Abstract
Background:
The antileukemic activity of hot water extract of plant parts of some Japanese willow tree species grown at different levels of nitrogen were examined.
Materials and Methods:
Water extracts of willow leaves were prepared for this studies in different level of nitrogen nutrition.
Results:
The extracts obtained from the leaves and stem exhibited anti-leukemic activities prominently. The crude hot water extracts of the young growing parts including apex, matured leaves and stem, killed the blasts of acute myeloid leukemia (AML) cells, (HL60 and NB4) after 48h incubation, however, such desperation was far less in the root extract. Similar to the plant parts, response of extracts obtained from different willow species was not identical; the proportion of dead cells relative to whole cells of the culture medium ranged from 21% to 93% among the species. Leaf extracts obtained from the responsive willow species decreased the live cell percentage and increased the dead cell percentage at higher level of nitrogen nutrition. The mode of desperation of leaf extract treated AML cells in such species appeared to be cell apoptosis as shown by binding with fluorescein isothiocyanate (FITC) -labeled Annexin V.
Conclusion:
Differentiation of alive AML cells continued unabated and apoptosis was poor when extract of an unresponsive species added to the culture medium.
Keywords: Annexin V, apoptosis, differentiation, leukemia cell, Japanese willow tree species
INTRODUCTION
The genus Salix contains about 350 species in the world, and about 40 species thrive in Japan. The willow trees belonging to different Salix species have been utilized for various purposes, such as biodiesel fuel,[1] soil erosion control,[2,3] and conservation of river stream bank from inundation.[2] More recently, the plant is used as a potential source for anticancer and anti-oxidant phytochemicals.[4] Several authors observed antitumor and antioxidant activities in the plant extracts obtained from Aloe vera,[5] Syzygium cumini (pomposia),[6] red grapes,[7,8] and Euphorbiacae family[9] in the past. However, this property could be noticed in willow leaf extracts very recently.[10,11] There are different types of phytochemicals responsible for the anti-leukemic activity. It was reported that fucoxanthin extracted from the edible seaweed (Undariapinnatifida) induced apoptosis on human leukemia cells, HL-60.[12,13] Resveratrol obtained from red grapes expressed robust antioxidant activity in cancer prevention.[8] Cell differentiation activity of all trans-retinoic acid led to the development of therapies for controlling the cancerous cells active in human acute promyelocytic leukemia.[14,15,16,17] Similarly, docosahexaenoic acid-containing phosphatidylethanolamine could be involved in differentiation on human leukemia cells.[18] But information is scant on the nature of anti-leukemic phytochemicals present in the willow trees. Willow trees grow in widely contrasting environments across the globe under different geographic locations, climatic conditions, and soil types. The contribution of environmental variation on production of anti-leukemic phytochemicals in the plant has not been assessed hitherto. Besides, the role of cytokinin as a potential anti-leukemic phytochemical has not been assessed adequately, although some cytokinin species inhibit growth of human leukemic cells.[19] Cytokinins are hormones unique to all plants and willow species are no different. The concentration of the hormone varies with differences in environmental conditions. Nitrogen nutrition regulates synthesis of the chemical[20] and its concentration in plant parts. Cytokinin concentration in the shoot apex is high at high N level[21,22] and elevated CO2 concentration.[23,24] In the present study, we investigated the effects of hot water extracts obtained from different parts of some willow tree species native to Japan on growth of acute myeloid human leukemic cells and attempted to unravel the possible role of anti-leukemic chemicals like salicin and cytokinin controlling the disease.
MATERIALS AND METHODS
Experiment 1: The effect of willow leaf extract on viability of leukemia cells
Preparation for plant part extract
Ten major willow tree species thriving in Japan were used to examine the effect of plant part extracts on acute promyeloid leukemia cells (APL) (1- Kogomi-Yanagi, 2- Ezonokinu-Yanagi, 3- Inukori-Yanagi, 4- Tachi-Yanagi, 5- Shidore-Yanagi, 6- Neko-Yanagi, 7-Shiro-Yanagi, 8-Yoshino-Yanagi, 9-Ootachi-Yanagi, 10- Onoe-Yanagi). Species Shiore-Yanagi originated from China, while the rest belonged exclusively to Japan. The plants were growing in the Botanical garden of Graduate School of Science, Tohoku University, Japan. Stem cuttings were used for propagation of willow tree species.[2] The cuttings about 20 cm long were defoliated except one leaf and then brought to the glass house of Graduate School of Biosphere Science, Hiroshima University. A few cuttings were inserted into 4L plastic pots filled with sand and irrigated with tap water. The pots were covered with plastic bags for maintaining high humidity around the cuttings. After root initiation, the culture medium of the cuttings was changed from sand without fertilizer to a mixture of mud bolls (clay 60% and sand 40%) and nitrogen fertilizer (40 kgN/ha equivalent). The cuttings were allowed to grow in pots for 14 months before sampling. Leaves, stem, and roots were collected from the plants at different time intervals for obtaining extracts. Seven grams of fresh leaves was boiled at 97°C in 100 ml distilled water for 20 min, then filtered through a membrane filter (2 μm) and centrifuged at 15,000 rpm for 15 min. The supernatant (extract) was collected in a conical flask and kept at -80°C until utilized for assay of the inhibitory effect on leukemia cell. The extracts of stem and roots were also collected similarly.
Measurement of cell viability
The HL-60 cell lines containing acute APL were maintained in the culture media. The culture medium was prepared using Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum and 80 μg/ml gentamicin at 37°Cin a humidified atmosphere with 5% CO2 in air in the incubator. The medium was then filtered through 0.22 μm Millipore filters. One ml of the filtrate was transferred into a 1.8 ml screw- capped sterile plastic tube. Six glass tubes each having 0.1 ml of the cell suspension containing 105 AML cells were taken for observation. 0.1 ml of the willow extract was added to three of these tubes, while three other tubes containing no extract served as control. The tubes were allowed incubation for 24 h more. The cells were tested for their viability using the trypan blue exclusion test.[25] Two hundreds cells were examined for estimation of percentage of viable cells. The other humanleukemia cell line, NB4 (acute promyeloid leukemia cell) was also cultured under the same conditions[16] and the percentage of viable cells was estimated.
Experiment 2: Effect of nitrogen application on cell growth and differentiation
Twenty plants each of two willow tree species, Kogome-yanagi and Ezonokinu-yanagi were grown for 14 months as described in Experiment 1. Thereafter, half of the plants of each variety received 0.5 liter of nutrient solution including 5 mM nitrate for 10 consecutive days, while the other half received the same quantity of solution without nitrate during the same period. Ten days after initiation of nitrogen treatment, 10 plants in each species were harvested and separated into young growing parts (apical meristem plus folded leaves), unfolded leaves, stem and roots and then weighed. An aliquot of each part was used for the extraction with hot distilled water, and its effect on viability of HL-60 was measured as described in Experimental 1.
NBT reduction assay
The activity of cells to reduce nitrobluetetrazolium (NBT) was determined.[17] Approximately 106 viable cells were suspended in 1 ml of RPMI 1640 containing 0.5 mg/ml NBT (Sigma) and 162 nM of 2-O-tetradecanoyl-phorbol-13-acetate (TPA) (Sigma). After incubation at 37°C, 5% CO2 for 25 min, the reaction was stopped by application of 5 M HCl (1 M, final concentration). The suspension was kept at room temperature for 20 min and then centrifuged. The percentage of formazan-positive cells was counted on cytospin slides by Wright-Giemsa staining. With exclusion of dead cells, 150 intact cells per specimen were counted under light microscopy.
Experiment 3: The effect of young leaf extract of willow tree species, Tachiyanagion desperation of human leukemia cells, HL-60, and NB4
Materials
The young leaves were collected from a willow tree species, Tachiyanagi (Salix subfragilis, Anderss), growing on a street in Hiroshima city. The leaf extract of the variety was obtained by a vacuum extraction method with help of an extraction machine.
Measurement of cell viability
An aliquot of the extract was employed for examining its effect on desperation of leukemia cells HL-60 and NB4. Suspensions of the leukemia cells (0.5 × 106 cells/ml) in 2 ml of culturemedium were incubated with or without the willow leaf extract inmulti-dishes (Costar, Cambridge, MA). Treatments were applied by varying the extract concentration, i.e., 50 μl/ml (5%), 100 μl/ml (10%), 150 μl/ml (15%), 200 μl/ml (20%), and no extract (control). At 48 h after incubation, the treated solution was removed by centrifugation and cells in the sedimentation were stained with Propidium iodide (PI) by suspending in 100 μl of binding buffer (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer containing 150 m MNaCl, 5 m MKCl, 1 m M MgCl2, 1,8 m MCaCl2 and FITC-labeled annexin V dye at pH 7.4. The cells were counted in a Model ZM Coulter Counter (Coulter Electronics, Luton, United Kingdom) to estimate their viability percentage.
Detection of apoptotic cells with Annexin V binding
Aliquots of cells collected after the 48 h treatments were labeled with fluorescein isothiocyanate (FITC) -labeled Annexin V (Genzyme Co., Cambridge, MA) for 30 min on ice according to the method of Sato et al.[17] FITC-conjugated murine IgG monoclonal antibodies of unrelated specificity were used as controls. After staining, cellswere washed and numbers of apoptotic cells were countedby flow cytometry (FACSCalibur, Becton Dickinson, Tokyo, Japan). The labeled cells were discriminated because they were glittering under microscope and the response was better at higher concentration of Annexin V.
Statistical analysis
The effect of treatments was assessed according to the expected mean squares given by McIntosh.[26] The standard deviation was calculated according to the methods described by Snedecor.[27]
RESULTS AND DISCUSSION
The current results showed that the total AML cell number increased considerably within 24 h from the initiation of treatment in the control, while no such increase of cell number was observed in the tubes treated with hot water extract of leaves of the Salix species [Figure 1]. The treatment suppressed AML cell multiplication and the effect was identical among different species used in the experiment; no significant difference in total cell number among species was observed [Figure 1]. However, the effect of willow leaf extract on cell survival was different among the willow tree species utilized, the proportion of dead AML ranged from 21% to 93%. No. 1, 5, 7, 9 species had higher percentage of desperate cells, while No. 2, 4, 10 species showed the opposite trend [Figure 2]. The effect of Chinese species (species no. 5) on AML cell desperation was similar to that of the Japanese species. These results exhibited that Salix species thriving in Japan produce substances effective in killing APL cell, HL60, and NB4 although the magnitude of the effectiveness differs widely among them. El-Shemy, et al.,[10] reported that extract of Salix species growing in semi-tropical climate of Egypt possess ability to kill leukemia cells and our findings corroborated this observation. It is also found that the species growing in temperate climate of Japan are no different from that of the Egyptian species of semi-tropical climate in cell desperation of actively growing promyeloid leukemia cells. In spite of the differences in climate conditions, leaves of Salix species retain anti-leukemic capability.
Figure 1.
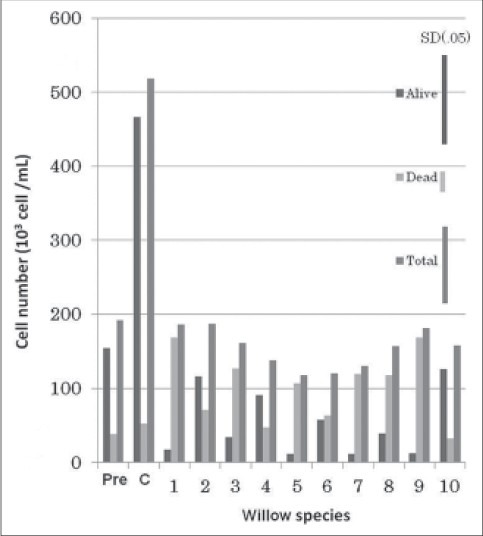
The effect of willow young leaves extract on the viability of AML. Pre: At the initiation of the treatments; C: Control (none treated). Scientific name of Willow species examined are indicated as follows: (1) kogomi-Yanagi, (2) Ezonokinu-Yanagi, (3) Inukori-Yanagi, (4) Tachi-Yanagi, (5) Shidore-Yanagi, (6) Neko-Yanagi, (7) Shiro-Yanagi, (8) Yoshino-Yanagi, (9) Ootachi-Yanagi, (10) Onoe-Yanagi. Species Shiore-Yanagi originated from China, while the rest belonged exclusively to Japan
Figure 2.
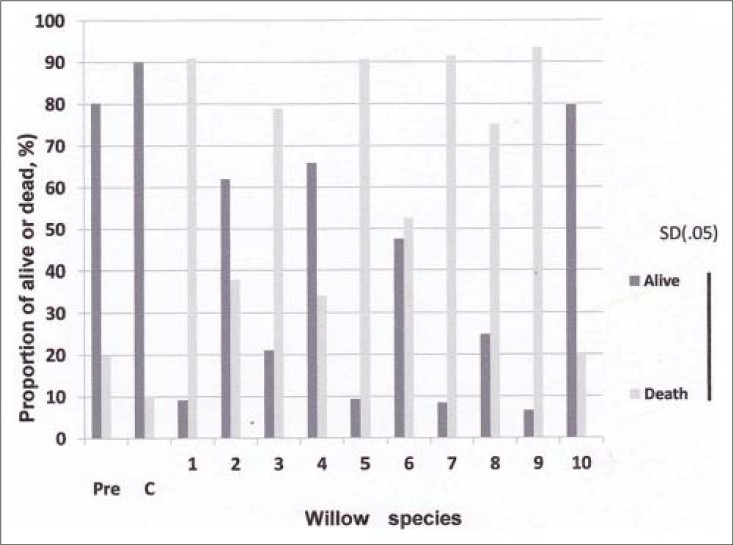
Willow young leaves extracts on tlle viability of AML Pre: at the initiation of the treatment; C: Control
A few substances of plant origin such as all-trance retinoic acids[14] and cytokinin[19] have been reported to be effective for desperation of leukemia cells. However, information is scant on the chemical controlling anti-leukemic activity in the plant part extracts of Japanese willow tree species. In this context, it was shown in our study that the proportion of dead AML cells was higher by application of the extracts derived from young growing leaves, matured leaves and stem than that from roots, and the magnitude of response did not vary significantly between young growing parts, leaves and stem [Figures 3 and 4]. This result showed that not only young leaves, but also matured leaves as well as stem have the ability to desperate AML cells, and the chemical involved in killing blasts exists in these plant parts and leaf age did not degrade the chemical. It was reported that salicin derived from the extract of leaves[10] and bark[28] of willow tree exhibit anti-leukemic activity. In our study, the presence of salicin in leaf and stem extracts might be an ingredient responsible for desperation of AML cells.
Figure 3.
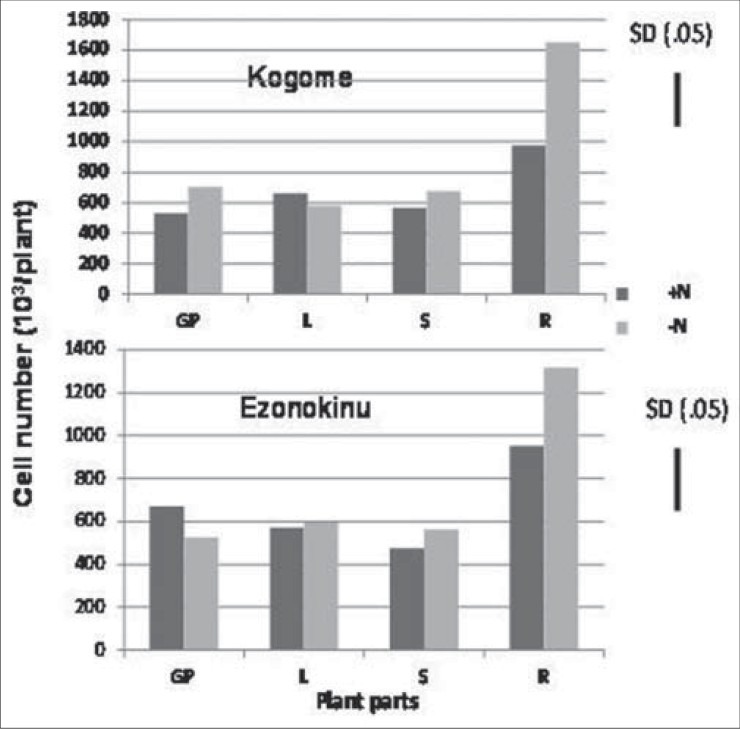
The effect of various plant parts extracts of willow species grown at different nitrogen rates on the number of AML cells. GP: Young growing leaves; L: Matured leaves; S: Stem; R: Roots
Figure 4.
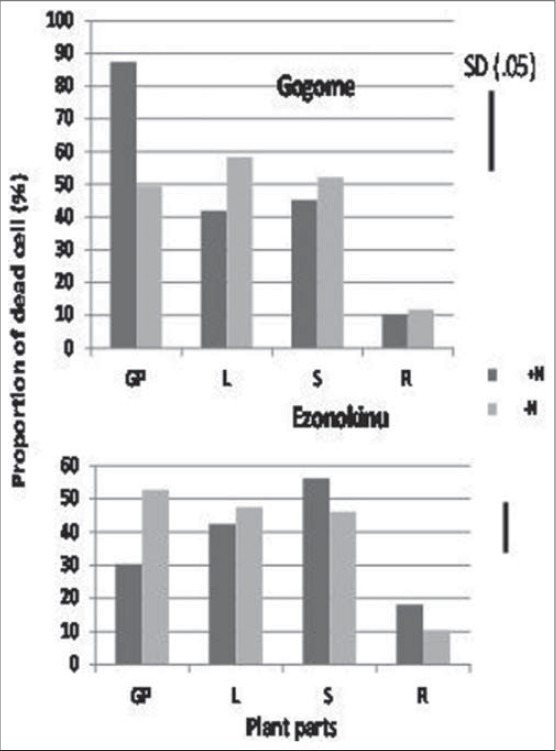
The effect of willow young and matured leaves, stem, and roots extracts on the proportion of live AML cells. GP: Young growing leaves; L: Matured leaves; S: Stem; R: Roots
The mode of action of willow leaf extract on desperation of leukemia blasts has been undermined so long. In our study leaf extract of Tachiyanagi species affected AML cell growth and the magnitude of response increased with increase in concentration of the extract. The number of living cells declined [Figures 2 and 5] and the number of undifferentiated early apoptotic cells increased [Figures 6 and 7] with increase in concentration of leaf extract. The response of leaf extract was identical for both HL60 and NB4 types of AML cells. These results corroborated our earlier findings that the mode of action of desperation of the blasts by the willow species leaves extract is apoptosis.[11]
Figure 5.
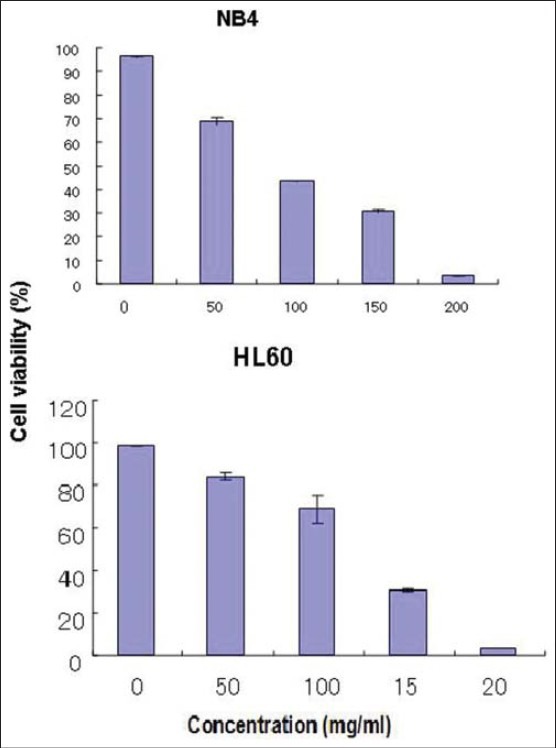
Effect of willow young leaf extract concentration on the proportion of live cell
Figure 6.
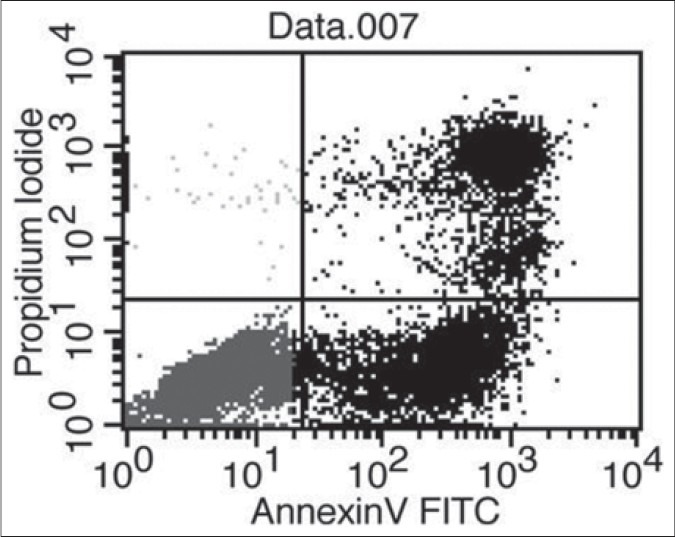
Flow cytometric detection of undifferentiated early-apoptoic cells. NB4 cells were cultured 6days in the presence of 5% willow leaf extract
Figure 7.
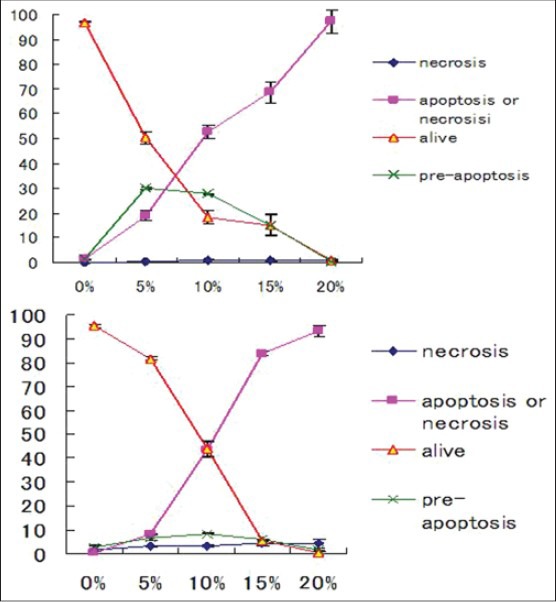
Undifferentiated early-apoptoic cell (pre-apoptosis) necrosis cells as affected by extract of willow leaves at the graded levels
In Experiment 2, willow species 1 and 2, which differed widely in response to leaf extract application on AML cell viability [Figure 2], were chosen for measuring difference in cell activity through NBT reduction capacity [Figure 6]. The data showed that 45% of cells were stained by application of 50 μM of the leaf extract in No. 2 species, while the percentage of such cells was only 13% at 10 μM concentration. The difference in response between 10 uM and 50 uM concentrations leaf extract applications were not found in species no. 1. These data suggested that differentiation and maturation of HL60 cells could be induced by the leaf extract of No. 2 (Ezonokinu-yanag) willow species alone, preferably at 50 uM concentration of leaf extract. Further, it was found that application of all trans-retinoic acid could stimulate the response higher than that of willow tree extract. These results showed that major willow tree species in Japan produce substances, which induce apoptosis and differentiation of AML cells.
In Experiment 2, we also observed that percentage of dead leukemia cells relative to whole plant cells increased in response to application of the extract from young growing parts of Kogome-yanagi when the plants were cultured at higher level of nitrogen, while such phenomenon was not observed for another willow species, Ezonokinu-yanagi [Figure 4]. Plant parts other than the growing leaves also did not show any response to nitrogen nutrition. This result suggested that in addition to genetic diversity and difference in plant parts, nitrogen level of soil is crucial for controlling desperation of leukemia cells. It might be possible higher nitrogen level enhanced cytokinin synthesis in the plant and, thereby, increased AML cell differentiation capacity of the leaf extract. A close correlation between nitrogennutrition and cytokinins has been demonstrated in the literature.[29,30] Higher nitrogen nutrition increases the delivery per unit leaf area of cytokinin to the leaf.[23] A nitrate specific signal is responsible for regulation of cytokinin biosynthesis.[22] More recently, nitrate has been identified as a factor regulating gene expression of a key enzyme of cytokinin biosynthesis pathway.[30] This evidence suggests that the fate of leukemia cells is associated with intrinsic concentration of cytokinin and cytokinin synthesis in willow tree depends on genetic variation of the species, location of plant parts, and status of nitrogen nutrition of the growth medium. It was reported that not all types of cytokinins are effective in the control of leukemia cell growth, kinetin was very effective in inducing differentiation as found by NBT [Figure 8] reduction in the cells into mature granulocytes, while such phenomenon was not observed by kinetin riboside which induced apoptosis prior to the differentiation process.[19] We conclude that death of leukemia cells through apoptosis in spite of involvement of differentiation is induced by willow tree leaf extract and intrinsic cytokinins of the extract may be responsible for action in addition to salicin. Das et al.,[31] reported that execution process of cell apoptosis involves integration of several phytochemicals and cytokinin is one of them. Our study supports this proposition.
Figure 8.
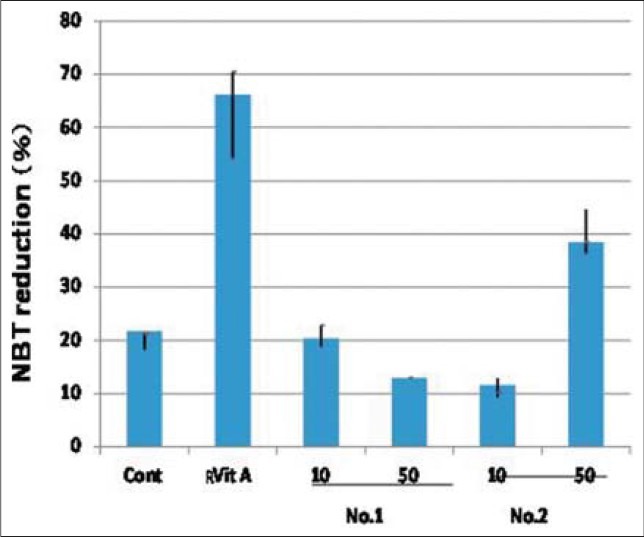
The effect of willow young leaves extracts at different concentration on the nitrobluetetrazolium (NBT) reduction. Control: Non treated; Retinoic acid: All trans retinoic acid, No.1: Kogome; No.2: Ezonokinu; Vertical bar denote SD (0.05)
ACKNOWLEDGMENT
We would like to deeply appreciate for presenting NB4 cells to Dr. Atsushi Sato. I would like to deeply appreciate for valuable suggestion and guidance for propagation and cultivation of willow tree species to staff members in botanical garden, Graduate School of Science, Tohoku University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Adegbidi HG, Volk TA, White EH, Abrahamson LP, Briggs RD, Bickelhaupt DH. Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenergy. 2001;20:399–411. [Google Scholar]
- 2.Kupchan MS, Uchida I, Branfman AR, Caily RG, Pezeshki SK, Li S, et al. Factors governing survival of black willow (Salix nigra) cuttings in a streambank restoration project. Ecol Eng. 2007;29:56–65. [Google Scholar]
- 3.Wilkinson AG. Poplars and willows for soil erosion control in New Zealand. Biomass Bioenergy. 1999;16:263–74. [Google Scholar]
- 4.Kumar AP, Graham H, Robson C, Garapati K, Ghosh R. An overview of anticancer herbal medicine. In: Cho WC, editor. Evidence-Based Anticancer Material Medica. Springer; 2011. pp. 1–36. [Google Scholar]
- 5.EL-Shemy HA, Aboul-Soud MA, Nassr-Allah AA, Aboul-Enein KM, Kabash A, Yagi A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via suoercritical carbon dioxide extraction. Curr Med Chem. 2010;17:129–38. doi: 10.2174/092986710790112620. [DOI] [PubMed] [Google Scholar]
- 6.Afify AE, Fayed SA, Shalaby EA, El-Shemy HA. Syzygium cumini (pomposia) active principles exhibit potent anticancer and antioxidant activities. Afr J Pharm Pharmacol. 2011;5:948–56. [Google Scholar]
- 7.Brisdelli F, D’Andrea G, Bozzi A. Resveratrol: A natural polyphenol with multiple chemopreventive properties. Curr Drug Metab. 2009;10:530–46. doi: 10.2174/138920009789375423. [DOI] [PubMed] [Google Scholar]
- 8.Al-Abd AM, El-Sherbiny AM, El-Moselhy MA, Nofal SM, El-Latif HA, El-Eraky WI, et al. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Proliferat. 2011;44:591–601. doi: 10.1111/j.1365-2184.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yufei B. Antileukemic principles isolated from Euphorbiaceae plants. Science. 1976;191:571–2. doi: 10.1126/science.1251193. [DOI] [PubMed] [Google Scholar]
- 10.El-Shemy HA, Aboul-Enein AM, Aboul-Enein MI, Fujita K. The effect of willow leaf extract on human leukemic cells, in-vitro. J Biochem Mol Biol. 2003;36:387–9. doi: 10.5483/bmbrep.2003.36.4.387. [DOI] [PubMed] [Google Scholar]
- 11.El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K. Willow leaves’ extracts contain anti-tumor agents effective against three cell types. PloS One. 2007;2:e178. doi: 10.1371/journal.pone.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta. 2004;1675:113–9. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Konishi I, Hosokawa M, Sashima T, Kobayashi H, Miyashita K. Halocynthiaxanthin and fucoxanthiol isolated from Halocynthiaroretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp Biochem Phys C. 2006;142:53–9. doi: 10.1016/j.cbpc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–72. [PubMed] [Google Scholar]
- 15.Imaizumi M, Sato A, Koizumi Y, Inoue S, Suzuki H, Suwabe N, et al. Potentiated maturation with a high proliferating activity of acute promyelocytic leukemia induced in vivo by granulocyte or granulocyte/macrophage colony-stimulating factors in combination with all-trans retinoic acid. Leukemia. 1994;8:1301–8. [PubMed] [Google Scholar]
- 16.Idres N, Benoit G, Flexor MA, Lanotte M, Chabot GG. Granulocytic differentiation of human NB4 promyelocytic leukemia cells induced by all-trans retinoic acid metabolites. Cancer Res. 2001;61:700–5. [PubMed] [Google Scholar]
- 17.Sato A, Imaizumi M, Hoshi Y, Rikiishi T, Fujii K, Kizaki M, et al. Alteration in the cellular response to retinoic acid of a human acute promyelocytic leukemia cell line, UF-1, carrying a patient-derived mutant PML-RARα chimeric gene. Leukemia Res. 2004;28:959–67. doi: 10.1016/j.leukres.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Ishigamori H, Hosokawa M, Kohno H, Tanaka T, Miyashita K, Takahashi K. Docosahexaenoic acid-containing phosphatidylethanolamine enhances HL-60 cell differentiation by regulation of c-jun and c-myc expression. Mol Cell Biochem. 2005;257:127–33. doi: 10.1007/s11010-005-1090-z. [DOI] [PubMed] [Google Scholar]
- 19.Ishii Y, Hori Y, Sakai S, Honma Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ. 2002;13:19–26. [PubMed] [Google Scholar]
- 20.Neuberg M, Pavlíková D, Žižková E, Motyka V, Pavlík M. Different types of N nutrition and their impact on endogenous cytokinin levels in Festulolium and Trifoliumpratense L. Plant Soil Environ. 2011;57:381–7. [Google Scholar]
- 21.Sattelmacher B, Marschner H. Relation between nitrogen nutrition, cytokinin activity and tuberization in Solanumtuberosum. Physiol Plant. 1978;44:65–8. [Google Scholar]
- 22.Sakakibara H, Takei K, Hirose N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci. 2006;11:440–8. doi: 10.1016/j.tplants.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Yong JW, Wong SC, Letham DS, Hocart CH, Farquhar GD. Effect of elevated [CO2] and nitrogen nutrition on cytokinins in xylem sap and leaves of cotton. Plant Physiol. 2000;124:762–80. doi: 10.1104/pp.124.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Groot CC, Marcells LF, Boogaard RV, Kaiser WM, Lambers H. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil. 2003;248:257–68. [Google Scholar]
- 25.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute myeloid leukemia. Br J Hematol. 1976;33:451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 26.Mcintosh MS. Analysis of combined experiments. Agron J. 1983;75:153–5. [Google Scholar]
- 27.Snedeccor GW. 5th ed. Ames, Iowa: Iowa State College Press; 1959. Statistical Methods. [Google Scholar]
- 28.Kammerer B, Kahlich R, Biegert C, Gleiter CH, Heide L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem Anal. 2005;16:470–8. doi: 10.1002/pca.873. [DOI] [PubMed] [Google Scholar]
- 29.Wagner H, Michael G. The influence of varied nitrogen supply on the production of cytokinins in the roots of sunflower plants. Biochem Physiol Plants. 1971;162:147–58. [Google Scholar]
- 30.Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 31.Das A, Kawai-Yamada M, Uchimiya H. Pragrammed cell death in plants. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee, editors. Abiotic Stress Adaptation in Plants. Dordrecht: Springer; 2010. p. 371e386. [Google Scholar]


