Abstract
OBJECTIVE
Patients with idiopathic intracranial hypertension (IIH) have elevated intracranial pressure (ICP) without identifiable cause. The clinical course is variable, resulting in irreversible vision loss in some, and a benign course in others. While MRI findings have been described in IIH, their association with visual outcome has not been evaluated.
MATERIALS AND METHODS
46 patients with IIH underwent funduscopic evaluation, visual field testing, lumbar puncture with opening pressure (OP) measurement, and MRI. Patients were stratified into: Group 1: no vision loss (n=28), Group 2: some vision loss (n=10), and Group 3: severe vision loss (n=8). MRI findings in the orbits, pituitary gland, and optic canals, and frequency of skull base cephaloceles and transverse sinus stenosis (TSS) were assessed blinded to the patients’ visual outcome. Demographic, clinical, and MRI findings were evaluated for association with visual outcome.
RESULTS
Patients in group 3 (worst visual outcome) were significantly younger (P=0.03) and had higher OP (P=0.04) than the other groups. There were no significant differences in gender, race, or body mass index. Despite worse visual outcomes and sometimes fulminant vision loss, there were no differences in the frequency of orbital MRI findings, TSS, or in optic canal diameter and pituitary appearance among the three groups. Group 3 had significantly lower cephalocele frequency than the other groups (P=0.04).
CONCLUSION
While MRI findings may suggest elevated ICP and the diagnosis of IIH, they are not predictive of visual outcome in IIH.
Introduction
Idiopathic intracranial hypertension (IIH) is a syndrome of unknown etiology that results in elevated intracranial pressure (ICP) without an intracranial mass lesion, hydrocephalus, or abnormality of CSF composition.[1, 2] Patients with IIH may present with headaches, tinnitus, diplopia, or transient visual obscurations associated with papilledema.[3, 4] Symptoms can be improved with reduction of CSF pressure through pharmacologic therapy or CSF diversion procedures,[5] however, the overall prognosis in IIH is variable with chronically untreated elevated ICP leading to permanent vision loss in up to 10% of patients.[5, 6]
While by definition no structural cause for elevated ICP is identified on brain imaging,[1, 2] additional findings on CT and MRI are commonly seen in patients with IIH. In the orbits, these include flattening of the posterior sclera, distension of the peri-optic nerve subarachnoid CSF space, vertical tortuosity of the optic nerve sheath complex, and protrusion and/or enhancement of the prelaminar optic nerve.[7] An “empty” sella turcica manifest by varying degrees of flattening of the superior surface of the pituitary gland, CSF intensity signal in the sellar confines, and enlargement and remodeling of bony sella turcica, is frequently seen, presumably as an imaging correlate of chronically elevated intracranial pressure.[8, 9] Venographic imaging (commonly MRV) is performed to exclude dural venous sinus thrombosis which can mimic IIH in clinical presentation.[10] A large proportion of patients with IIH have bilateral TSS on MRV,[11–13] although it is still debatable whether it is the cause or the effect of elevated ICP.[14, 15] While the sensitivity and specificity of these MRI findings has been evaluated for the diagnosis of IIH,[9, 16] whether or not they portend a better or worse visual outcome has not been assessed. The purpose of this study is to determine the association of MRI findings with visual outcome in IIH.
Methods
Patients
Approval for this study was obtained from the institutional review board. Patients were identified using our clinical database of patients with IIH from January 2007 through January 2012. Patients were included if they had an MRI of the brain performed at our institution, and had visual field and lumbar puncture data available for review. All included patients were evaluated in a standardized fashion by one of three experienced neuro-ophthalmologists (BBB, VB, NJN), including documentation of body habitus, detailed neuro-ophthalmic examination with formal visual field testing (static perimetry using a Humphrey automated perimeter or kinetic perimetry using a Goldmann perimeter, and review of lumbar puncture data (including CSF OP).
Management strategies were similar for all patients included in this study, including diagnostic lumbar puncture performed in the lateral decubitus position, discontinuation of any medications possibly associated with the development of raised ICP, the use of acetazolamide with total daily dosages between 500 and 1500 mg, and recommendation for weight loss. Surgery (either CSF-shunting procedure or optic nerve sheath fenestration) was performed in fulminant forms, [17] patients with severe visual loss at presentation, when the visual function was abnormal at baseline and did not improve within a few weeks, or for intractable headaches clearly related to elevated ICP. None of our patients underwent endovascular venous stenting.
In order to assess Humphrey visual field and Goldmann visual field tests, we used a three-point scale to evaluate the severity of visual field loss in our patient population: the degree of final visual field loss was graded on a 0 to 2 point scale: 0) normal or enlarged blind spot, 1) mild or moderate visual field deficit, or 2) severe visual field deficit by consensus review of two neuro-ophthalmologists (BDR and BBB) masked to radiologic findings. Charts of eligible patients were reviewed to determine the clinical course. We classified all eligible patients into three groups: Group 1: good visual outcome with no visual field deficits, Group 2: good visual outcome with some visual field deficits, and Group 3: poor visual outcome with severe visual field deficits. Group 3, those considered to a have “poor” clinical course, included patients who had fulminant disease, had progressive visual field defects, or required a surgical procedure for IIH management. Patients with a “good” clinical course had none of the “poor” clinical features.
MRI Technique
Magnetic resonance imaging was performed at either 3.0-Tesla (Siemens Trio, Erlangen, Germany) or 1.5-Tesla (Siemens Avanto, Erlangen, Germany or GE Signa, Milwaukie, Wisconsin) using a standard head coil. All patients underwent standardized brain MRI protocol including pre-contrast axial DWI, T1-weighted, T2*-weighted, T2-weighted, and sagittal T1-weighted images. Images obtained were at 0.7 × 0.7-mm in plane resolution at a slice thickness of 5-mm. All but four (42/46) patients received intravenous gadolinium contrast material at a standard dose (0.1 mmol/kg) intravenous gadolinium based contrast agent (Multihance, Bracco Diagnostics Inc.), followed by post-contrast axial T1-weighted and sagittal volumetric magnetization prepared gradient recalled echo T1-weighted images (TR of 1900 ms, TE of 2–3 ms, and flip angle of 9–12 degrees with 1-mm isotropic voxels).
All but four (42/46) patients also underwent MRV concurrent with their MRI evaluation. 23 patients underwent contrast-enhanced MRV, and 19 patients underwent non-contrast MRV. The contrast-enhanced MRV protocol included an axial pre-contrast MRV mask (TR of 4–6 ms, TE of 1–2 ms, and flip angle of 22–30 degrees with slice thickness of 0.8–1.4 mm), followed by repetition of the sequence 60 seconds after contrast administration. The pre-contrast MRV dataset was subtracted from the post-contrast dataset, and multiple oblique maximum intensity projections (MIPs) were generated from this subtracted dataset with rotation around the craniocaudal axis (“spin”) or the transverse axis (“nod”) at 6 degree increments. The non-contrast MRV protocol consisted of an oblique sagittal 2D TOF (TR of 20, TE of 4.83, with slice thickness of 2.5-mm) from which rotating MIPs were generated.
Image Analysis
All MRI examinations were reviewed by an experienced neuroradiologist (AS) blinded to the clinical course of the patient and knowledge of demographic information or OP data. The imaging findings evaluated are depicted in figure 1. Previously described orbital imaging findings of protrusion of the ON head, enhancement of the ON head, flattening of the posterior sclera, increased peri-ON CSF, and vertical tortuosity of the intro-orbital ON were recorded for all patients (except enhancement of the ON head in the four patients who did not receive intravenous contrast material for their MRI).
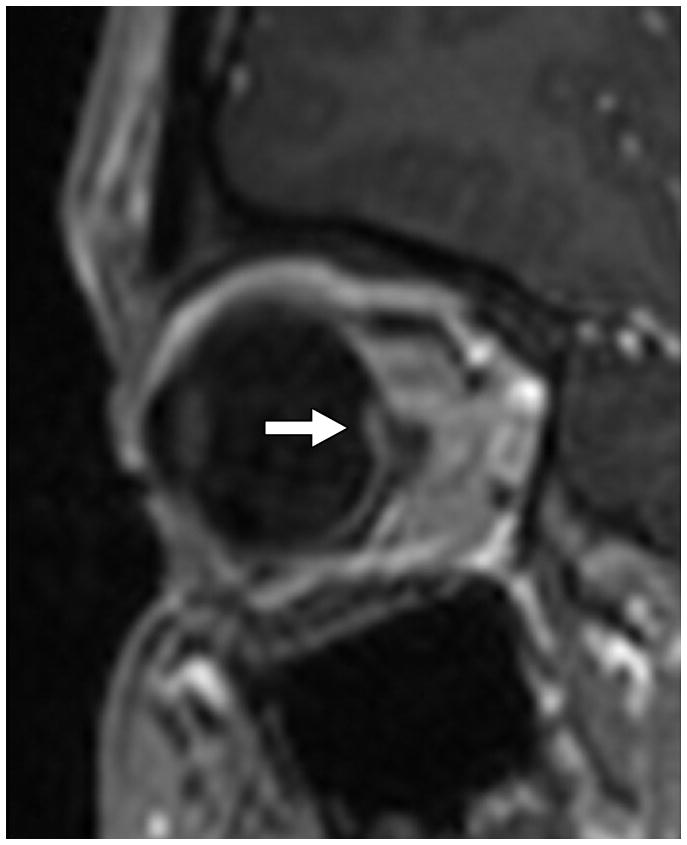
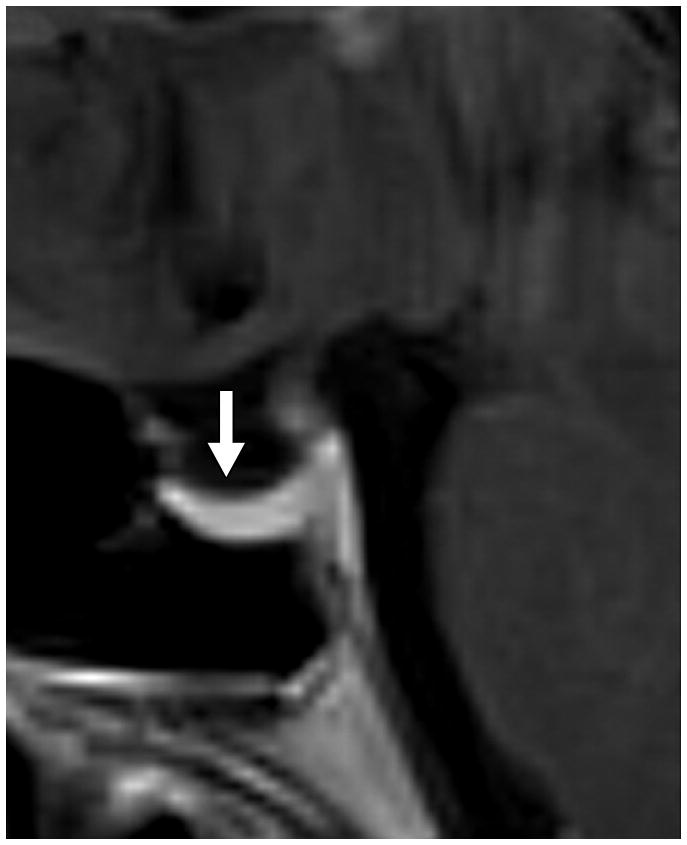
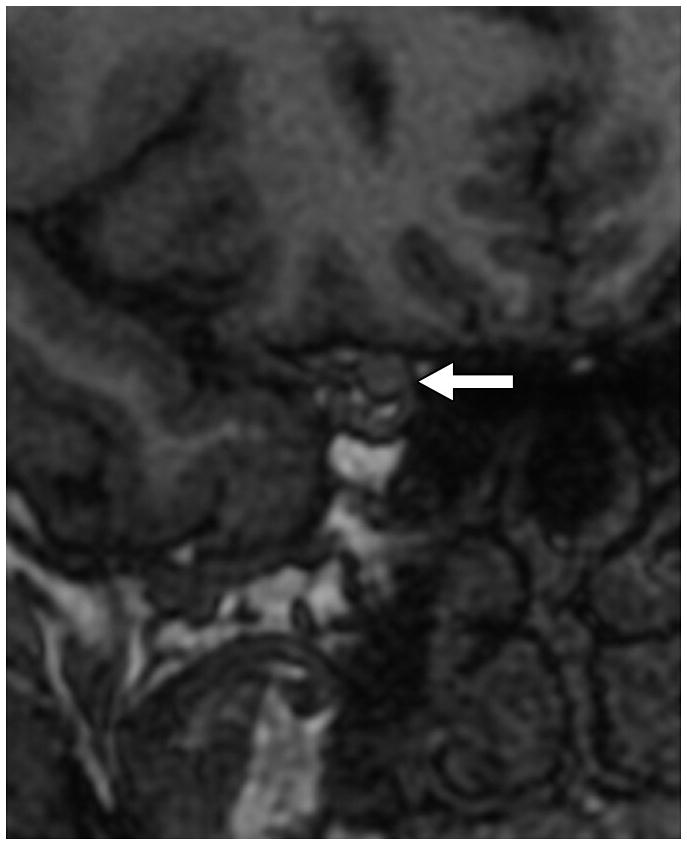
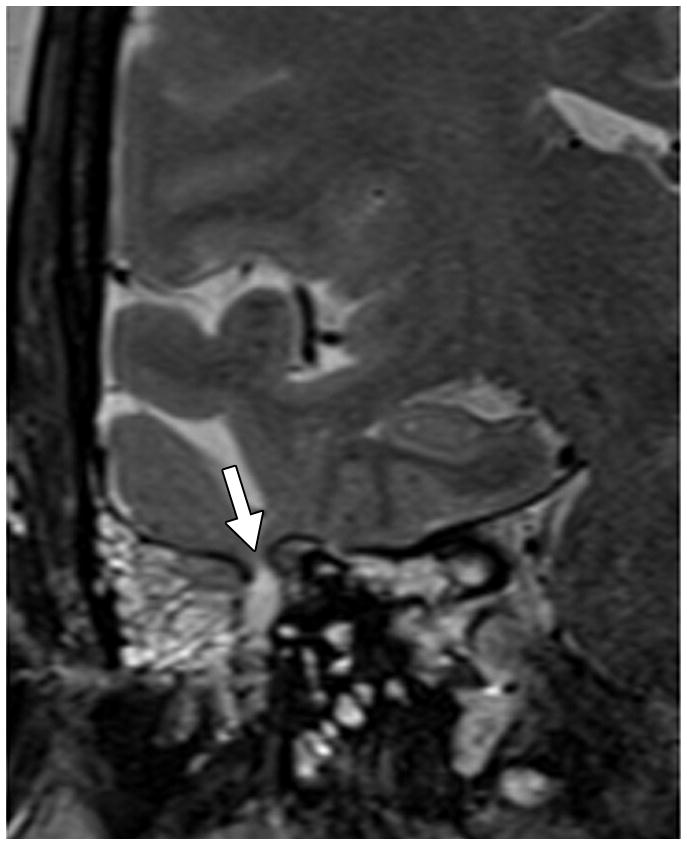
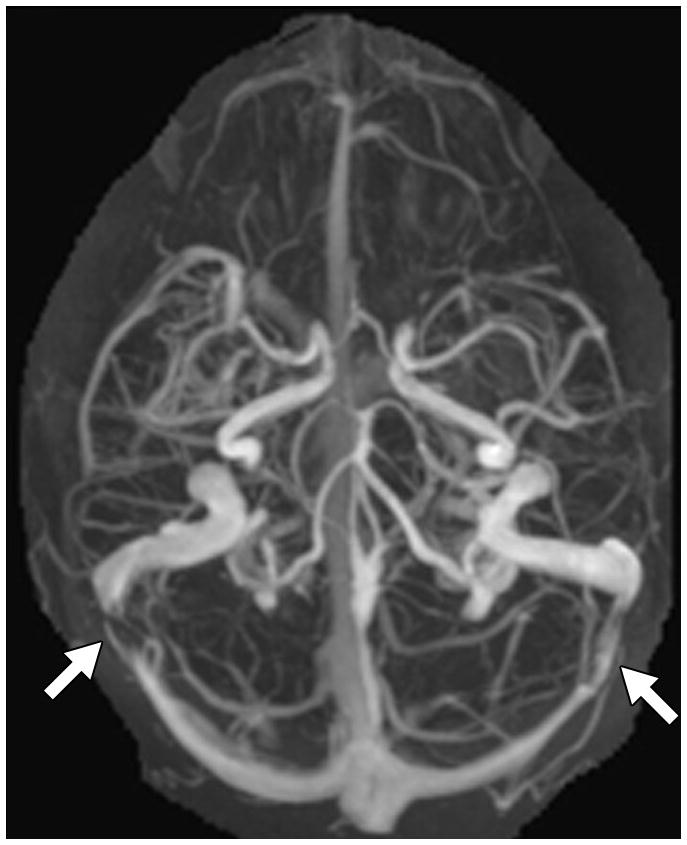
Fig. 1. MRI findings evaluated for in each patient with IIH.
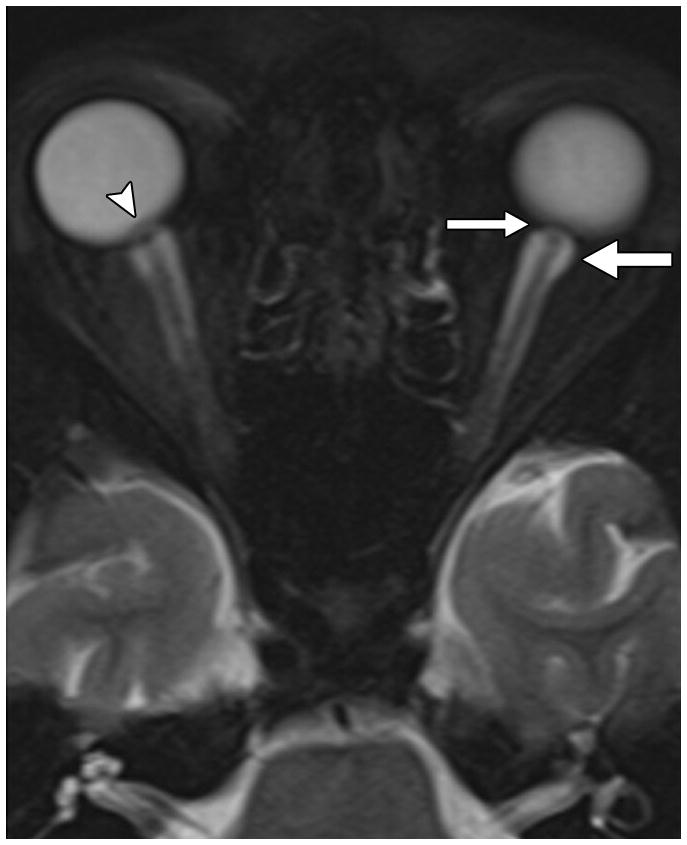
A. Orbital findings of ON head protrusion (arrowhead), posterior scleral flattening (thin arrow), and increased orbital peri-ON CSF (thick arrow) on a magnified axial T2-weighted fat-saturated image.
B. ON head enhancement and protrusion (arrow) on a magnified sagittal post-contrast volumetric T1-weighted image.
C. Pituitary grade. This was graded from I to V using the method of Yuh et al. The pituitary on this magnified mid-sagittal T1-weighted image depicted would be a category IV: superior concavity mild (>2/3) the height of the sella (arrow)
D. Optic canal width. Oblique coronal reformats of the volumetric Magnified reformatted volumetric T1-weighted dataset orthogonal to the axis of the intracanalicular ON were generated and the minimal diameter was measured. Arrow points to the ON.
E. Cephaloceles. These were defined as CSF or brain and CSF containing structures outside of the expected confines of the cranial vault. This coronal T2-weighted image shows multiple osteodural defects with CSF filling the right mastoid air cells.
F. TSS on MRV. This was based on MIP images generated from either contrast-enhanced MRV (n=23) or 2D TOF MRVs (n=19). The image shows a cranio-caudal projection of a contrast-enhanced MRV with bilateral TSS (arrows).
Pituitary tissue height on the sagittal T1-weighted image was classified into five categories based on the system of Yuh et al[8]: I = normal, II = superior concavity that was mild (<(1/3) the height of the sella), III = moderate (between (1/3) and (2/3) concavity of height of sella), IV = severe (>(2/3) concavity of height of sella), and V = no visible pituitary tissue. The minimal optic canal diameter was measured on oblique coronal multiplanar reconstructions of the volumetric T1-weighted dataset. The frequency of cephaloceles, as defined by CSF containing or brain and CSF containing structures outside the expected confines of the cranial vault was recorded in each patient. Finally, the presence or absence of TSS was evaluated on MIP images from either the contrast-enhanced MRV or 2D TOF MRV datasets in all but four patients (who did not undergo MRV evaluation).
Statistical Analysis
Statistical analysis was performed with R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). Data analysis assessed associations of demographic, clinical, and MRI findings with visual outcome using the Fisher exact test for categorical variables and the Spearman correlation for continuous variables.
Results
Differences in demographic, clinical, and MRI findings among visual outcome groups
Of 134 IIH patients screened for eligibility, 61 did not have MRIs available for review, 12 did not have a verified opening pressure, and 15 were lost to follow-up after initial visit and did not complete workup for this study. The median duration of symptoms prior to presentation was 5 weeks (interquartile range: 3–12 weeks). Median age for the 46 patients in this study was 32.2 years (range of 18.4 to 60.5 years). There were 42 females and 4 males. Twenty four patients were black, 1 Hispanic, and 21 white. Overall, there were 28 patients that met our criteria for inclusion in group 1 (good visual outcome with no visual field deficits), 10 patients in group 2 (good visual outcome with some visual field deficits), and 8 patients in group 3 (poor visual outcome with severe visual field deficits).
Differences in demographic, clinical, and MRI findings among the three visual outcome groups are summarized in table 1. Patients in group 3 (severe vision loss) were significantly younger (P=0.03) and had significantly higher OP (P=0.04) than the other groups with more favorable visual outcomes. There were no significant differences in gender, race, or body mass index. There were no significant differences in the frequency of orbital MRI findings, pituitary appearance, minimal optic canal width, or frequency of TSS among the three groups. Group 3 had significantly lower cephalocele frequency than the other groups (P=0.04).
Table 1.
Differences in demographic, clinical, and MRI findings among visual outcome groups
| Group 1 | Group 2 | Group 2 | |||
|---|---|---|---|---|---|
| N | No Vision Loss (N=28) |
Some Vision Loss (N=10) |
Severe Vision Loss (N=8) |
P | |
| Demographic and Clinical Findings | |||||
| Age (Years) | 46 | 31.8 (26.2–39.7) | 30.6 (27.8–43.7) | 21.5 (20.1–27.4) | 0.03 |
| Gender (Male) | 46 | 11% (3) | 0% (0) | 12% (1) | 0.61 |
| Race (Non-Black) | 46 | 57% (16) | 30% (3) | 38% (3) | 0.32 |
| Body Mass Index | 44 | 39.4 (34.9–42.7) | 37.9 (34.4–41.0) | 33.3 (30.4–39.0) | 0.45 |
| OP (cm H2O) | 46 | 31.0 (29.0–37.3) | 31.4 (28.3–36.9) | 48.5 (39.5–55.3) | 0.04 |
| Frequency of Orbital Findings on MRI | |||||
| ON Head Protrusion | 46 | 57% (16) | 70% (7) | 50% (4) | 0.77 |
| ON Head Enhancement | 42 | 28% (7) | 10% (1) | 43% (3) | 0.33 |
| Scleral Flattening | 46 | 82% (23) | 80% (8) | 100% (8) | 0.61 |
| Increased Perioptic CSF | 46 | 89% (25) | 80% (8) | 100% (8) | 0.54 |
| ON Tortuosity | 46 | 57% (16) | 70% (7) | 50% (4) | 0.77 |
| Osseous and Venous Findings on MRI | |||||
| Pituitary Grade | |||||
| 1 | 46 | 4% (1) | 0% (0) | 12% (1) | 0.29 |
| 2 | 11% (3) | 20% (2) | 12% (1) | ||
| 3 | 32% (9) | 10% (1) | 38% (3) | ||
| 4 | 46% (13) | 50% (5) | 38% (3) | ||
| 5 | 7% (2) | 20% (2) | 0% (0) | ||
| Optic Canal Width (mm) | 46 | 4.0 (3.7–4.1) | 3.6 (3.5–4.0) | 3.9 (3.7–4.0) | 0.27 |
| Cephaloceles | 46 | 21% (6) | 50% (5) | 0% (0) | 0.04 |
| TSS on MRV | 46 | 96% (27) | 90% (9) | 100% (8) | 0.64 |
Continuous data are listed as median (25th percentile–75th percentile). Categorical data are listed as percentage (frequency). Data analysis assessed associations with the Fisher exact test for categorical data and the Spearman correlation for data with continuous outcomes.
Differences in demographic, clinical, and MRI findings between patients with and without cephaloceles
Results are summarized in table 2. IIH patients with cephaloceles had significantly lower OP (30 vs. 34 cm H2O, P=0.05) than those without cephaloceles and a trend toward an older age (39.2 vs. 28.5 years; P=.06). Patients with cephaloceles were also significantly more likely to have a higher pituitary grade (greater superior surface flattening/concavity) than patients without cephaloceles (P=0.01). No significant difference in other clinical or MRI findings were present between the two groups.
Table 2.
Differences in demographic, clinical, and MRI findings between IIH patients with and without cephaloceles
| N | Cephalocele Absent (N=35) | Cephalocele Present (N=11) | P | |
|---|---|---|---|---|
| Demographic and Clinical Findings | ||||
| Age (Years) | 46 | 28.5 (25.0–37.2) | 39.2 (28.8–44.1) | 0.06 |
| Gender (Male) | 46 | 9% (3) | 9% (1) | 1.00 |
| Race (Non-Black) | 46 | 51% (18) | 36% (4) | 0.38 |
| Body Mass Index | 44 | 38.8 (32.2–42.2) | 37.3 (34.7–39.3) | 0.66 |
| Fulminant Course | 46 | 9% (3) | 0% (0) | 1.00 |
| Any Visual Field Deficit | 46 | 37% (13) | 45% (5) | 0.73 |
| OP (cm Water) | 46 | 34.0 (29.8–48.5) | 30.0 (27.5–34.5) | 0.05 |
| Frequency of Orbital Findings on MRI | ||||
| ON Head Protrusion | 46 | 54% (19) | 73% (8) | 0.32 |
| ON Head Enhancement | 42 | 28% (9) | 20% (2) | 1.00 |
| Scleral Flattening | 46 | 80% (28) | 100% (11) | 0.17 |
| Increased Perioptic CSF | 46 | 89% (31) | 91% (10) | 1.00 |
| ON Tortuosity | 46 | 60% (21) | 55% (6) | 1.00 |
| Osseous and Venous Findings on MRI | ||||
| Pituitary Grade | ||||
| 1 | 46 | 6% (2) | 0% (0) | 0.01 |
| 2 | 17% (6) | 0% (0) | ||
| 3 | 34% (12) | 9% (1) | ||
| 4 | 34% (12) | 82% (9) | ||
| 5 | 9% (3) | 9% (1) | ||
| Optic Canal Width (mm) | 46 | 4.0 (3.6–4.0) | 4.0 (3.6–4.3) | 0.76 |
| TSS on MRV | 46 | 94% (33) | 100% (11) | 1.00 |
Continuous data are listed as median (25th percentile–75th percentile). Categorical data are listed as percentage (frequency). Data analysis assessed associations with the Fisher exact test for categorical data and the Spearman correlation for data with continuous outcomes.
Discussion
This study evaluated whether or not MRI findings that have been described in patients with IIH are associated with vision loss. We found that while these findings are common among IIH patients, and indeed suggestive of elevated ICP, none of these imaging findings, except perhaps cephaloceles, were associated with visual outcome.
Most patients with IIH will have a favorable course without long-term visual impairment. However, a small number of patients can follow a more aggressive course, with blindness occurring in one or both eyes in 8–10% of patients,[18] and some loss of vision in almost half of patients in some studies.[5, 19] Severe vision loss at presentation suggests a higher risk of permanent vision loss.[5, 20] Male gender, black race, younger age of onset, more severe obesity, higher OP on lumbar puncture, and severe papilledema have been variably identified as risk factors for vision loss. [20–24] Our results support that younger patient age and higher OP are associated with more severe vision loss.
Previous studies have described a higher frequency of several orbital and sellar findings in patients with IIH than in controls. Brodsky and Vaphiades [7] in a group of 20 IIH patients found that flattening of the posterior sclera, enhancement of the prelaminar optic nerve, distension of the perioptic subarachnoid space, intraocular protrusion of the prelaminar optic nerve, and vertical tortuosity of the orbital optic nerve, as well as an “empty” sella, were all present at significantly higher rates in IIH patients than in controls, in whom they were seen less than 5% of the time. In 30 patients with IIH, Agid et al [9] found similar significantly higher frequencies of these findings, but concluded that only flattening of the posterior aspect of the globes, if present, was clinically useful for strongly suggesting the diagnosis of IIH. Maralani et al [16] found that an empty sella turcica and posterior globe flattening were both highly specific for IIH in a study of 43 IIH patients and 43 controls.[16]
None of the orbital findings previously described in IIH, nor the optic canal diameter were significantly associated with visual outcome in patients with IIH. A high frequency of scleral flattening and increased perioptic CSF was seen in all three visual outcome groups, consistent with prior studies demonstrating that these are the most frequent orbital findings seen on MRI in IIH.[9] However, while these orbital findings may correlate with the elevated ICP seen in IIH, our findings indicate that they do not portend a worse visual prognosis, nor does the absence of these imaging signs suggest a better visual prognosis.
An empty sella turcica is characterized by intrasellar herniation of suprasellar arachnoid and subarachnoid space CSF, resulting in flattening of the pituitary gland, with chronically transmitted CSF pulsations often leading to bony expansion and remodeling of the sella turcia. Depending on how it is defined, the empty sella turcica is one of the most common imaging signs of IIH, seen in up to 70% of patients;[7] however, empty sella turcica is also thought to be a normal variant, seen in up to 12% of asymptomatic individuals, presumably the result of a deficiency in the diaphragm sella.[25, 26] If duration of chronically elevated ICP impacts the likelihood of vision loss, and the extent of pituitary flattening reflects the cumulative duration/extent of elevated ICP, then patients with higher pituitary grades would be expected to have greater vision loss, but we did not find such an association.
Patients with spontaneous CSF otorrhea[27] and rhinorrhea[28] share many of the demographic features of IIH, including obesity and female predominance, and have a high incidence of empty sella turcica,[27] but these patients tend not to have the typical visual problems of IIH, possibly because their ICP has normalized or is low due to their CSF leak. We evaluated the presence or absence of skull base cephaloceles, which were typically present at the floor of the middle cranial fossa or involve Meckel cave or the petrous apex. The group with the worst vision loss did not have any cephaloceles, a significantly lower prevelance than that of patients with little or no vision loss. It is intriguing to hypothesize that the development of cephaloceles and CSF leaks may provide an alternative route to the optic nerve for decompression of elevated ICP and may thus be protective against vision loss. However, patients without cephaloceles tended to also be younger and to have higher OP that those with cephaloceles, placing them at higher risk of visual loss. Because of the limitation of small numbers of patients, we were not able to perform a stratified analysis to explore this issue further; however, the data strongly suggest that confounding by age and OP may explain the association of cephaloceles with visual loss seen in this study. Patients with cephaloceles were significantly more likely to have higher pituitary grades than those without, suggesting that sellar expansion and formation of skull base cephaloceles may share similar etiologies.
TSS has been described in IIH, although to varying degrees based on the imaging technique utilized. TOF and phase contrast MRV frequently suffers from artifacts in the region of the distal transverse sinus due to in-plane, turbulent, and tortuous flow, and is highly dependent on acquisition parameters.[13] Contrast-enhanced MRV techniques have shown a higher percentage of TSS in IIH patients. For example, Farb et al. [11] found 93% of IIH patients and 7% of controls had TSS, suggesting that this is a common and relatively specific finding in IIH. Use of TSS on MRV in combination with conventional MRI findings has also been shown to have extremely high specificity for diagnosis of IIH.[16] It remains controversial whether or not TSS is etiologically related to IIH, since some studies have demonstrated resolution of TSS after CSF diversion procedures to lower CSF OP,[29, 30] while others have shown that stenting of the TSS can result in lowering of CSF OP and resolution of symptoms.[14, 31] Our study encompassed both patients who had 2D TOF MRVs and those who had contrast-enhanced MRVs. Regardless of technique, patients in all three visual outcome groups had statistically similar rates of TSS, all >90% in frequency. Therefore, while the presence of TSS may be useful in the diagnosis of IIH, it is not a good predictor of vision loss, and it does not appear that its presence or absence should guide specific management.
While this represents the first study to evaluate associations between imaging findings and visual outcomes in IIH, it does have limitations. The study is retrospective with a relatively small number of IIH patients evaluated. A single neuroradiologist reviewed the images, rather than multiple reviewers in concensus. The acquisition parameters of the MRI examinations were slightly different across scanner platforms and field strengths; however, slice thickness and overall imaging protocol were standardized for all MRI studies. There was variability in the MRV technique used, with 19 patients undergoing 2D TOF MRV, and 23 patients undergoing contrast-enhanced MRVs. Additionally, some of the patients with complete flow gaps on TOF MRV could have actually had flow-related artifacts rather than TSS. These were interpreted without knowledge of patient visual status, so any bias or limitation due to the technique used should not be expected to have a systematic effect. More quantitative evaluations of the degree of TSS would likely have been more useful for determining effects of TSS on visual outcome, although grading or quantitative evaluation of TSS on the TOF MRVs would have been difficult. Future studies using automated morphometric evaluation of degree of TSS on contrast-enhanced MRV, similar to evaluation of carotid stenosis, and analysis of flow quantification data from cine-phase contrast MRI may provide important information. Finally, accurate measurement of the optic canal width can be difficult on volumetric T1-weighted gradient-recalled echo images, and may be better performed on thin-section CT with multiplanar reconstruction through the optic canal.
In conclusion, while MRI findings may suggest the presence of elevated ICP and the diagnosis of IIH, they are not predictive of visual outcome in IIH. Particularly, the absence of imaging findings does not exclude a diagnosis of IIH or portend a better visual prognosis. Although these distinctive imaging findings may facilitate our eventual understanding of the pathophysiology of IIH, the presence or absence of orbital findings, an empty sella turcica, or TSS should not guide specific management of patients with IIH.
Acknowledgments
This study was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology). Dr. Bruce receives research support from the NIH/NEI (K23-EY019341). Dr. Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnotes
Disclosures: None of the authors have relevant disclosures or conflicts of interest with regard to this manuscript submission.
The findings were presented at the American Society of Neuroradiology (ASNR) 50th annual meeting.
References
- 1.Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488–494. doi: 10.1136/jnnp-2011-302029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 3.Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991;41:239–244. doi: 10.1212/wnl.41.2_part_1.239. [DOI] [PubMed] [Google Scholar]
- 4.Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28:593–617. doi: 10.1016/j.ncl.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–474. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 6.Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol. 2006;5:433–442. doi: 10.1016/S1474-4422(06)70442-2. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 8.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging. 2000;12:808–813. doi: 10.1002/1522-2586(200012)12:6<808::aid-jmri3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521–527. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]
- 10.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53:1537–1542. doi: 10.1212/wnl.53.7.1537. [DOI] [PubMed] [Google Scholar]
- 11.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–1424. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JN, Gillard JH, Owler BK, Harkness K, Pickard JD. MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Psychiatry. 2004;75:621–625. doi: 10.1136/jnnp.2003.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol. 2005;252:1021–1025. doi: 10.1007/s00415-005-0710-6. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32:1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohr A, Dorner L, Stingele R, Buhl R, Alfke K, Jansen O. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2007;28:656–659. [PMC free article] [PubMed] [Google Scholar]
- 16.Maralani PJ, Hassanlou M, Torres C, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012 doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–232. doi: 10.1212/01.wnl.0000251312.19452.ec. [DOI] [PubMed] [Google Scholar]
- 18.Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991;114 (Pt 1A):155–180. [PubMed] [Google Scholar]
- 19.Rowe FJ, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye (Lond) 1998;12 (Pt 1):111–118. doi: 10.1038/eye.1998.18. [DOI] [PubMed] [Google Scholar]
- 20.Wall M. Sensory visual testing in idiopathic intracranial hypertension: measures sensitive to change. Neurology. 1990;40:1859–1864. doi: 10.1212/wnl.40.12.1859. [DOI] [PubMed] [Google Scholar]
- 21.Szewka A, Bruce B, Newman N, Biousse V. Idiopathic Intracranial Hypertension: Relation Between Obesity and Visual Outcomes. J Neuroophthalmol. 2012 doi: 10.1097/WNO.0b013e31823f852d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe FJ, Sarkies NJ. Visual outcome in a prospective study of idiopathic intracranial hypertension. Arch Ophthalmol. 1999;117:1571. [PubMed] [Google Scholar]
- 23.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–867. doi: 10.1212/01.wnl.0000304746.92913.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foresti M, Guidali A, Susanna P. Primary empty sella. Incidence in 500 asymptomatic subjects examined with magnetic resonance. Radiol Med. 1991;81:803–807. [PubMed] [Google Scholar]
- 26.Saindane AM, Lim PP, Aiken A, Chen Z, Hudgins PA. Factors Determining the Clinical Significance of an “Empty” Sella Turcica. Am J Roentgenol. doi: 10.2214/AJR.12.9013. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Goddard JC, Meyer T, Nguyen S, Lambert PR. New considerations in the cause of spontaneous cerebrospinal fluid otorrhea. Otol Neurotol. 2010;31:940–945. doi: 10.1097/mao.0b013e3181e8f36c. [DOI] [PubMed] [Google Scholar]
- 28.Schlosser RJ, Woodworth BA, Wilensky EM, Grady MS, Bolger WE. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol. 2006;115:495–500. doi: 10.1177/000348940611500703. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JN, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology. 2004;62:1907–1908. doi: 10.1212/01.wnl.0000125285.44539.d7. [DOI] [PubMed] [Google Scholar]
- 30.King JO, Mitchell PJ, Thomson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology. 1995;45:2224–2228. doi: 10.1212/wnl.45.12.2224. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JN, Cousins C, Owler BK, Sarkies N, Pickard JD. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74:1662–1666. doi: 10.1136/jnnp.74.12.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]


