Abstract
Background:
131-radioiodine has been widely used as an effective radionuclide for treatment of patients with thyroid diseases. The purpose of the present study is to investigate the radioprotective effects of curcumin as a natural product that protects against the genotoxic effects of 131I in human cultured lymphocytes.
Materials and Methods:
Whole blood samples from human volunteers were incubated with curcumin at doses of 5, 10, and 50 μg/mL. After 1-hour incubation, the lymphocytes were incubated with 131I (100 μCi/1.5 ml) for 2 hours. The lymphocyte cultures were then mitogenically stimulated to allow for evaluation of the number of micronuclei in cytokinesis-blocked binucleated cells.
Results:
Incubation of lymphocytes with 131I at dose 100 μCi/1.5 mL induced genotoxicity shown by increase in micronuclei frequency in human lymphocytes. Curcumin at 5, 10, and 50 μg/mL doses significantly reduced the micronuclei frequency. Maximal protective effects and greatest decrease in micronuclei frequency were observed when whole blood was incubated with 50 μg/mL dose of curcumin with 52%.
Conclusion:
This study has important implications for patients undergoing 131I therapy. Our results indicate a protective role for curcumin against the genetic damage and side effects induced by 131I administration.
Keywords: Curcumin, genotoxicity, 131I, micronucleus, radioiodine
INTRODUCTION
Radioiodine-131 (131I) has been widely used for treatment of patients with thyroid diseases such as hyperthyroidism and thyroid cancer. The wide usage of 131I in nuclear medicine is due to its physical and radiochemical properties. It emits beta particles (Emax = 0.61 MeV and Eavg = 0.20 MeV) and gamma rays (E = 0.36 MeV), with a physical half-life of approximately 8 days. It accumulates at high concentration in the thyroid tissue with high target to nontarget ratio.[1] The therapy effect of 131I is related to beta particle, which is damaging to cells with direct or indirect effects. In direct action, radiation may directly hit a particularly sensitive atom or molecule and disrupt chemical bonds in the cell. In the indirect action, radiation can damage a cell indirectly by interacting with water molecules in the body through reactive oxygen species (ROS).[2] ROS can attach to critical macromolecules such as DNA, resulting in DNA damage, chromosomal breaks, and cell death.[3] Although 131I acts with this mechanism to damage the tumor cells, it can have side effects on normal tissues due to unwanted accumulation of this radionuclide in healthy organs. Short- and long-term side effects related to radioiodine therapy are nausea, sialadenitis, and hematological depression. Since iodine is secreted in salivary glands at high concentration, salivary gland dysfunction is reported in patients undergoing 131I therapy.[4] In addition to these side effects, there is an induction of secondary primary cancer and genetic damage following in 131I therapy. An increased risk of leukemia, bladder cancer, and colorectal cancer were reported in patients after 131I therapy.[5,6,7,8,9]. There are several studies showing that genetic damage was increased in patients after 131I therapy. The frequency of micronuclei as breaks of chromosome was elevated in patients after 131I therapy.[10,11,12] Thus, protection of DNA may reduce side effects induced by 131I which reduces incidence of secondary cancer. Curcumin is a dietary antioxidant derived from a herb Curcuma longa and possesses therapeutic properties. It has been reported to scavenge free radicals and inhibit lipid peroxidation, with these mechanisms, curcumin protects critical macromolecules such as DNA from oxidative stress.[13,14] Several studies reported the radioprotective effects of curcumin against cytotoxicity induced by external gamma rays. Pretreatment with curcumin protected hepatocytes against gamma rays-induced cellular damage.[15] Curcumin protected human lymphocytes against genotoxicity induced by gamma radiation.[16] With respect to protective effects of curcumin, the aim of this study is to determine the radioprotective effects of curcumin against genotoxicity induced by 131I in human peripheral blood lymphocyte cells in vitro.
MATERIALS AND METHODS
Chemicals
All chemicals were obtained from Merck and Sigma. Curcumin was prepared in ethanol and diluted with medium culture. 131I Na in sterile solution was prepared from AEOI, Tehran, Iran, it was used freshly.
Irradiation protocol
The study protocol was approved by the ethical committee of the university. After obtaining written informed consent, 12 mL whole blood samples were collected in heparinized tubes from three healthy, nonsmoking male volunteers aged 25–35 years. The whole blood was divided into six 1.5-mL tubes, one for each of the six study groups: Control; 131I only; curcumin at doses 5, 10, and 50 μg/mL with 131I; and curcumin only. First, blood samples were incubated with curcumin for 1 hour, after which 100 μCi of 131I was added to the blood samples and incubated at 37 °C for 2 hours. After incubation, RPMI 1640 medium was added to each tube and the cultures were centrifuged at 1500 g for 8 minutes. To separate 131I from the whole blood, the upper (less dense) solution was removed and blood was transferred for micronucleus assay.
Micronuclei assay
From each sample, an aliquot of 750 μL (control and irradiated groups) was added to 4.15 mL of RPMI 1640 culture medium (Gibco, USA), which contained 10% fetal calf serum, 0.1 mL/5 mL phytohemagglutinin (Gibco, USA), antibiotics (penicillin 100 IU/mL, streptomycin 100 μg/mL), and 2 mM glutamine (Sigma, USA) at final concentration. All cultures were set up in duplicate and incubated at 37 ± 1°C in a humidified atmosphere of 5% CO2/ 95% air. Cytochalasin B (Fluka, final concentration: 6 μg/mL) was added after 44 hours of culture incubation. At the end of 72 hour of incubation, the cells were collected by centrifugation and resuspended in 0.075 M cold potassium chloride for 8 min at 1500 g. They were then immediately treated with a fixative solution three times (methanol: acetic acid). Fixed cells were dropped onto clean microscopic slides, air-dried, and stained with Giemsa solution (20%). All slides were coded by an individual other than the scorer, and were evaluated at × 100 magnification for the micronuclei frequency in cytokinesis-blocked binucleated cells with well-preserved cytoplasm. To be scored as micronuclei, candidates had to have a diameter between 1/16th and 1/3rd of main nuclei, be non-refractile, and not be linked to or overlap with the main nuclei.[17] At each blood collection time, at least 1000 binucleated cells from duplicate irradiated and control cultures from each volunteer were examined; then, the frequency of micronuclei was recorded.
Statistical analysis
At each blood collection, the prevalence of micronuclei was recorded for each volunteer. The data were analyzed using analysis of variance (ANOVA) with Tukey's HSD Post-hoc test.
RESULTS
The mean percentage of micronuclei in the lymphocytes of volunteers treated with 100 μCi of 131I was 3.38 ± 0.33, whereas the percentage of nontreated control lymphocytes was 1 ± 0.6 (P < 0.001) [Table 1]. The frequency of micronuclei (an indication of the genotoxic effects of internal irradiation) after preincubation with curcumin at doses of 5, 10, and 50 μg/mL were, 2.44 ± 0.49, 2.0 ± 0.61, and 1.63 ± 0.21, respectively [Figure 1]. The data demonstrate that the frequencies of micronuclei found in the curcumin-treated samples were significantly lower than that of samples cultured with only 131I. Whole blood samples incubated with 5, 10, and 50 μg/mL of curcumin, and then exposed in vitro to 131I radiation, exhibited a significant decrease in micronuclei frequency compared with samples incubated with only 131I (P < 0.01). Total micronuclei values were 28, 41 and 52% less in the samples treated with curcumin at concentrations of 5, 10, and 50 μg/mL, respectively than in controls [Table 1]. Curcumin alone did not cause genotoxicity in cultured lymphocytes at concentrations of 50 μg.
Table 1.
The percentages of micronuclei induced in vitro by 131I on cultured blood lymphocytes from human volunteers and protective effects of curcumin
Figure 1.
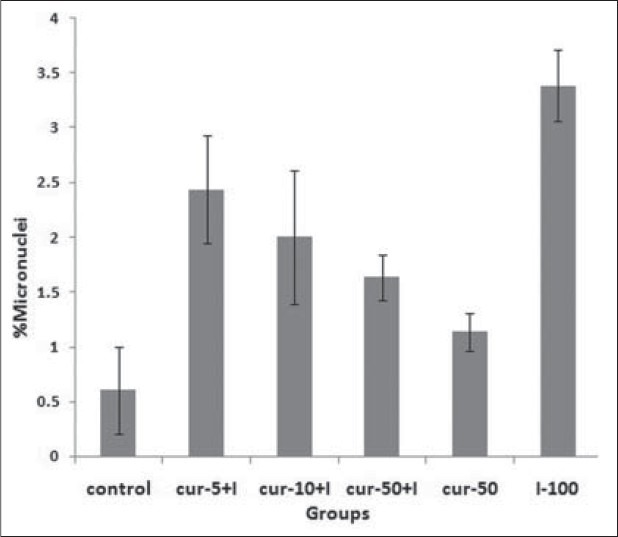
In vitro protection by curcumin (Cur) at different concentration (5, 10 and 50 ìg) against radiation-induced genetic damage induced by 131I (I) in cultured whole blood lymphocyte. The data represent average ± standard deviation of three human volunteers. P<0.001: Sample at control compared with similarly irradiated lymphocytes from the blood sample treated with I. P<0.05: I sample compared to I-Cur-5+I, Cur-10+I and Cur-50+I samples. P>0.05: Cur-50 sample compared control sample. P>0.05: Between groups of I-Cur-5+I, Cur-10+I and Cur-50+I samples
A typical depiction of binucleated cells with micronuclei is given in Figure 2.
Figure 2.
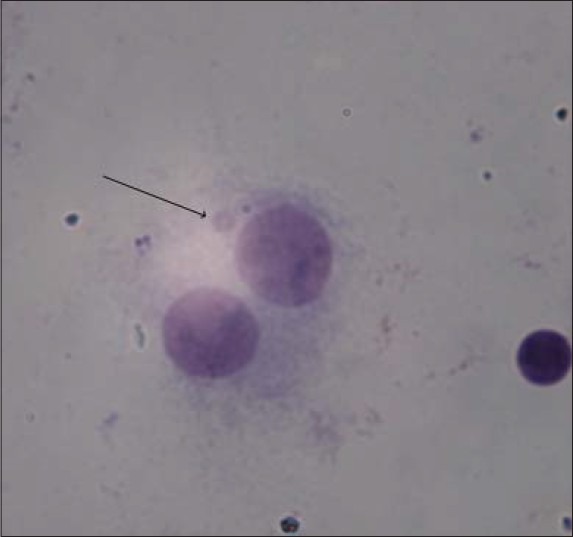
A typical binucleated lymphocyte with micronuclei in our study
DISCUSSION
In the present study, we show that curcumin significantly protects lymphocytes against genotoxicity induced by 131I. In vitro incubation of human blood with the natural compound curcumin reduced the frequency of micronuclei induced by internal irradiation by the radiopharmaceutical 131I. Measures of genotoxicity have been used to estimate the risk of damage induced by internal irradiation from the radiopharmaceutical. Genotoxic agents can cause cancer, hereditary disorders, and abnormalities in developing embryos.[18]
In this study, we have observed genotoxic effects induced by 131I at dose 100 μCi in lymphocytes. Gil et al.,[19] assessed DNA damage, namely micronuclei in peripheral lymphocytes in patients with thyroid cancer after 131I therapy. The number of micronuclei in cells increased significantly 1 month after the treatment.[19] Ballardin et al.,[10] reported a 4-fold increase in the frequency of micronuclei at 7 days after radioiodion therapy in patients; however, micronuclei frequency declined slowly and reached baseline at 180 days after 131I therapy.[10] Other studies reported a significantly higher frequency of micronuclei in patients after 131I therapy.[12,20] The micronuclei frequency assay can be used as a valuable endpoint and sensitive method for studying radiation biology of 131I in patients for assessment of genetic damage. However, 131I is considered a very useful radionuclide in reducing thyroid activity, nevertheless, as a genotoxic agent that may produce secondary cancer incidence in patients.[21,22,23] Incidence of leukemia was significantly increased in patients after iodine therapy.[23] 131I emits gamma and beta rays. Beta rays have short range board with higher destroying effects on cells as compared with gamma rays. Induction of oxidative stress is one of the main mechanisms for therapeutic and/or side effects of 131I. Oxidative stress may be related to DNA damage.[19] In this study, we showed that curcumin protects human lymphocytes against genotoxicity induced by internal irradiation. For all three volunteers, the 131I-treated lymphocytes incubated with curcumin showed a reduction in micronuclei frequency as compared with 131I-treated samples without curcumin. This finding suggests that curcumin acts effectively as a free radical scavenger. Curcumin treatment at 50 μg/mL provides maximal leukocyte protection. Curcumin has been reported to have several beneficial health effects, including anti-inflammatory, neuroprotective, antioxidant, anticancer, and radiosensitizer.[24,25,26,27,28,29,30] Curcumin has been shown to affect several cell signaling pathways, including apoptosis (activation of caspases and downregulation of anti-apoptotic gene products), proliferation (HER-2, EGFR, and AP-1), angiogenesis (VEGF), and inflammation (NF-κB, TNF, IL-6, IL-1, COX-2, and 5-LOX).[24] Also, it has been shown to sensitize human cancer cells to gamma radiation, which is a dual benefit effect of curcumin in patients for cancer therapy.[28,31,32]
Curcumin has been shown to have antioxidant activity against the cellular oxidative stress associated with diseases. Curcumin showed a powerful capacity for scavenging intracellular small oxidative molecules such as H2O2, HO·, ROO·. Curcumin can readily transfer electron or easily donate H-atom from two phenolic sites to scavenge free radicals. The excellent electron transfer capability of curcumin is because of its unique structure and different functional groups, including a β-diketone and several π electrons that have the capacity to conjugate between two phenyl rings.[33,34] Pharmacokinetics parameters of curcumin were measured in patients after the consumption of 8 g of curcumin. No toxicity was observed in patients with curcumin ingestion.[35,36] Curcumin plasma level range was from 29 to 419 ng/mL,[36] and 22 to 42 ng/mL after 2–6 hours after single dose.[35] Curcumin has poor oral bioavailability, recently many investigations focused on nanoformulation and water soluble derivatives of this natural product to increase absorption by intestinal tract.[37,38,39]
Because curcumin has been studied extensively as a promising pharmacological drug, it may be a useful protective candidate for patients undergoing 131I therapy. In this study, we showed that curcumin significantly protects human lymphocytes against genotoxicity induced by 131I.
ACKNOWLEDGMENTS
This research was the subject of a Pharm D thesis of Nayereh Shafaghati as a student of Mazandaran University of Medical Sciences. It was supported by Mazandaran University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this study.
REFERENCES
- 1.Parthasarathy KL, Crawford ES. Treatment of thyroid carcinoma: Emphasis on high-dose 131I outpatient therapy. J Nucl Med Technol. 2002;30:165–71. [PubMed] [Google Scholar]
- 2.Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov Today. 2010;15:907–18. doi: 10.1016/j.drudis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Nakada K, Ishibashi T, Takei T, Hirata K, Shinohara K, Katoh S, et al. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J Nucl Med. 2005;46:261–6. [PubMed] [Google Scholar]
- 5.Grudeva-Popova J, Yaneva M, Zisov K, Ananoshtev N. Therapy-related acute promyelocytic leukemia after treatment with radioiodine for thyroid cancer: Case report with literature review. J BUON. 2007;12:129–32. [PubMed] [Google Scholar]
- 6.Schroeder T, Kuendgen A, Kayser S, Kroger N, Braulke F, Platzbecker U, et al. Therapy-related myeloid neoplasms following treatment with radioiodine. Haematologica. 2012;97:206–12. doi: 10.3324/haematol.2011.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolade VO, Bosinski TJ, Ruffy EL. Acute promyelocytic leukemia after iodine-131 therapy for Graves’ disease. Pharmacotherapy. 2005;25:1017–20. doi: 10.1592/phco.2005.25.7.1017. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds CJ, Smith T. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br J Radiol. 1986;59:45–51. doi: 10.1259/0007-1285-59-697-45. [DOI] [PubMed] [Google Scholar]
- 9.de Vathaire F, Schlumberger M, Delisle MJ, Francese C, Challeton C, de la Genardiere E, et al. Leukaemias and cancers following iodine-131 administration for thyroid cancer. Br J Cancer. 1997;75:734–9. doi: 10.1038/bjc.1997.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballardin M, Gemignani F, Bodei L, Mariani G, Ferdeghini M, Rossi AM, et al. Formation of micronuclei and of clastogenic factor(s) in patients receiving therapeutic doses of iodine-131. Mutat Res. 2002;514:77–85. doi: 10.1016/s1383-5718(01)00323-0. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez S, Carbonell E, Galofre P, Creus A, Marcos R. Cytogenetic damage after 131-iodine treatment for hyperthyroidism and thyroid cancer. A study using the micronucleus test. Eur J Nucl Med. 1999;26:1589–96. doi: 10.1007/s002590050499. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe N, Kanegane H, Kinuya S, Shuke N, Yokoyama K, Kato H, et al. The radiotoxicity of 131I therapy of thyroid cancer: Assessment by micronucleus assay of B lymphocytes. J Nucl Med. 2004;45:608–11. [PubMed] [Google Scholar]
- 13.Polasa K, Naidu AN, Ravindranath I, Krishnaswamy K. Inhibition of B (a) P induced strand breaks in presence of curcumin. Mutat Res. 2004;557:203–13. doi: 10.1016/j.mrgentox.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Rong S, Zhao Y, Bao W, Xiao X, Wang D, Nussler AK, et al. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine. 2012;19:545–50. doi: 10.1016/j.phymed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Modulatory effects of curcumin on gamma-radiation-induced cellular damage in primary culture of isolated rat hepatocytes. Environ Toxicol Pharmacol. 2007;24:98–105. doi: 10.1016/j.etap.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Sebastia N, Montoro A, Montoro A, Almonacid M, Villaescusa JI, Cervera J, et al. Assessment in vitro of radioprotective efficacy of curcumin and resveratrol. Radiat Meas. 2011;46:962–6. [Google Scholar]
- 17.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 18.Lundqvist H, Antoni G, Langstrom B. Genotoxic hazard of radiopharmaceuticals in humans: Chemical and radiation aspects coupled to microdosing. Eur J Clin Pharmacol. 2007;63:641–5. doi: 10.1007/s00228-007-0304-6. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro Gil O, Oliveira NG, Rodrigues AS, Laires A, Ferreira TC, Limbert E, et al. Cytogenetic alterations and oxidative stress in thyroid cancer patients after iodine-131 therapy. Mutagenesis. 2000;15:69–75. doi: 10.1093/mutage/15.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez S, Carbonell E, Galofre P, Creus A, Marcos R. Micronuclei induction by 131I exposure: Study in hyperthyroidism patients. Mutat Res. 1997;373:39–45. doi: 10.1016/s0027-5107(96)00185-6. [DOI] [PubMed] [Google Scholar]
- 21.Fallahi B, Adabi K, Majidi M, Fard-Esfahani A, Heshmat R, Larijani B, et al. Incidence of second primary malignancies during a long-term surveillance of patients with differentiated thyroid carcinoma in relation to radioiodine treatment. Clin Nucl Med. 2011;36:277–82. doi: 10.1097/RLU.0b013e31820a9fe3. [DOI] [PubMed] [Google Scholar]
- 22.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–46. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid. 2009;19:451–7. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal DK, Mishra PK. Curcumin and its analogues: Potential anticancer agents. Med Res Rev. 2010;30:818–60. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 25.Ali T, Shakir F, Morton J. Curcumin and inflammatory bowel disease: Biological mechanisms and clinical implication. Digestion. 2012;85:249–55. doi: 10.1159/000336720. [DOI] [PubMed] [Google Scholar]
- 26.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin-from molecule to biological function. Angew Chem Int Ed Engl. 2012;51:5308–32. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 27.Kelkel M, Jacob C, Dicato M, Diederich M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules. 2010;15:7035–74. doi: 10.3390/molecules15107035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 29.Mythri RB, Bharath MM. Curcumin: A potential neuroprotective agent in Parkinson's disease. Curr Pharm Des. 2012;18:91–9. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RA, Leonard MC. Curcumin for inflammatory bowel disease: A review of human studies. Altern Med Rev. 2011;16:152–6. [PubMed] [Google Scholar]
- 31.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–30. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Jornet P, Camacho-Alonso F, Gomez-Garcia F. Effect of curcumin and irradiation in PE/CA-PJ15 oral squamous cell carcinoma. Acta Odontol Scand. 2011;69:269–73. doi: 10.3109/00016357.2011.554864. [DOI] [PubMed] [Google Scholar]
- 33.Agnihotri N, Mishra PC. Scavenging mechanism of curcumin toward the hydroxyl radical: A theoretical study of reactions producing ferulic acid and vanillin. J Phys Chem A. 2011;115:14221–32. doi: 10.1021/jp209318f. [DOI] [PubMed] [Google Scholar]
- 34.Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. 2011;6:e26012. doi: 10.1371/journal.pone.0026012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 36.Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–64. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 37.Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–8. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Wahlang B, Kabra D, Pawar YB, Tikoo K, Bansal AK. Contribution of formulation and excipients towards enhanced permeation of curcumin. Arzneimittelforschung. 2012;62:88–93. doi: 10.1055/s-0031-1295487. [DOI] [PubMed] [Google Scholar]
- 39.Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2012;69:679–89. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


