Abstract
Background:
North East India is a rich source of medicinal plants and a number of plant extracts are used by tribal peoples living in this area for various disorders. L.aspera is such a plant, traditionally used as an antitumor agent.
Aim:
In the present study, aerial parts of L.aspera were investigated for antitumor activity in Dalton's lymphoma (DAL) bearing mice. The ability of plant extract in free radical scavenging, neoangiogenesis inhibition and macrophage stimulation were also checked.
Materials and Methods:
Based on the preliminary in vitro cytotoxicity studies ethyl acetate fraction of L.aspera (EALA) was selected for the detailed study. DAL ascites tumor model was performed to check the antitumor activity of EALA (200 and 400mg/kg of body weight). Hematological and histopathological parameters were estimated. Antioxidant levels, neoangiogenesis and peritoneal macrophage count were also determined.
Results:
In vitro MTT and Trypan blue assay results showed the cytotoxic effect of EALA in DAL cells lines. EALA treatment resulted in significant decrease in ascites tumor volume and viable cell count. Hematological and liver antioxidant parameters were normalised by EALA treatment. It was also found that EALA treatment inhibits neovascularisation and produce macrophage stimulation in treated mice.
Conclusion:
The results showed that EALA is a promising anticancer agent and its activity is comparable to the standard drug 5-Flouro uracil (5-FU).
Keywords: Antioxidant, antitumor, L.aspera, macrophage stimulation, neoangiogenesis
INTRODUCTION
Cancer is one of the most serious diseases that damage human health in the modern world and the second largest deadly disease just below heart disease.[1] Currently available anticancer drugs like alkylating agents, anti-metabolites, apoptosis inducers, cell cycle inhibitors and hormonal regulators are harmful to the normal cells in great extent. So, there is an increased interest in new drug molecules of plant origin as they are less likely to cause serious side effects.[2] Recent studies show that oxidative stress and neoangiogenesis plays good role in the initiation and development of cancer.[3] It is also reported that activated macrophages have an important role in cancer therapy, since they can kill tumor cells.[4] Studies are claiming that ingestion of natural antioxidants reduces the risk of arthritis, cardiovascular diseases, cancer and other diseases associated with ageing.[5,6] Hence; the development of an anticancer plant molecule with antioxidant, neoangiogenesis inhibition and macrophage stimulatory properties may improve the conventional anticancer treatment.
L.aspera is a weed plant, widely available in Assam and used in indigenous system of medicine for variety of ailments.[7] Literature survey reveals that extracts of L.aspera exhibit analgesic, anti-inflammatory, anti-arthritic and anti-pyretic efficacies.[8] Extract prepared from root parts of the plant showed significant antinociceptive, antioxidant and cytotoxic activities.[9] The present study was designed to evaluate the antitumor effect of L.aspera in DAL bearing mice, with emphasis on antioxidant, anti-angiogenesis and macrophage stimulatory properties of the plant extract.
MATERIALS AND METHODS
Drugs and chemicals
Trypan blue, MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide), RPMI medium, Foetal bovine serum (FBS), 5-FU, Lowry reagent, Folin ciocaltease reagent, DTNB (5, 5’- dithio-bis-(2-nitrobenzoic acid), Catalase assay kit (Lot#062M4144V) and SOD assay kit (Lot#BCBJ2137V) were purchased from Sigma Aldrich. All other chemicals used were of analytical grade.
Animals
The study was carried out using Swiss albino mice weighing 20 ± 5 gm. They were obtained from the Central Animal Facility of National Institute of Pharmaceutical Education and Research, Guwahati. The mice were grouped, housed in polyacrylic cages and maintained under standard laboratory conditions (temperature 25 ± 2°C) with light/dark cycle (12/12 h). They were allowed free access to standard dry pellet diet and water ad libitum. All experimental studies were done after getting permission from the Institutional Animal Ethics Committee, Gawahati Medical College, Guwahati (MC/32/2012).
In vitro cytotoxicity studies
A Pilot study (Trypan blue and MTT assay) of various extracts of L.aspera were performed in DAL cells and the ethyl acetate fraction showed good results as compared to the other extracts. Hence EALA was selected for the detailed study.
Evaluation of in vitro antitumor property
Experimental design
Swiss albino mice (n = 6) were divided in to 5 groups:
Group 1: Normal control (0.5% CMC 10 ml/kg p.o.)
Group 2: Tumor control (0.5% CMC 10 ml/kg p.o.)
Group 3: Standard control (5-FU 20 mg/kg i.p.)
Group 4: Test1 (EALA 200 mg/kg p.o.)
Group 5: Test2 (EALA 400 mg/kg p.o.).
All the animals were injected with DAL cells (1 × 106 cells/mouse) i.p, except the normal group, for the development of ascites tumor. After 24h of tumor inoculation, treatments were given daily for a period of 14 days as described above.
Tumor growth response
All treatments were given continuously for 14 days as described in the experimental design. On 15th day tumor fluid aspirated from peritoneum and measured. The viability of the DAL cells was checked by try pan blue assay.
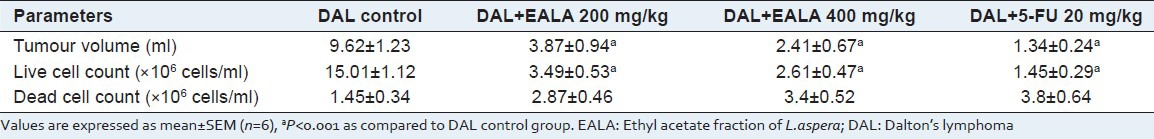
Hematological parameters
On 15th day blood was collected from the above animals by retro orbital plexure method. Whole blood was used for the estimation of hemoglobin (Hb) content,[10] red blood cells (RBC) count, and white blood cells (WBC) count. RBC and WBC counting was performed using Countess cell counter.
Antioxidant parameters
After collection of blood samples, the mice were sacrificed. Then the liver was excised, rinsed in ice cold normal saline followed by ice cold 10% KCl solution, blotted, dried and weighed. A 10% (w/v) homogenate was prepared in ice cold KCl solution and centrifuged at 1500rpm for 15 min at 4°C. The supernatant thus obtained was used for the estimation of lipid peroxidation (LPO),[11] glutathione (GSH),[12] and total protein (TP).[13] Super oxide dismutase (SOD) and Catalase (CAT) level were checked using respective kits according to manufacturer's instruction. A part of the dissected liver from all the groups were cleared off of the surrounding tissues and used for histological study.
Antiangiogenic study
After sacrificing the animals on 15th day, peritoneum of the mice were removed and the inner lining of the peritoneal cavity were examined for angiogenesis. Photographs of the peritoneal linings were captured. Histopathological specimen of peritoneum was prepared as described above. The sections were observed under low power light microscope to identify the highly vascularised areas. The micro vessel density (MVD) was counted in ten fields of these vascularised areas under low power focus and the average MVD was noted.[14]
Macrophage stimulation study
Swiss albino mice were divided in to 3 groups containing 6 animals. Group 1 was kept as control (0.5% CMC p.o). Group 2 and group 3 mice were treated with EALA 200 mg/kg and 400 mg/kg p.o. All treatments were given for a period of 7 days. On the 8th day, peritoneal cavities of the animals were washed with PBS and cells collected. Peritoneal cells were counted using Countess cell counter.[15]
Statistical analysis
Values were expressed as mean ± SEM. Statistical analysis was performed using one way ANOVA (Graph pad prism version 6) followed by Dunnets post hoc test and values of P < 0.05 were considered to be statistically significant.
RESULTS
Evaluation of in vitro antitumor property
Tumor growth response
Following the inoculation of DAL cells, there was profound proliferation of tumor cells along with tumor fluid in the peritoneal cavity of the mice. Both doses of EALA and 5-FU significantly reduced the amount of tumor fluid and live tumor cell count (aP < 0.001) as compared to tumor control. Moreover, plant extract treatment increased the number of dead cell count [Table 1].
Table 1.
Effect of EALA on tumour growth response in DAL mice
Hematological parameters
On 15th day, hematological parameters were estimated. It was found that in tumor control group, the RBC and Hb counts were drastically reduced, but WBC count became doubled as compared to negative control. In treatment group (EALA 400 mg/kg) and 5-FU group, there were no significant changes in hematological parameters [Figure 1].
Figure 1.
In vitro cytotoxic effect of EALA on DAL cells by trypan blue exclusion assay. All values are mean ± SEM, n=6. IC50 for EALA = 29.4 μg/ml; and 5-FU = 28.2 μg/ml
Antioxidant parameters
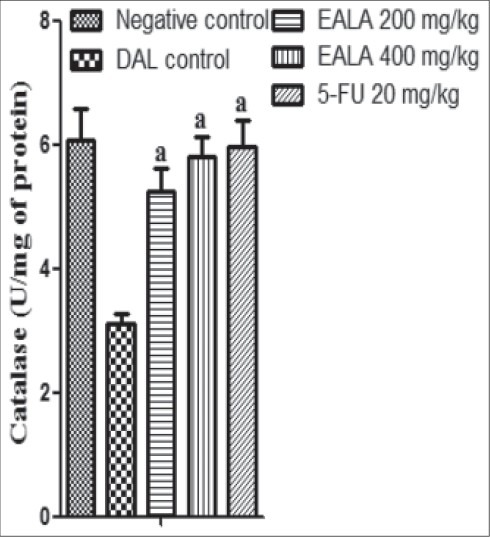
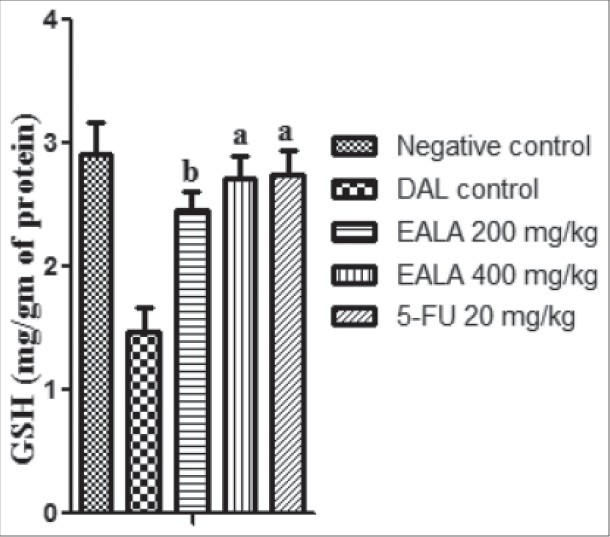
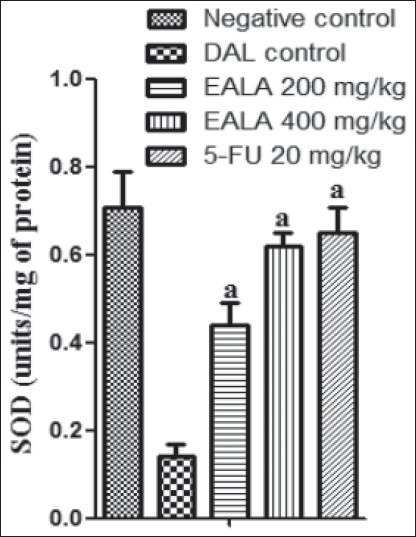
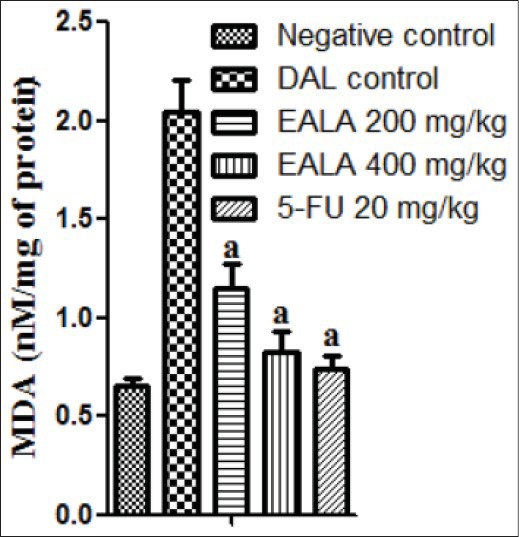
It was found that liver antioxidant markers like Catalase, SOD and GSH level were reduced significantly (aP < 0.001) in DAL control group as compared to negative control. But the Malon di aldehyde (MDA) content of DAL control group was elevated markedly. It was observed that, treatment with both doses of EALA and standard drug were restored the liver enzyme levels [Figures 2–5]. Histological examination of the liver tissue was performed to check the hepatoprotective effect of EALA. Steatosis and lymphocyte accumulation were widely observed in DAL tumor group while animals treated with EALA 400 mg/kg showed almost normal liver histology [Figure 6].
Figure 2.
Effect of EALA and 5-FU on mean survival time in DAL bearing mice. Values are expressed as mean ± SEM (n=6), aP<0.001 as compared to DAL control group
Figure 5.
Effect of EALA and 5-FU on antioxidant parameters (Catalase) in DAL bearing mice. Values are expressed as mean± SEM (n=6). aP<0.001 as compared to DAL control group
Figure 6.
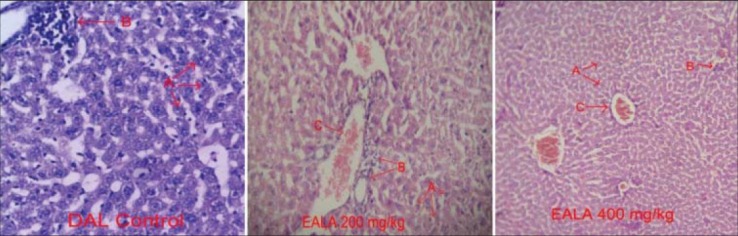
Histology of liver stained with H and E (×100) of mice from the different groups, on day 15 of the experiment (n=6). A: Hepatocytes, B: lymphocytes, C: Portal area
Figure 3.
Effect of EALA and 5-FU on percentage increase in life span in DAL bearing mice. Values are expressed as mean (n=6)
Figure 4.
Effect of EALA and 5-FU on change of body weight in DAL bearing mice. Values are expressed as mean± SEM (n=6), aP<0.001 as compared to DAL control group
Anti-angiogenic study
The peritoneum of DAL tumor control mice showed greater number of blood vessels. The peritoneum photographs showed that both doses of EALA inhibited the tumor induced new blood vessel synthesis [Figure 7]. The hematoxylin and eosin stained peritoneal tissue sections showed increased MVD in DAL tumor group as compared to the treatment group [Figure 8]. MVD count analysis showed that EALA 400 mg/kg and EALA 200 mg/kg significantly inhibited the neoangiogenesis (aP < 0.001), (bP < 0.01) respectively [Figure 9].
Figure 7.
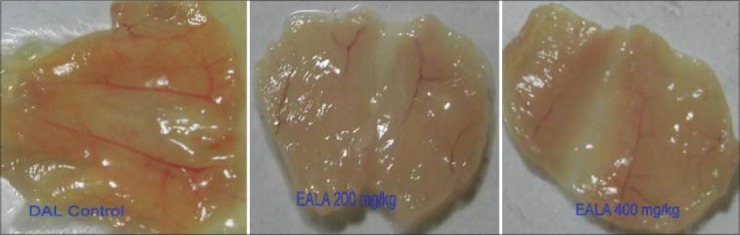
Effect of EALA on neoangiogenesis in peritoneal tissue of DAL bearing mice
Figure 8.
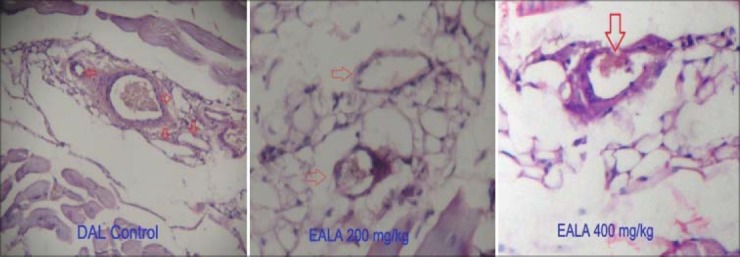
Histopathology of the peritoneal wall sections (×100) of DAL bearing mice treated or untreated with EALA on day 15 of the experiment (n=6). The arrow head indicates neoangiogenesis
Figure 9.
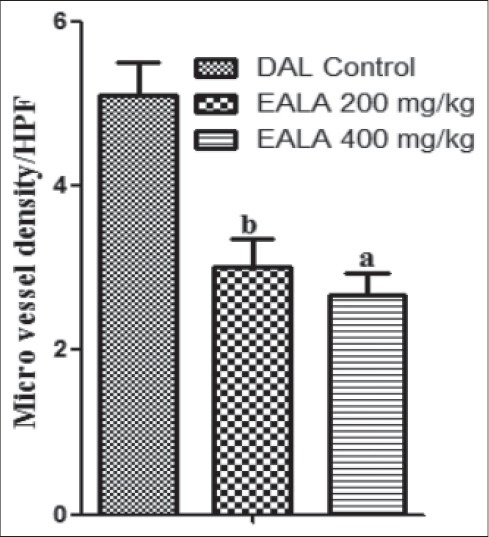
Effect of EALA on MVD count of the peritoneal section of tumor bearing mice. Values are expressed as mean± SEM (n=6). aP<0.001, bP<0.01 as compared to DAL control group
Macrophage stimulation study
Average peritoneal cell count of normal mouse was found to be 5.1 × 106 cells/ml. The treatment with EALA 200 mg/kg and EALA 400 mg/kg significantly increased the peritoneal cell count (bP < 0.001) and (aP < 0.001) respectively. Seven days continuous treatment with EALA 400 mg/kg elevated the peritoneal cell count to 7.8 × 106 cells/ml [Figure 10].
Figure 10.
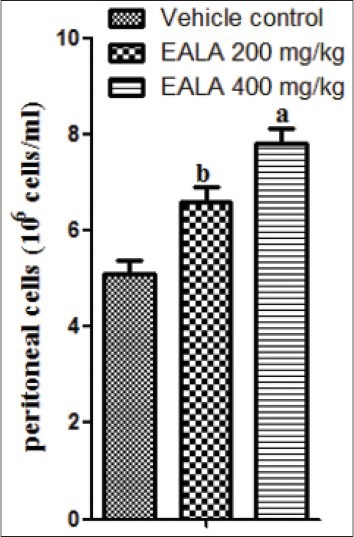
Effect of EALA in macrophage stimulation. Values are expressed as mean± SEM (n=6). aP<0.001, bP<0.01 as compared to vehicle control group
DISCUSSION
Plants have served as a good source of antitumor agents and a large number of plants possessing anticancer properties have been documented.[16] It is well established that oxidative free radicals are promoting carcinogenesis and many plant extracts containing antioxidant principles have been reported to possess antitumor activity.[17] So we have selected L.aspera, a folklore plant in which antioxidant and cytotoxic properties have been already reported.[9,18]
On the basis of in vitro cytotoxicity study results EALA was forwarded to further in vivo studies. Ascitic tumor study showed that, EALA lowered the tumor volume and live cell count as compared to tumor control group. Moreover EALA treatment increased the no of dead cells also. These results could indicate either a direct cytotoxic effect of EALA on tumor cells as evidenced by the in vitro studies or an indirect local effect, which may involve macrophage activation.[19]
Hematological data of tumor control group showed that animals were suffering from loss of RBCs, severe anemia and accumulation of WBCs. The anemia encountered in DAL mice is mainly due to reduction in RBC or hemoglobin percentage and this may occur either due to iron deficiency or due to hemolytic or myelopathic conditions.[20] It was found that EALA therapy restored the RBC and Hb level in treated mice. The significant rise of WBC in DAL induced group might be a defensive mechanism against cancer cells.[21] As the tumor development was reduced by EALA treatment, the WBC count also become normal.
DAL tumor group showed a sharp fall in antioxidant parameters like GSH, Catalase and SOD levels with elevated levels of lipid peroxidation. It was observed that tumor cells produced more peroxides when they proliferate actively after inoculation of tumor. This rise in peroxides indicated the occurrence of intensification of oxygen free radical production.[22] Antioxidant molecules, such as GSH and vitamin E and C as well as antioxidant enzymes such as SOD, Catalase and glutathione peroxidase, have been long believed to have protective and anticancer activities by scavenging excess of free radicals. In the current study, L.aspera therapy restored the antioxidant levels and reduced the MDA content in tumor animals which may be due to the antioxidant and free radicles scavenging ability of the plant. Many studies reported that plant derived extracts containing antioxidant principles showed cytotoxicity towards tumor cells and antitumor activity in experimental animals.[17]
The histological examination of the liver of DAL control animals showed marked changes indicating the toxic effects of the tumor. The normalization of these effects observed in the tissues treated with EALA further supported the potent hepato protective and antioxidant effects of the extract.
Antiangiogenic activity of EALA was studied in DAL bearing mice. A greater number of blood vessels were observed in the peritoneum of DAL bearing control mice. The peritoneal wall tissue of EALA treated mice exhibited significant decreases in blood vessel formation compared to the DAL control; especially large blood vessels were absent. Furthermore the micro vessel density (MVD) analysis of peritoneal wall sections demonstrated significant reduction in MVD count in EALA treated group as compared to DAL control. All these findings strongly indicated the Antiangiogenic effects of L.aspera.
Indirect antitumor activity of EALA was also checked using macrophage stimulation study. Peritoneal fluid of normal mouse contains about 5 × 106 peritoneal cells/ml, among which 50% are macrophages only.[23] EALA treatment for 7 days was found to enhance peritoneal cell count significantly as compared to normal animals. These results demonstrated the indirect effect of L.aspera on DAL cells, probably mediated through enhancement and activation of macrophages.
CONCLUSION
In conclusion, the ethyl acetate extract of L.aspera aerial parts are effective in inhibiting the Daltons lymphoma in vitro and in vivo. The biochemical and histological studies revealed that anti cancer effect of L.aspera mediated through macrophage stimulation, anti angiogenesis and free radicles scavenging. Further investigations are going in our laboratory for the isolation and characterisation of the active principle and to find the mechanism of action in molecular level.
ACKNOWLEDGMENT
This study was financially supported by NIPER-Guwahati (Grant No: 02/PhD/PC/2011), Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India.
Footnotes
Source of Support: NIPER-Guwahati, Department of Pharmaceuticals, Ministry of Chemicals and Fertilisers, Government of India
Conflict of Interest: None declared.
REFERENCES
- 1.Heron MP, Hoyert DL, Xu J, Scott C, Tejada-Vera B. Deaths: Preliminary data for 2006. Natl Vital Stat Rep. 2008;56:1–52. [Google Scholar]
- 2.Babu TD, Kuttan G, Padikkala J. Cytotoxic and anti-tumor properties of certain taxa of umbelliferae with special reference to Centella asiatica urban. J Ethnopharmacol. 1995;48:53–7. doi: 10.1016/0378-8741(95)01284-k. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh HP, Yamaki K, Tsushida T. Effect of fenugreek (Trigonella foenumgraecum) galactomannan fractions on phagocytosis in rat macrophages and on proliferation and IgM secretion in HB4C5 cells. Carbohydr Polym. 2002;50:79–83. [Google Scholar]
- 5.Augustine BB, Dash S, Lahkar M, Lihite RJ, Kumar PS, Sathish P. Effect of Mirabilis jalapa linn flowers in experimentally induced arthritis and consecutive oxidative stress. Int J Pharm Pharm Sci. 2013;5:190–3. [Google Scholar]
- 6.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M. Separation of L. aspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitory and antioxidant activities. Chem Pharm Bull (Tokyo) 2003;51:595–8. doi: 10.1248/cpb.51.595. [DOI] [PubMed] [Google Scholar]
- 8.Kripa KG, Chamundeeswari D, Thanka J, Uma Maheswara Reddy C. Modulation of inflammatory markers by the ethanolic extract of L. aspera in adjuvant arthritis. J Ethnopharmacol. 2011;134:1024–7. doi: 10.1016/j.jep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MS, Sadhu SK, Hasan CM. Preliminary antinociceptive, antioxidant and cytotoxic activities of L. aspera root. Fitoterapia. 2007;78:552–5. doi: 10.1016/j.fitote.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Sunila ES, Kuttan R, Preethi KC, Kuttan G. Dynamized preparations in cell culture. Evid Based Complement Alternat Med. 2009;6:257–63. doi: 10.1093/ecam/nem082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 12.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Chandru H, Sharada AC, Kumar CS, Rangappa KS. Antiangiogenic and growth inhibitory effects of synthetic novel 1, 5-diphenyl-1, 4 pentadiene-3-one-3-yl-ethanone pyridine curcumin analogues on Ehrlich ascites tumor in vivo. Med Chem Res. 2008;17:515–29. [Google Scholar]
- 15.Sur P, Ganguly DK. Tea plant root extract (tre) as an antineoplastic agent. Planta Med. 2007;60:106–9. doi: 10.1055/s-2006-959427. [DOI] [PubMed] [Google Scholar]
- 16.Gupta M, Mazumder UK, Kumar RS, Sivakumar T, Vamsi ML. Antitumor activity and antioxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in swiss albino mice. J Pharmacol Sci. 2004;94:177–84. doi: 10.1254/jphs.94.177. [DOI] [PubMed] [Google Scholar]
- 17.Ruby AJ, Kuttan G, Dinesh Babu K, Rajasekharan KN, Kuttan R. Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 18.da Silva JK, Sousa PJ, Andrade EH, Maia JG. Antioxidant capacity and cytotoxicity of essential oil and methanol extract of Aniba canelilla (H.B.K) Mez J Agric Food Chem. 2007;55:9422–6. doi: 10.1021/jf071928e. [DOI] [PubMed] [Google Scholar]
- 19.Natesan S, Badami S, Dongre SH, Godavarthi A. Antitumor activity and antioxidant status of the methanol extract of Careya arborea bark against Dalton's lymphoma ascites induced ascitic and solid tumor in mice. J Pharmacol Sci. 2007;103:12–23. doi: 10.1254/jphs.fp0060907. [DOI] [PubMed] [Google Scholar]
- 20.Leonard D. Energy and nitrogen metabolism in cancer. Adv Cancer Res. 1954;2:229–53. doi: 10.1016/s0065-230x(08)60496-0. [DOI] [PubMed] [Google Scholar]
- 21.Manjula SN, Kenganora M, Parihar VK, Kumar S, Nayak PG, Kumar N, et al. Antitumor and antioxidant activity of Polyalthia longifolia stem bark ethanol extract. Pharm Biol. 2010;48:690–6. doi: 10.3109/13880200903257974. [DOI] [PubMed] [Google Scholar]
- 22.Navarro J, Obrador E, Pellicer JA, Asensi M, Vina J, Estrela JM. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic Biol Med. 1997;22:1203–9. doi: 10.1016/s0891-5849(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 23.Rajkapoor B, Jayakar B, Murugesh N. Antitumor activity of Bauhinia variegata on Dalton's ascitic lymphoma. J Ethnopharmacol. 2003;89:107–9. doi: 10.1016/s0378-8741(03)00264-2. [DOI] [PubMed] [Google Scholar]


