Leader M. Prevalence of abnormal involuntary movements (“spontaneous dyskinesias”) in the normal elderly. Psychopharmacology. 1982;77:105–108. doi: 10.1007/BF00431929. [DOI] [PubMed] [Google Scholar]
- Kubis N, Faucheux BA, Ransmayr G, Damier P, Duyckaerts C, Henin D, Forette B, Le Charpentier Y, Hauw JJ, Agid Y, Hirsch EC. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain. 2000;123:366–373. doi: 10.1093/brain/123.2.366. [DOI] [PubMed] [Google Scholar]
- Leonard BW, Amaral DG, Squire LR, Zola-Morgan S. Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. J Neurosci. 1995;15:5637–5659. doi: 10.1523/JNEUROSCI.15-08-05637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J Comp Neurol. 1999a;409:25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Ma SY, Roytt M, Collan Y, Rinne JO. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol. 1999b;25:394–399. doi: 10.1046/j.1365-2990.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH. Update on dopamine receptors. Ann Clin Psychiatry. 1994;6:79–90. doi: 10.3109/10401239409148986. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, Sanchez-Barcelo E. Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Neural Transmiss Gen Sect. 1999;106:1089–1098. doi: 10.1007/s007020050225. [DOI] [PubMed] [Google Scholar]
- Muthane U, Yasha TC, Shankar SK. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann Neurol. 1998;43:283–287. doi: 10.1002/ana.410430304. [DOI] [PubMed] [Google Scholar]
- Odenheimer G, Funkenstein HH, Beckett L, Chown M, Pilgrim D, Evans D, Albert M. Comparison of neurologic changes in ‘successfully aging’ persons vs the total aging population. Arch Neurol. 1994;51:573–580. doi: 10.1001/archneur.1994.00540180051013. [DOI] [PubMed] [Google Scholar]
- Rinne JO. Muscarinic and dopaminergic receptors in the aging human brain. Brain Res. 1987;404:162–168. doi: 10.1016/0006-8993(87)91367-9. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res. 1990;508:349–352. doi: 10.1016/0006-8993(90)90423-9. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- Ryoo HL, Joyce JN. Loss of dopamine D2 receptors varies along the rostrocaudal axis of the hippocampal complex in Alzheimer’s disease. J Comp Neurol. 1994;348:94–110. doi: 10.1002/cne.903480105. [DOI] [PubMed] [Google Scholar]
- Scatton B, Simon H, Le Moal M, Bischoff S. Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980;18:125–131. doi: 10.1016/0304-3940(80)90314-6. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399– 404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Severson JA, Marcusson J, Winblad B, Finch CE. Age-correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem. 1982;39:1623–1631. doi: 10.1111/j.1471-4159.1982.tb07996.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Barchas JD, Eberwine JH. In situ transcription: specific synthesis of complementary DNA in fixed tissue sections. Science. 1988;240:1661–1664. doi: 10.1126/science.2454508. [DOI] [PubMed] [Google Scholar]
- Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985;14:1039–1052. doi: 10.1016/0306-4522(85)90275-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, Pappas N. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998;44:143–147. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, Pappas N. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56– 61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wong DF, Pearlson GD, Tune LE, Young LT, Meltzer CC, Dannals RF, Ravert HT, Reith J, Kuhar MJ, Gjedde A. Quantification of neuroreceptors in the living human brain: IV. Effect of aging and elevations of D2-like receptors in schizophrenia and bipolar illness. J Cereb Blood Flow Metab. 1997;17:331–342. doi: 10.1097/00004647-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Xiberas X, Martinot JL, Mallet L, Artiges E, Loc HC, Maziere B, Paillere-Martinot ML. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503–508. doi: 10.1192/bjp.179.6.503. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]