Abstract
Background:
Mesaconitine is the main active component of genus aconitum plants that are widely used in clinics in China. However, little has been known about the metabolic pathway of mesaconitine.
Objective:
To explore the metabolites and propose the pathway of mesaconitine.
Materials and Methods:
In the present study, mesaconitine (4 mg kg−1) was orally administered to male rats. Then, blood samples collected were pretreated using solid-phase extraction technique with C18 cartridges, and analyzed using LC/MS/MS method with electrospray ionization. Both positive ion mode and collision induced dissociation (CID) were used to elucidate the structures of the major metabolites of mesaconitine.
Results:
Ten compounds were identified, among which seven were new metabolites, and the metabolic pathway was proposed. The protonated molecular ions of seven new metabolites were at m/z 648, 618, 616, 602, 572, 468, and 542, multistage fragment ions with neutral loss of 28 u (CO), 60 u (CH3COOH), 18 u (H2O), and 32 u (CH3OH). These new metabolites detected firstly in vivo, were named 10-hydroxyl-mesaconitine, hypaconitine, dehydrated mesaconitine 16-O-demethylmesaconitine, 16-O-demethylhypaconitine, and 16-O-demethyl-dehydrated hypaconitine, respectively. Furthermore, the breaking sequence of methoxyl was obtained using quantum chemistry.
Conclusion:
The study proved that the method of solid-phase extraction technique coupled with MS and quantum chemistry can be applied to the analysis of metabolites in plasma quickly and conveniently.
Keywords: Liquid chromatography-electrospray ion trap-mass spectrometry, mesaconitine, metabolite, quantum chemistry
INTRODUCTION
Mesaconitine is one of the important alkaloids of genus aconitum plants used in traditional Chinese medicine and has potential toxicity and wide bioactivities. Mesaconitine is classified into diester diterpenoid alkaloids, which share a common C19-norditerpenoid skeleton, and have anti-inflammatory activities and analgesic functions, accompanied by strong toxicity and narrow therapeutic window. At cellular level, aconitine-type compounds interact with activated sodium channels with high affinity and shift the conformational equilibrium toward the activated state.[1] Studies on aconitine metabolites have been reported,[2,3,4,5,6,7,8,9] indicating the main metabolites include benzoylaconine, 16-O-demethylaconitine, and aconine. However, additional study is needed to confirm whether or not the three metabolites are only ultimate ones. Currently, liquid chromatography mass spectrometry is an extremely sensitive technique that can detect traces of metabolites in a metabolome sample.[10] In the present study, we found not only some reported metabolites but also seven compounds that were not detected previously using this method. These seven compounds were identified as 10-hydroxyl-mesaconitine, 16-O-demethylmesaconitine, hypaconitine, 16-O-demethylhypaconitine, dehydrated mesaconitine, 16-O-demethyl-dehydrated hypaconitine, and dehydrated mesaconine, respectively. A novel metabolic pathway was presented based on the present findings.
MATERIALS AND METHODS
Standards and samples
Standards of aconitine (>98%) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Six male Sprague Dawley rats were supplied from the Animal Service of Health Science Center, Peking University.
Solvents and reagents
HPLC-grade acetonitrile (MeCN) was purchased from E. Merck (Darmstadt, Germany) and ammonia (AR grade) was obtained from Beihua Fine Chemicals Co., Ltd. (Beijing, China). The water used for HPLC was purified by a Milli-Q system (Millipore, Milford, MA, U.S.A.).
Rat blood samples preparation
After 12-hour overnight fast, rats were treated with mesaconitine (4 mg·kg−1, intragastric administration). Fifteen minutes after the dose, the whole blood samples were collected from abdominal aorta, and then the plasma was separated and stored at −20°C prior to analysis by HPLC/MS/MS. Before analysis, 200 μl of each plasma sample was mixed with 200 μl of methanol and centrifuged at 16,000 × g for 10 min, and then the supernatant was transferred to vials for assay.
Mass spectrometry analysis
All experiments were performed using an AB SCIEX QTRAP; 5500 LC/MS/MS with a Shimadzu UFLC. The chromatogram flow rate was 0.36 ml·min−1 acetonitrile and 0.04 ml·min−1 aqueous phase. The MS parameters were optimized using chemical reference substance and confirmed to be effective in compounds identification. The ion source was electrospray ionization source with the mode of positive ion. The ion-spray voltage was 5500 V, capillary temperature was set at 250°C, ion source gas was 20 V, declustering potential was 80 V, entrance potential was 10 V, and collision cell exit potential was 13 V.
RESULTS AND DISCUSSION
Identification of metabolites
It was reported that pseudomolecules [M + H]+ of aconitine-type alkaloids were often detected easily under the positive ion mode,[11] which was further confirmed in this study. The mass spectrum of each metabolite of mesaconitine is shown in Figure 1. The metabolic pathway is shown in Figure 2. The pseudomolecules of reaction products of aconitine were at m/z 648, 632, 618, 616, 602, 590, 572, 542, 468, and 486, respectively [Table 1].
Figure 1.
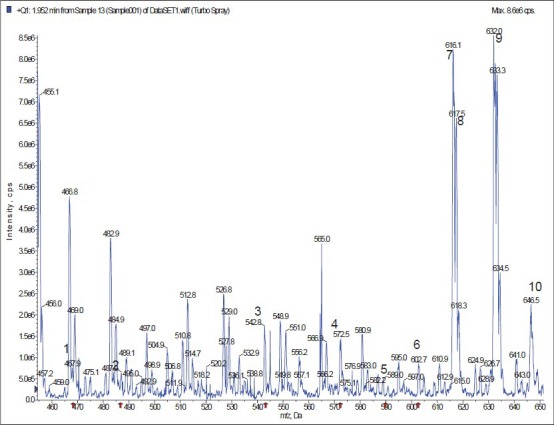
The MS of blood sample in rat.1: dehydrated mesaconine; 2: mesaconine; 3: 16-O-demethyl-dehydrated hypaconitine; 4: dehydrated mesaconitine; 5: Benzoyl-mesaconine; 6:16-O-demethylhypaconitine; 7: hypaconitine; 8: 16-O-demethylmesaconitine; 9:mesaconitine; 10: hydroxyl-mesaconitine
Figure 2.
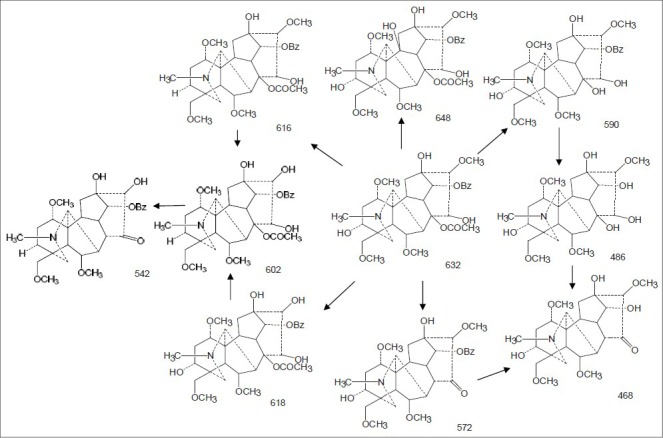
The metabolic pathway of mesaconitine in rat
Table 1.
Identification of metabolites by MSn
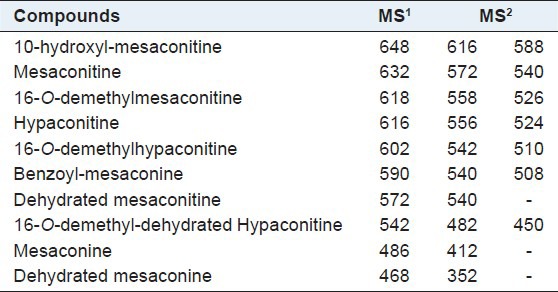
The pseudomolecule of 648 is speculated to be 10-hydroxyl-mesaconitine with the MS2 at m/z 616 and m/z 588. According to the literature,[6] the fragmentation ion was formed by loss of methanol (CH3OH) and acetic acid (CH3COOH). The fragmentation pathway is identical to that of aconitine.
The pseudomolecule of 632 is likely to be mesaconitine with MS2 at m/z 572 and m/z 540. According to the literature,[6] the fragmentation ion was loss of methanol and acetic acid.
The pseudomolecule of 618 may be 10-O-demethyl- mesaconitine. Its MS2 was at m/z 558 and 526; 558 was loss of CH3COOH, whereas 526 was loss of CH3OH and CH3COOH.
The pseudomolecule of 616 is likely to be hypaconitine, which is consistent with the previous study. Its MS2 was at m/z 556 and 524; 556 was loss of CH3COOH, whereas 524 was loss of CH3COOH and CH3OH.
According to the literature, the pseudomolecule of 602 is 16-O-demethylhypaconitine. Its MS2 was at m/z 542 and 510; 542 was loss of CH3COOH, whereas 510 was loss of CH3COOH and CH3OH.
According to the literature,[6] the pseudo-molecule of 590 is benzoyl-mesaconine. Its MS2 was at m/z 540 and 508; 508 was loss of CH3OH.
The pseudomolecule of 572, according to the literature,[11] we think it is dehydrated mesaconitine. Its MS2 was at m/z 542 and 510; 542 was loss of CH3COOH, whereas 510 was loss of CH3COOH and CH3OH.
The pseudomolecule of 542, according to the literature,[11] we think it is 16-O-demethyl-dehydrated hypaconitine. Its MS2 was at m/z 482 and 450; 482 was loss of CH3OH and CO, whereas 450 was loss of 2 CH3OH and CO.
The pseudomolecule of 486, according to the literature,[11] we think it is mesaconine. Its MS2 was at m/z 412; 412 was loss of two CH3OH.
The pseudomolecule of 468, according to the literature,[12,13,14] we think it is dehydrated mesaconine. Its MS2 was at m/z 352; 352 was loss of two CH3OH, two H2O, and O.
The loss order of four methoxyl groups
At first, we speculated that the loss neutral molecule (m/z 32) was CH3OH. However, there are four methoxyl groups in mesaconitine, and the loss order of the four methoxyl groups was unknown. In the present study, quantum chemistry software Gaussian 03W was used to reveal the law of loss. Using density functional theory and the 6-31G* basis sets, geometry promoting and shaking rate were analyzed under HF/STO-3G level, we used the B3LYP/6-31G* technology, and the geometrical model standard is the aconitine model, and the bond length U of aconitine and mesaconitine is international standard. The results showed that the length order of the bonds that connects the four methoxy groups is as follows: C16 (1.440 Å) > C1 (1.422 Å) > C6 (1.411Å) > C18 (1.408Å); the results of mesaconitine are C16 (1.421 Å) >C1 (1.422 Å) > C6 (1.411Å) > C18 (1.408Å). According to these data, the conclusion is obtained that the breaking sequence of chemical bond is probably C16 or C1.
CONCLUSIONS
Numerous aconitum plants, such as aconite root, Aconitum carmichaeli Debx., Aconitum szechenyianum, are widely used in clinics, especially in China.[15,16,17,18] However, these herbs may be fatal if improperly used or used as poisons. To clarify the mechanism of toxicity, the metabolites of alkaloids, which are the main bioactive compounds of those herbs mentioned above, are perfect proof for taking these herbs. It is widely believed that aconitine-type alkaloids are unstable, especially in vivo, and thus it is extremely important to detect more metabolites and elucidate the metabolic pathway. In this study, seven new metabolites and two metabolic pathways are found in vivo for the first time. Since this method in the study is exploratory, more effort is needed to confirm the findings using this method. For example, the identification of these compounds should be further studied.
In the present study, blood samples at a series of time points were detected, and the results indicated that varied sample data appeared at different time points.[19,20] For example, there was no mesaconine in the sample of 0.5 hour, whereas it was found in 2-hour sample. This result further validated the instability of the alkaloid. So it is very important that a database of metabolites was built, which is the goal of our next work. During the experiment, we found an interesting thing that different fragments were produced under different CID voltage; for example, mesaconine was broken under 40 eV, whereas mesaconine under 50 eV. These data tell us that CID voltage is an important parameter in MS experiment.
Footnotes
Source of Support: This study was supported by National Natural Science Foundation of China (No. 30901959, No.81102807)
Conflict of Interest: None declared.
REFERENCES
- 1.Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 2.Tan P, Liu YG, Li F, Qiao YJ. Reaction products analysis of aconitine in dilute ethanol using ESI-Q-ToF-MS. Pharmazie. 2012;67:274–6. [PubMed] [Google Scholar]
- 3.Zhao Y, Song F, Wang X, Guo X, Liu Z, Liu S. Studies on the biotransformation of 16-O0-demethylaconitine and electrospray ionization tandem mass spectrometry. Acta Chim Sinica. 2008;66:525–30. [Google Scholar]
- 4.Wada K, Nihira M, Hayakawa H, Tomita Y, Hayashida M, Ohno Y. Effects of long term administrations of aconitine on electrocardiogram and tissue concentrations of aconitine and its metabolites in mice. Forensic Sci Int. 2005;148:21–9. doi: 10.1016/j.forsciint.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang S, Liu Y, Yan L, Dou G, Gao Y. Characterization of metabolites and cytochrome P450 isoforms involved in the microsomal metabolism of aconitine. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:292–300. doi: 10.1016/j.jchromb.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HG, Sun Y, Duan MY, Chen YJ, Zhong DF, Zhang HQ. Separation and identification of aconitum alkaloids and their metabolites in human urine. Toxicon. 2005;46:500–6. doi: 10.1016/j.toxicon.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HG, Shi XG, Sun Y, Zhong DF, Zhang HQ. Study on the metabolites of aconitine in rabbit blood. Jilin Da Xue Xue Bao Li Xue Ban. 2006;44:284–6. [Google Scholar]
- 8.Zhang HG, Shi XG, Sun Y, Duan MY, Zhong DF. New metabolites of aconitine in rabbit urine. Chin Chem Lett. 2002;13:758–60. [PubMed] [Google Scholar]
- 9.Xie FM, Wang HC, Shu HL, Li JH, Jiang JR, Chang JP, et al. Separation and characterization of the metabolic products of lappaconitine in rat urine by high-performance liquid chromatography. J Chromatogr. 1990;526:109–18. doi: 10.1016/s0378-4347(00)82488-3. [DOI] [PubMed] [Google Scholar]
- 10.Lewis-Stanislaus AE, Li L. A method for comprehensive analysis of urinary acylglycines by using ultra-performance liquid chromatography quadrupole linear ion trap mass spectrometry. J Am Soc Mass Spectrom. 2010;21:2105–6. doi: 10.1016/j.jasms.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Yue H, Pi Z, Song F, Liu Z, Cai Z, Liu S. Studies on the aconitine-type alkaloids in the roots of Aconitum Carmichaeli Debx. by HPLC/ESIMS/MS n. Talanta. 2009;77:1800–7. doi: 10.1016/j.talanta.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Tan P, Li F, Qiao Y. Study on the reaction products of hyaconitine in methanol and water using electrospray ionization mass spectrometry. Afr J Pharm Pharmacol. 2011;5:2575–80. [Google Scholar]
- 13.Tan G, Lou Z, Jing J, Li W, Zhu Z, Zhao L, et al. Screening and analysis of aconitum alkaloids and their metabolites in rat urine after oral administration of aconite roots extract using LC-TOFMS-based metabolomics. Biomed Chromatogr. 2011;25:1343–51. doi: 10.1002/bmc.1607. [DOI] [PubMed] [Google Scholar]
- 14.Mozhayeva GN, Naumov AP, Negulyaev YA, Nosyreva ED. The permeability of aconitine-modified sodium channels to univalent cations in myelinated nerve. Biochim Biophys Acta. 1977;466:461–73. doi: 10.1016/0005-2736(77)90339-x. [DOI] [PubMed] [Google Scholar]
- 15.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, Ommen B, et al. Mass-spectrometry-based metabolmics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5:435–58. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Liu Q, Zhang H, Qiao Y. Studies on hydrolysates of aconitine by HPLC-MSn. Chin J New Drugs. 2007;16:303–5. [Google Scholar]
- 17.Zhao Y, Song F, Yue H, Guo X, Li H, Liu Z, et al. Studies on the biotransformation of 16-O-demethyldeoxyaconitine of the metabolite of aconitine in human intestinal bacteria. Chin J Anal Chem. 2007;35:1711–5. [Google Scholar]
- 18.Zhao Y, Song F, Guo X, Liu S. Studies on the biotransformation of aconitine in human intestinal bacteria using soft-ionization mass spectrometry. Chem J Chin Univ. 2008;29:55–9. [Google Scholar]
- 19.Yoshioka N, Gonmori K, Tagashira A, Boonhooi O, Hayashi M, Saito Y, et al. A case of aconitine poisoning with analysis of aconitine alkaloids by GC/SIM. Forensic Sci Int. 1996;81:117–23. doi: 10.1016/s0379-0738(96)01980-9. [DOI] [PubMed] [Google Scholar]
- 20.Sui Z, Liu Z, Liu Z, Hu X, Li N, Wang R. Study on metabolites of aconitine in rabbit urine collected at different times after administration. Liaoning J Tradit Chin Med. 2009;36:644–6. [Google Scholar]


