Abstract
Background:
Tamarix hohenackeri Bunge is a salt cedar that grows widespread in the desert mountains in Xinjiang. T. hohenackeri has not been investigated earlier, although there are many reports of phytochemical work on other Tamarix species.
Materials and Methods:
To find out natural angiotensin-converting enzyme (ACE) inhibitor and platelet aggregation inhibitors, the bioactive extract (ethyl acetate [EtOAc] fraction) from the dried aerial parts of T. hohenackeri were investigated. The active fraction was purified by repeated column chromatography, including silica gel, Sephadex LH-20 column, medium-pressure liquid chromatography (MPLC) (polyamide column) and high-performance liquid chromatography (HPLC). The isolated major constituents were tested for their anti-platelet aggregation activity.
Results:
Bioassay-directed separation of the EtOAc fraction of the 70% ethanol extract from the air-dried aerial parts of T. hohenackeri led to the isolation of a new triterpenoid lactone (1), together with 13 known compounds (2-14). It was the first time to focus on screening bioactive constituents for this plant. The chemical structures were established on the basis of spectral data (ESI-MS and NMR). The results showed that the flavonoid compounds (7 and 8) and phenolic compounds (9, 10, 11, and 14) were potential ACE inhibitors. And the flavonoid compounds (5 and 7) showed significant anti-platelet aggregation activities.
Conclusion:
On the basis of the chemical and biological data, the material basis of ACE inhibitory activity for the active part was the phenolic constituents. However, the flavonoid compounds were responsible for the anti-platelet aggregation. The primary structure and activity relationship were also discussed respectively.
Keywords: ACE inhibitors, bioassay-directed separation, new triterpenoid lactone, platelet aggregation inhibitors, Tamarix hohenackeri Bunge
INTRODUCTION
Tamarix L. is an important member of Tamaricaceae family, consisting of 20 species and one variety of Tamarix growing in China. They usually distribute in arid or semiarid desert area and saline-alkali fields in the various Northwest provinces of China.[1] Tamarix species are traditional medicinal plants used for the treatment of leukoderma, spleen troubles, and eye diseases,[2] as well as an astringent, aperitif, stimulus of perspiration, and diuretic.[3] The pharmacological and biological activities study revealed that the extract of some species of Tamarix plants showed hepatoprotective,[4,5,6] antioxidant,[7] antibacterial,[8] anti-inflammatory,[9] antineoplastic,[10] and inhibitive on α-glucosidase[11] effects. The plants of this genus are also famous as the main host plants of valuable and rare traditional Chinese herbal medicine Herba Cistanches, which has obvious effects of supplementing kidney. Now, Tamarix hohenackeri Bunge and Tamarix ramosissima Ledeb are widely used as host plants in large-scale cultivation of Herba Cistanches in Xinjiang. The phytochemical research has revealed that some species of Tamarix plants are rich in flavonoids, triterpenes, phenylpropanoids, organic acids, steroids, tanins, and lignans.[12,13] However, there are few reports on T. hohenackeri Bunge.
As we know, angiotensin I-converting enzyme (ACE) plays a critical physiological role in the regulation of blood pressure.[14] ACE can increase blood pressure by converting an inactive form of decapeptide (angiotensin I) into a potent vasoconstrictor angiotensin-II (anoctapeptide) and inactivating catalytic function of bradykinin, which has depressor action.[15]
Blood platelets are implicated in the hemostatic process and also in thrombus formation, which is one of the most important contributors to pathogenesis of many circulatory diseases and inflammatory conditions.[16,17,18,19] Thus, anti-platelet compounds have wide therapeutic potential for various circulatory diseases.
In our previous research on screening ACE inhibitory and anti-platelet aggregation active extracts from herbs distributing in Xinjiang, the 70% EtOH extract and EtOAc-soluble part of T. hohenackeri showed significant ACE inhibitory and anti-platelet aggregation activities. In order to reveal the natural ACE and platelet aggregation inhibitors from T. hohenackeri, the bioactivity-guided fractionation and chemical identification were carried out. In the present study, we just want to discuss the separation and characterization of main constituents including a new triterpenoid lactone (1), together with 13 known compounds 2-14. It is the first time to focus on the biochemical constituents of this plant, and all the compounds described in this manuscript were reported firstly. Furthermore, the ACE inhibitory and anti-platelet aggregation activities of the extracts and purified compounds from the active fraction (EtOAc extract) were evaluated systematically and the material–activity relationship and structure–activity relationship were discussed according to the result.
MATERIALS AND METHODS
Mass spectra were measured using Shimadzu QP-2010 Plus (Japan). NMR spectra were recorded on Bruker ARX-300 and ARX-600 spectrometers, used CDCl3 or DMSO-d6 as solvents with TMS as the internal standard. HR-ESI-MS spectra were obtained using Bruker APEX 7.0 Tesla FT-MS apparatus; in m/z (rel. %). Silica gel (SiO2: 200-300 mesh, Qingdao Marine Chemical Company, P. R. China), Sephadex LH-20 (Pharmacia, Co., Switzerland), macroporous resin HPD-100 (Cangzhou Bonchem Co., Ltd., P. R. China), polyamide (Taizhou City Luqiao Sijia Biochemical Plastic Factory, P. R. China) were used for column chromatography. Prep. semi-preparative HPLC was carried out on a YMC ODS C-18 column (250 mm × 10 mm, 10 μm, ODS-A, Co., Ltd. Japan) and Shimadzu SPD-10A UV detector. Silica gel GF254 was used for TLC (SiO2: 200-300 mesh, Qingdao Marine Chemical Company, P.R. China). The chromatograms were visualized under UV light (at 254 and 365 nm) before and after spraying with sulfuric acid-ethanol (10%) spray reagent, ferric chloride-ethanol spray reagent and bromocresol green-ethanol-sodium hydroxide spray reagent. Other chemicals and solvents in this experiment were all analytical grade or higher and from Shandong Yuwang Co., Ltd. Chemical Engineering Branch and Tianjin Damao Chemical Reagent Co.
Plant material
The aerial parts of T. hohenackeri Bunge were collected from Hetian district of Xinjiang province in China, in June 2011. The plant material was identified by Prof. Xiaoguang Jia. (Xinjiang Institute of Chinese Materia Medica and Ethnodrug). A voucher specimen (No. 20110613) is deposited in School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University.
Extraction and isolation
The air-dried aerial parts (6.8 kg) were powdered and refluxed with 70% ethanol (3 × 9 L) to yield the ethanol extract (1850 g). The residue (1800 g) was suspended in distilled water and partitioned with petroleum ether, ethyl acetate, and n-butanol (saturated with water). The solvents were evaporated separately under reduced pressure to yield petroleum ether extract (56 g), ethyl acetate extract (75 g), n-butanol extract (235 g), respectively. The ethyl acetate fraction (73 g) was subjected to column chromatography on silica gel (200-300 mesh, 130 × 8 cm, 662 g) gradiently eluted with CH2Cl2/MeOH (100:0-0:1) to give 12 fractions (F1 -F12). Compound 1 (16.8 mg) was recrystallized in acetone from F2 (1.5 g). F3 (2.0 g) was subjected to Sephedax LH-20 (100 × 2 cm) eluted with MeOH to give compound 3 (18.6 mg). F5 (7.5 g) was purified on silica gel column (60 × 3.5 cm, 85 g) eluted with PE/acetone (in gradient, 100:0-0:1) to yield subfractions (Subfr. 5-1-Subfr. 5-8). Compound 9 (13.3 mg) was isolated from Subfr. 5-3 using recrystallization from acetone. Subfr. 5-4 was separated over MPLC (MeOH/H2O 10:90-100:0) to give compound 4 (32.1 mg). Subfr. 5-6 was subjected to column chromatography over Sephadex LH-20, eluting with gradient MeOH/H2O to obtain compound 5 (11.8 mg). Compound 6 (18.0 mg) and 7 (25.6 mg) were isolated from F6 (8.6 g) by chromatography on macroporous resin eluted with MeOH/H2O (6:4 and 9:1), and silica gel eluted with PE/acetone (10:3). F7 (14.7 g) was applied to CC on silica gel column (80 × 5 cm, 150 g) with PE/acetone gradient system, MPLC (MeOH/H2O 8:2) and then purified by Sephedax LH-20 to give compound 2 (17.3 mg). F8 (2.3 g) was separated by macroporous resin column chromatography using MeOH/H2O (0:1, 3:7, 6:4, 9:1) to obtain compound 10 (201.3 mg). F9 (5.5 g) was chromatographied on macroporous resin column eluted with MeOH/H2O (3:7, 6:4, 9:1) and then purified by Sephadex LH-20 (MeOH) to give compound 8 (37.6 mg). F10 (7.2 g) was subjected to MPLC on polyamide column (MeOH/H2O 10:90-100:0), silica gel (CH2Cl2/MeOH, 25:1-1:1), Sephadex LH-20 (MeOH), was purified by HPLC (RP-18, 11:89 MeOH/H2O + 0.05% HAc) to give compound 12 (tR = 47 min, 12.4 mg) and 14 (tR = 115 min, 10.1 mg). F11 (4.9 g) was subjected to polyamide column (MeOH/H2O 10:90-100:0) and purified by Sephadex LH-20 (MeOH) to give compound 11 (253.1 mg).
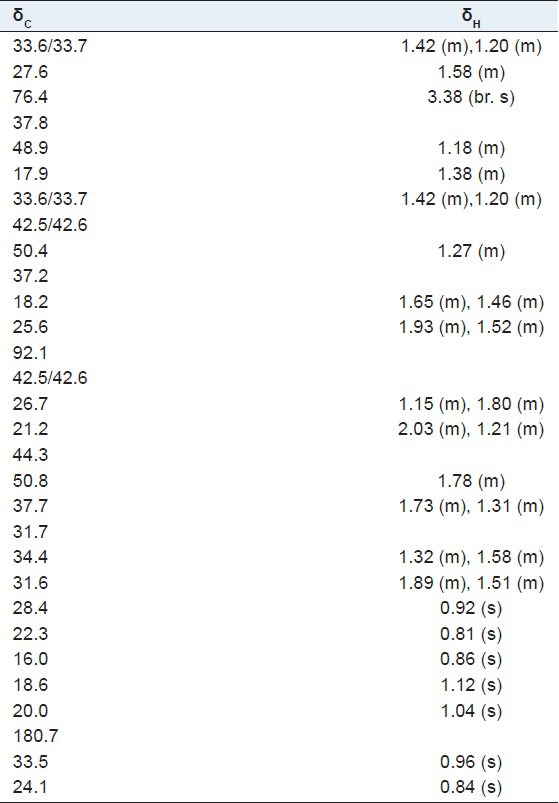
3α-hydroxyolean-28,13-olide (1): White amorphous powder; 1H-NMR (600 MHz, DMSO-d6) and 13C-NMR (150 MHz, DMSO-d6) spectral data, see Table 1; HR-ESI-MS: m/z 479.3496 [M + Na]+ (calcd. for C30H48O3Na, 479.3475).
Table 1.
1H-NMR (600 MHz), 13C-NMR (150 MHz) spectral data in CDCl3 of compound 1
Angiotensin I-converting enzyme assay
For the assay, 1U ACE was dissolved in 5 mL borate buffer (pH 8.3), and each 0.5 mL of enzyme solution was added 1 mL of borate buffer (pH 8.3) before working. Hip-His-Leu (7.6 mmol/L) were prepared in borate buffer (0.2 mol/L, pH 8.3, containing 608 mmol/L of NaCl). Briefly, 15 μL ACE extract was incubated with 5 μL samples for 30 mins at 37°C in 0.5 mL centrifuge tube. Then, 25 μL HHL was added and incubated for 15 mins at 37°C. Afterward, the action was terminated by adding 5 μL 10% TFA.[20] The reaction buffer was directly analyzed in HPLC autoinjection system (mobile phase: 30% methol-1 mL TFA-0.5 mL glacial acetic acid (pH 3-3.3), 70% methol-1 mL TFA-0.5 mL glacial acetic acid (pH 3-3.3) eluted in gradient, AR; detection wavelength: 228 nm; flow rate: 1 mL/min). The resulting hippuric acid was quantitative analyzed by HPLC. The results are listed in Figure 1 and Table 2.
Figure 1.
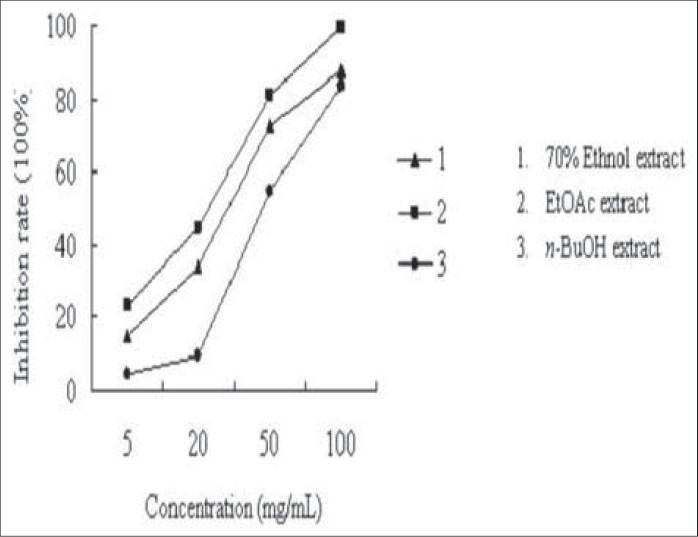
The ACE inhibiting activities of different extracts of Tamarix hohenackeri Bunge (1. 70% ethanol extract; 2. EtOAc extract; 3. n-BuOH extract)
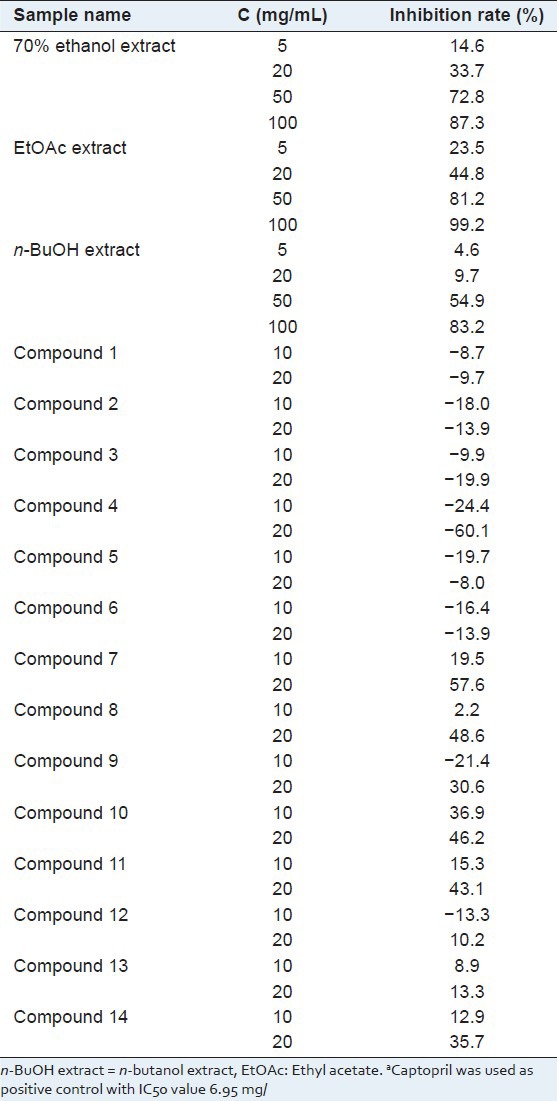
Table 2.
The ACE inhibiting activities of extracts and compounds isolated from the active fraction (EtOAc extract)a
Blood collection and preparation of platelet rich plasma (PRP) and platelet poor plasma[21]
Platelets were drawn from healthy male rat (215 g) into 15% (v/v) acid–citrate–dextrose (ACD: 1.5 mM citric acid, 75 mM sodium citrate, 124 mM dextrose). platelet rich plasma (PRP) was prepared by centrifugation of citrated blood at 800 g for 10 min. Platelets were washed and resuspended in buffer A (130 mM NaCl, 10 mM sodium citrate, 9 mM NaHCO3, 6 mM dextrose, 9 mM MgCl2, 0.81 mM KH2PO4 3 mM KCl, 10 mM Tris [tris (hydroxymethyl) aminomethane], pH 7.4), adjusted to 3 × 105/μl, and supplemented with 1.8 mM CaCl2.
Inhibition ratio for platelet aggregation
Platelets were resuspended in buffer A to a consistent concentration (OD value: 1.02) and were allowed to rest for 15 min prior to use. Calcium (1.8 mM) and ADP (20 mM) were added just prior to assay. Platelet aggregations were performed at 37°C. The platelet suspension was added to the 96-well plate with the amount of 100 μl/well. The tested samples were dissolved in DMSO and diluted with buffer A to different concentration. Aspirin was used as positive control and the buffer A (0.1% DMSO) was used as negative control. The OD values were recorded in Flexstation® Enzyme-labelled meter (molecular device) at 405 nm. All the samples were pre-incubated at 37°C for 10 min by sustained oscillation. Then 50 μl antagonists (containing ADP and calcium) were added to the samples. Aggregation was monitored for 30 min after the addition of agonist. The OD values were recorded every 45 s in Flexstation® Enzyme-labelled meter (molecular device) at 405 nm and the aging kinetic curves were expressed. Platelet aggregation rate was calculated by the calculation formula as follows:
At = (OD0 - ODt/ODt × 100%
The maximal platelet aggregations of test compounds were selected to calculate the inhibition ratio, which expressed as percentages maximal platelet aggregation of negative normalized to 100% by the calculation formula as follows:
Inhibition rate = (ANmax − ATmax)/ANmax × 100%
RESULTS AND DISCUSSION
The EtOAc extraction was identified as active part because of its significant ACE inhibitory activity (100 mg/mL, 99.2% inhibiting rate) and anti-platelet aggregation activity (100 μg/mL, 99.2% inhibiting rate). In turn, the active part was purified for ACE and platelet aggregation inhibitors. Fourteen main constituents were isolated and elucidated. Meanwhile, the potential activities were investigated systematically.
Compound 1 was obtained as white amorphous powder. The positive HR-ESI-MS of compound 1 showed a molecular ion peak at m/z 479.3496 [M + Na]+, which indicated a molecular formula of C30H48O3, in conjunction with the 13C-NMR data. The 1H and 13C-NMR spectra of 1 showed characteristics of the oleanane skeleton. The 1H-NMR spectrum displayed seven singlets for δ 0.81, 0.84, 0.85, 0.92, 0.96, 1.04, 1.12 (7 × 3H, s) and 30 carbon signals were observed in the 13C-NMR spectrum. The presence of a quaternary carbon signal at δ 180.7 (C-28) suggested a carboxyl ester or γ-lactone. While the signal at δ 92.1 in the 13C-NMR spectrum showed that the oxygen-bearing carbon in the lactone group was tertiary carbon on either C-13 or C-18. Heteronuclear multiple-bond correlation (HMBC) [Figure 2] between δ 1.04 (3H, s, H-27), 1.15 (1H, m, H-15), 1.78 (1H, m, H-18), and δ 92.1 (C-13); δ 2.03 (1H, m, H-16) and δ 180.7 (C-28) indicated the presence of 28,13-lactone. In the 1H-NMR spectrum, the significant downfield were observed in the methyl region at δ 1.12 (m, H-26) due to the strong anisotropic effect of the lactone group at C-28, while the C-27 methyl appeared at δ 1.04. The facts provided further evidence for the 28, 13-lactone.[22,23] The HMBC spectrum [Figure 2] showed the long-range correlations between methyl proton signals at δ 0.92 (3H, s, H-23), 0.81 (3H, s, H-24), and δ 76.4 (C-3), 48.9 (C-5). The relative configuration of 1 was established by the NOESY spectrum [Figure 3], and a comparison with pertinent reference values.[24,25,26] The configuration of the hydroxyl group at C-3 could be determined as α-oriented on the basis of the coupling pattern of H-3 (δ 3.38, br. s). In the NOESY spectrum, the correlations of H-1β/H-3, H-9/H-27, H-18/H-29, H-5/H-23 indicated H-3, H-9, H-18 were β-oriented, while OH-3, H-5 were α-oriented. Based on above data, the structure of 1 was elucidated as 3α-hydroxyolean-28, 13-olide. The assignment of 1H and 13C-NMR spectroscopic data of 1 was based on HSQC, HMBC spectra and listed in Table 1.
Figure 2.
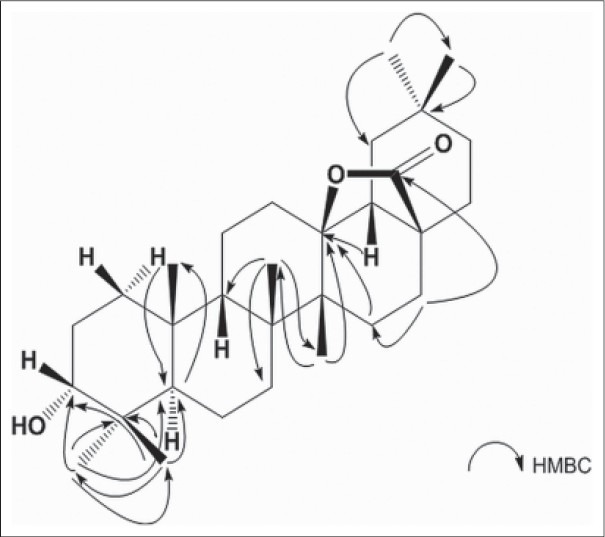
Selected correlations observed in the HMBC spectra of 1
Figure 3.
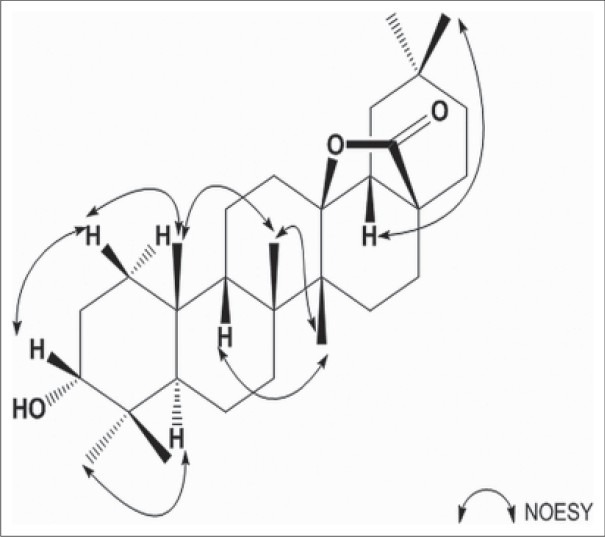
Key correlations observed in the NOESY spectra of 1
By comparison their physical and spectral data (ESI-MS, 1H-NMR, 13C-NMR) with those reported, the known compounds were characterized to be isoaleuritolic acid 3-(3’,4’- dihydroxy) caffeate (2),[27] 7, 4’- dimethoxykaemferol (3),[28] 4’- methoxykaempferol (4),[10] dillenetin (5),[29] tamarixetin (6),[30] chrysoeriol (7),[31] querceti (8),[32] isoferulic acid (9),[33] 3,5-dihydroxy-4- methoxybenzoic acid (10),[34] gallic acid (11),[35] protocatechuic acid (12),[36] gallic acid 3-methyl ether (13),[37] methyl gallate (14).[38] Structures of the isolated compounds are shown in Figure 4.
Figure 4.
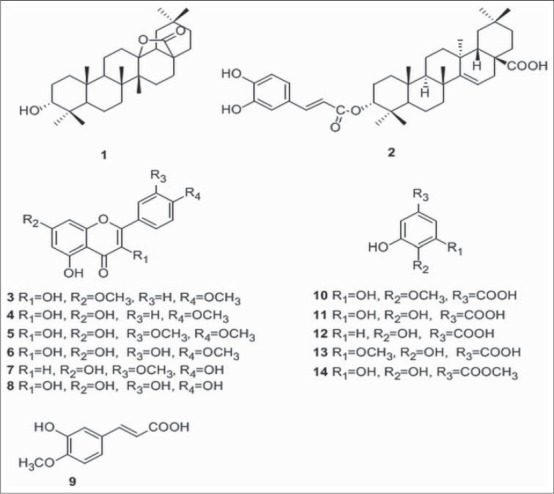
Structures of compounds 1-14
Then, the ACE inhibitory activity of the extracts and compounds isolated was investigated at different concentrations. The results are listed in Figure 1 and Table 2. The flavonoid compounds 7 and 8, and phenolic compounds 9, 10, 11, 14 showed strong ACE inhibitory activity. The phenolic compounds 12 and 13 exhibited moderate ACE inhibiting activity. However, the isolated triterpene did not give any ACE inhibitory activity. Considering the concentration of the isolated compounds, it is obvious that 3, 5-dihydroxy-4-methoxybenzoic acid (10) and gallic acid (11) make sense in ACE inhibiting activity. By analyzing the structures of tested compounds, the primary structure and activity relationship was concluded. First, comparing the activities of flavonoid compounds, it is suggested that 4′-hydroxy substitution might play an important role in flavonoid compounds for ACE inhibitory activity. Second, according to the activity result of compounds 10–14, we can summarize that the benzoic acids with 3,5-dihydroxy substitution exhibit better activity. Third, by comparing compounds 11 and 14, it can also be conferred that the benzoic acids showed stronger activity than benzoate.
Meanwhile, the anti-platelet aggregation activities were also investigated systematically Table 3. Dillenetin (5) and chrysoeriol (7) showed significant anti-platelet aggregation activity, respectively by 40.05% and 50.36% at a concentration of 200 μM/mL, which is much better than that of aspirin as a positive control in our experiment. However, the compounds 6, 8, 11, and 14 showed moderate inhibiting activities. Compounds 1, 9, 10, 12, and 13 exhibited weak activities. Obviously, the flavonoid compounds are responsible for the anti-platelet aggregation activities of this plant. The analysis of structure and activity relationship revealed that the 3′-methoxyl substitution might play a significant role in flavonoid compounds for anti-platelet aggregation activities. Moreover, according to the activities of compounds 10-14, we can conclude that benzoic acids with 3, 4, 5-trihydroxy substitution exhibit better activity.
Table 3.
The anti-platelet aggregation activities of extracts and compounds isolated from the active fraction (EtOAc extract)
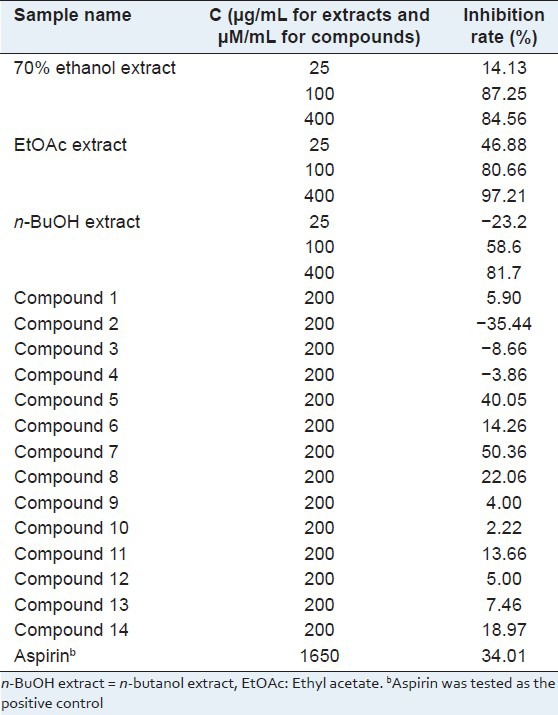
Above all, compounds 7, 8, and 11 are both natural potential ACE and platelet aggregation inhibitors.
Footnotes
Source of Support: This work was supported by National Natural Science Foundation of China (81001371), HYPERLINK “javascript:showjdsw(‘jd_t’, ‘j_’)” International Cooperation Project of Xinjiang Science and Technology Bureau (2010DFB30540), Special Fund of Technology for Xinjiang (201191152), Urumqi Scientific and Technological Projects (Y111120004), Science and Technology Foundation of Dalian city (2011J21DW012)
Conflict of Interest: None declared.
REFERENCES
- 1.Zhang DY, Pan BR, Yin LK. The photogeographical studies of Tamarix (Tamaricaceae) Acta Bot Yunnanica. 2003;25:415–27. [Google Scholar]
- 2.Sharma SK, Parmar VS. Novel constitutes of Tamarix species. J Sci Ind Res. 1998;57:873–90. [Google Scholar]
- 3.Gaston B. Vol. 1. Paris: Berlin Publishing House; 1998. The high flora in colors. France, Switzerland, Belgium and country neighbors; p. 373. [Google Scholar]
- 4.Assiri AM. Protective effect of Tamarix amplexicaulis seeds against hepatotoxicity induced by carbon tetrachloride, paracetamol, or D-galactosamine. J Saudi Chem Soc. 2006;10:437–46. [Google Scholar]
- 5.Sehrawat A, Sultana S. Tamarix gallica ameliorates thioacetamide–induced hepatic oxidative stress and hyperproliferative response in Wistar rat. J Enzyme Inhib Med Chem. 2006;21:215–23. doi: 10.1080/14756360500480673. [DOI] [PubMed] [Google Scholar]
- 6.Abouzid S, Sleem A. Hepatoprotective and antioxidant activities of Tamarix nilotica flowers. Pharm Biol. 2011;49:392–5. doi: 10.3109/13880209.2010.518971. [DOI] [PubMed] [Google Scholar]
- 7.Abouzid SF, Ali SA, Choudhary MI. A new ferulic acid ester and other constituents from Tamarix nilotica Leaves. Chem Pharm Bull (Tokyo) 2009;57:740–2. doi: 10.1248/cpb.57.740. [DOI] [PubMed] [Google Scholar]
- 8.Saidana D, Mahjoub MA, Boussaada O, Chriaa J, Cheraif I, Daami M, et al. Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveana. Microbiol Res. 2008;163:445–55. doi: 10.1016/j.micres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Barakat HH, Nada SA. Chemical and biological investigations of the constitutive phenolics of two enyptian folk-medicinal plants: A novel phenolic from the galls of Tamarix aphylla. Nat Prod Sci. 1996;2:96–101. [Google Scholar]
- 10.Wang B, Ren SW, Li GQ, Guan HS. Studies on antitumor steroids and flavonoids from Tamarix chinensis Lour. Chin Pharm J. 2009;44:576–80. [Google Scholar]
- 11.Chang X, Cui WH, Zhang JK, Wu YH, Kang WY. Glucosidase inhibitory activity of Tamarix chinensis Lour. and Tamarix ramosissima Ledeb. in Nei Meng-gu. Nat Prod Res Dev. 2011;23:146–8. [Google Scholar]
- 12.Zhang Y, Tu PF. Advances in studies on medicinal plants of Tamarix Linn. Chin Tradit Herb Drugs. 2008;39:947–51. [Google Scholar]
- 13.Huang SW, Liang JY. Progress of studies on Tamarix Linn. Strait Pharm J. 2007;19:5–9. [Google Scholar]
- 14.Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–9. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin converting enzyme: A new class of orally active antihypertensive agents. Science. 1977;196:441–4. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 16.Helft G, Worthley SG. Anti-thrombotic, anti-platelet and fibrinolytic therapy: Current management of acute myocardial infarction. Heart Lung Circ. 2001;10:68–71. doi: 10.1046/j.1444-2892.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 17.Rand ML, Leung R, Packham MA. Platelet function assays. Transfus Apher Sci. 2003;28:307–17. doi: 10.1016/S1473-0502(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 18.Savage B, Cattaneo M, Ruggeri ZM. Mechanisms of platelet aggregation. Curr Opin Hematol. 2001;8:270–6. doi: 10.1097/00062752-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Steinhubl SR, Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. Am J Cardiovasc Drugs. 2005;5:399–408. doi: 10.2165/00129784-200505060-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 21.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 22.Katai M, Terai T, Meguri H. Triterpenoids of the bark of Pieris japonica D. Don. C Nuclear magnetic resonance of the γ-Lactones of Ursane- and Oleanane-type Triterpenes. Chem Pharm Bull. 1983;31:1567–71. [Google Scholar]
- 23.Caldwell CG, Franzblau SG, Suarez E, Timmermann BN. Oleanane triterpenes from Junellia tridens. J Nat Prod. 2000;63:1611–4. doi: 10.1021/np0002233. [DOI] [PubMed] [Google Scholar]
- 24.Young MC, Potomata A, Chu EP, Haraguchi M, Yamamoto M, Kawano T. 13C-NMR analysis of monodesmosidic saponins from gomphrena macrocephala. Phytochemistry. 1997;46:1267–70. [Google Scholar]
- 25.Hu J, Shi X, Chen J, Huang H, Zhao C. Cytotoxic taraxerane triterpenoids from Saussurea graminea. Fitoterapia. 2012;83:55–9. doi: 10.1016/j.fitote.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell CG, Franzblau SG, Suarez E, Timmermann BN. Oleanane Triterpenes from Junellia tridens. J Nat Prod. 2000;63:1611–4. doi: 10.1021/np0002233. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Dai SJ, Chen RY, Yu DQ. Triterpenoids from the stems of Myricaria paniculata. J Asian Nat Prod Res. 2005;7:253–7. doi: 10.1080/10286020410001721168. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Tu PF. Chemical constituents of Tamarix ramosissima. Chin Tradit Herb Drugs. 2006;37:1764–8. [Google Scholar]
- 29.Zhang Y, Yuan Y, Cui B, Li S. Study on chemical constituents from ethyl acetate extract of Myricaria bracteata. Zhongguo Zhong Yao Za Zhi. 2011;36:1019–23. [PubMed] [Google Scholar]
- 30.Cao JQ, Sun SW, Chen H, Wang YN, Pei YH. Studies on flavonoids from Blumea riparia. Zhongguo Zhong Yao Za Zhi. 2008;33:782–4. [PubMed] [Google Scholar]
- 31.Yang NY, Ren AN, Hu WC, Qian SH, Duan JA, Tian LJ. Chemical constituents of Dendranthema nankingense. J Chin Pharm Univ. 2005;36:402–4. [Google Scholar]
- 32.Dong JY, Jia ZJ. Studies on chemical constituents of flavonoids in Hypericum attenuatun. Chin Pharm J. 2005;40:897–9. [Google Scholar]
- 33.Jiang HL, Yang XD, Zhang D, Xu LJ, Yang SL, Zou ZM. Chemical constituents of Siphonostegia chinensis. Chin Pharm J. 2003;38:97–9. [Google Scholar]
- 34.Kong LJ, Liang QL, Wu QN, Jiang JH. Chemical constituents of Sparganium stoloniferum. Chin Tradit Herb Drugs. 2011;42:440–2. [Google Scholar]
- 35.Zhang HL, Chen K, Pei YH, Hua HM. Research on the chemical constituents of Terminalia chebula Retz. J Shenyang Pharm Univ. 2011;18:417–8. [Google Scholar]
- 36.Chen P, Yang JS. Chemical constituents of Livistona chinensis seeds. Chin Pharm J. 2008;43:1669–70. [Google Scholar]
- 37.Souleman AM, Barakat HH, Hussein SA, el-Mousallamy A, Nawwar MA. Unique phenolic sulphate conjugates from the flowers of Tamarix amplexicaulis. Nat Prod Sci. 1998;4:245–52. [Google Scholar]
- 38.Shang XY, Li S, Wang YH, Wang SJ, Yang YC, Shi JG. Chemical constituents of Bauhinia aurea. Zhongguo Zhong Yao Za Zhi. 2006;31:1953–5. [PubMed] [Google Scholar]


