Abstract
Background:
Toona sinensis (A. Juss.) Roemer is an endemic species of Toona genus native to Asia. Its crude extract exhibits an effective anti-oxidant capacity against oxidative models, but the intrinsic substances responsible for this capacity in the extract remains unclear and is yet to be studied comprehensively.
Objective:
To investigate the chemical constituents of the young leaves of Toona sinensis and its anti-oxidant capacity.
Materials and Methods:
Silica gel column chromatography, preparative high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), and mass spectrometry (MS) were used to isolate and characterize the chemical constituents. Four chemical-induced oxidative models including DPPH free-radical scavenging assay, phenazine methosulphate (PMS) nicotinamide adenine dinucleotide (NADH) PMS-NADH-NBT superoxide anion scavenging assay, FeCl3-K3Fe (CN)6 reducing power assay, and FeCl2-FerroZine metal chelation assay were applied in the present study for evaluating anti-oxidant capacity.
Results:
Five flavonols and three derivatives of gallic acid, including quercetrin, kaempferol-3-O-α-L-rhamopyranoside, astragalin, quercetin, kaempferol, methyl gallate, ethyl gallate, and 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucopyranose were isolated from the leaves. Results showed that these compounds exhibited various antioxidant properties, markedly either as the strong scavengers for superoxide and free radicals or as molecules that were reducing or metal chelating in nature.
Conclusion:
The findings suggested that the 8 compounds in the young leaves of T. sinensis that were isolated in our study were the active compounds responsible for its antioxidant activity. These compounds can be utilized as a potential health supplement, as an available source of natural antioxidants, and as an effective material in pharmaceutical applications.
Keywords: Antioxidant capacity, derivatives of gallic acid, flavonols, Toona sinensis
INTRODUCTION
Toona sinensis (A. Juss.) Roemer, well-known as Red Toon, is an endemic species of Toona genus (Meliaceae family) native to Asia region and distributed from southern North Korea through most eastern, central, and south-western parts China to Thailand, Myanmar, India, Malaysia, and Indonesia. Since this deciduous arbor has been widely cultivated over China and India area, the young leaves of this plant is one of the most popular dietary vegetables with the locals.[1] In addition, for thousands of years, this leaves had been extensively consumed to treat oriental diseases such as halitosis, vomiting, dysentery, lack of appetite, enteritis, and itchiness because of its significant pharmacological effects on detoxification and anti-inflammation Furthermore, contrary to most clinical practices, such treatments showed no obvious irreversible adverse drug reactions.[2,3] Previous studies had shown that[4,5,6,7,8] the crude extract from the leaves of T. sinensis had a strong anti-proliferative effect on non-small cell lung cancer by regulating the expression of Bcl2, Bax, cyclin D1, and CDK4. It also exhibited an effective anti-oxidant capacity against several in vitro oxidative models such as scavenging for free and superoxide anion radicals, reducing and metal chelating nature. In order to reveal the intrinsic constituents responsible for these major activities of the extract, a few phytochemical investigations and instrumental analyses were carried out. Studies had showed that various compounds such as gallic acid, rutin, 3-hydroxy-5,6-epoxy-7-megastigmen-9-one, scopoletin, and 1,2,3,6-tetra-O-galloyl-β-D-glucopyranose had been isolated and identified from the leaves extract. However, till date, only a preliminary screening by DPPH assay had been conducted to examine the free radical scavenging ability of several isolated phenolic compounds. As such, the antioxidant capacities remained to be verified comprehensively.
In the present study, five flavonols and three derivatives of gallic acid, including quercetrin (1), kaempferol-3-O-α-L-rhamopyranoside (2), astragalin (3), quercetin (4), kaempferol (5), methyl gallate (6), ethyl gallate (7), and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (8) were isolated from the young leaves of T. sinensis. Their antioxidant capacities were evaluated and compared by using different chemical models such as DPPH-free radical scavenging assay, PMS-NADH-NBT superoxide anion scavenging assay, FeCl3-K3Fe(CN)6 reducing power assay, and FeCl2-FerroZine metal chelation assay. For the first time, to our knowledge, it was reported that compounds 2, 4, and 5 were isolated from this plant and the anti-oxidant capacities of these compounds were evaluated comprehensively by using various chemical methods.
MATERIALS AND METHODS
General procedures
1H-NMR and 13C-NMR spectra were obtained by a Burker AV-400 spectrometer using DMSO-d6 as solvent. Chemical shift was recorded in δ (ppm) with tetramethylsilane (TMS) used as an internal reference. All mass spectra were taken under electronic spray ionization (ESI) mode using a Thermo LXQ HPLC-Ion Trap-MS instrument.
Plant material
The fresh young leaves of T. sinensis were harvested from the city of Zhenjiang (Jiangsu Province, China) in May 2011. The voucher specimen (No.: SP20110513) had been authenticated by Prof. Jun Chen (Department of Chinese Materia Medica and Pharmacy, School of Pharmacy, Jiangsu University, Zhenjiang, Jiangsu, China) and was deposited at the Pharmacognosy Research Facility. After harvest, the leaves from the production site were immediately dried in shade in a well-ventilated place to remove most of water prior to a further 24 h lyophilization. The plant material was then grounded into powder form and passed through a 40-mesh sieve. The fine powder collected was stored in a dry cabinet (RH ≤ 50%) at room temperature.
Extraction and isolation of the constituents
Young leaves of T. sinensis, 2.0 kg, were macerated using 95% EtOH in an airtight glass cylinder at room temperature for 3 d (15 L × 3). During this period, the mixture was subjected to sonication with stirring for 30 min daily. The mixture was passed through suction filtration, and the collected solutions were pooled together. EtOH was removed under reduced pressure using rotor evaporator (Büchi R-200, Germany) below 50°C and 164.0 g of sticky fluid was collected. Then, 1.2 L of CH2Cl2, EtOAc, and n-BuOH was sequentially added into the crude extract to obtain the desired portions of different polarity. The EtOAc portion (41.0 g) or the n-BuOH portion (36.0 g) was subject to silica gel column chromatography, which was respectively flushed by the mixture of PE-EtOAc (100:0 → 0:100, v/v) or CH2Cl2-MeOH-H2O (100:10:1 → 10:100:10, v/v/v) with 0.2% (v/v) formic acid added to improve separation. Each 500 mL of the eluent was collected and solvents were removed completely by using rotor-vap under reduced pressure. Subsequently, to further purify the constituents, similar fractions collected were combined, followed by repeated column chromatography or subjected to preparative HPLC (Waters, USA) on a Lichrospher C18 column (30 mm × 250 mm, 5 μm; Hanbang, China) with linear gradient MeOH-H2O containing 0.1% TFA (v/v) as the eluting solvent driven at 20 mL/min. Following this series of extraction and purification, compounds 1-8 were successfully isolated from these two portions. The standard used in all following assays was either ascorbic acid (VC) or ethylenediaminetetraacetic acid (EDTA) as positive control.
Free-radical scavenging assay
The free-radical scavenging ability of these five flavonols and three derivatives of gallic acid isolated from the young leaves was individually evaluated by 1,1-diphenyl-2-picryl-hydrazil (DPPH) assay.[9] In brief, 100.0 μL of DPPH solution in EtOH (100 μM) was mixed with 50.0 μL of the solution of each compound at different concentrations for decolorization. The mixture was then incubated (shaking linear amplitude: 5 mm) in a 96-well microplate in shade at 25°C for 30 min, and the respective absorbance (Abs.) was measured at 519 nm using a microplate reader (Infinite 200, Tecan). A solution comprising of only DPPH solution was used as a negative control. The free-radical scavenging ability was expressed as percentage inhibition calculated by the following formula:
Percentage inhibition (%) = [(1 − Abs. of the sample)/(Abs. of the control)] ×100
Superoxide anion scavenging assay
The superoxide anion scavenging ability of these compounds was evaluated using the method described by Mohd A et al.[10] Superoxide radical required was produced in the nicotinamide adenine dinucleotide (NADH)-phenazine methosulphate (PMS) systems prior to the oxidation of NADH, and then assayed by reduction on addition of nitroblue tetrazolium (NBT). In this assay, the superoxide anion was generated in an aliquot of 3.00 mL of 100 mM phosphate buffer (pH 7.4) containing 750 μL of 300 μM NBT solution, 750 μL of 938 μM NADH solution, and 300 μL of tested compound solution at different concentrations. The desired reaction was initiated by the addition of 750 μL of 120 μM PMS solution into the mixture. The final reaction mixture was incubated at 25°C for 5 min and the Abs. at 560 nm was recorded against a blank sample using a UV-visible spectrophotometer (UV-2550, Shimadzu). Percentage inhibition was calculated using the formula mentioned above.
FeCl2-FerroZine metal chelation assay
Iron chelation potential of the compounds was determined by using FeCl2-FerroZine metal chelation assay.[11] Briefly, 100 μL of each compound solution in MeOH at different concentrations was added into 25.0 μL of 600 μM FeCl2 solution. The reaction was initiated by the addition of 25.0 μL of FerroZine solution (5 mM). Subsequently, the resulting mixture was shaken vigorously and left to react at room temperature for 10 min. After equilibrium had been established, Abs. of the mixture was read at 562 nm using the microplate reader. Negative control was prepared using FeCl2 and FerroZine only. The ability of tested anti-oxidant to disrupt the formation of the Fe2+-FerroZine complex was expressed as percentage of Fe2+ chelating activity, and it was calculated using the above formula.
Reducing power assay
The reducing power of these compounds had been determined using the method described.[9] The solution of each compound (200 μL) was added at different concentrations into the mixture of 500 μL of 200 mM Na2HPO4/KH2PO4 buffer (pH 6.6) and 500 μL of 1% K3Fe(CN)6. The resulting mixture was then incubated at 50°C for 20 min. Trichloroacetic acid (500 μL, 10% solution) was added into the mixture and then subjected to centrifugation at 2500 g for 10 min. A portion of the upper layer (50.0 μL) of the mixture was recovered and mixed with 50.0 μL of ultrapure water and 50.0 μL of 0.1% FeCl3 for 90 s. The Abs. was measured at 700 nm using the microplate reader. The phosphate buffer prepared earlier was used as a sample blank. The reducing power of the compound, which is proportional to the Abs of the reaction mixture, was expressed as the VC equivalent and calculated by Abs. of sample (50 μg/mL)/Abs. of VC (50 μg/mL).
Statistical analysis
All tests and analyses were performed in triplicate, and data collected was presented in the form of Mean ± SD. A dose-response linear curve was plotted to determine the IC50 values (μg/mL), which was defined as the concentration sufficient to achieve 50% of a maximum scavenging ability, for the free radical scavenging assay, superoxide anion scavenging assay, and metal chelation assay.
RESULTS
Structure elucidation of compounds 1-8
Quercetin-3-O-α-L-rhamopyranoside (quercetrin, 1): Yellow powder (methanol), 36 mg; ESI--MS m/z: 447[M-H]-, 301[M-Rha-H]-; 1H-NMR, and 13C-NMR data were similar to the reported.
Kaempferol-3-O-α-L-rhamopyranoside (2): Yellow powder (methanol), 34 mg; ESI--MS m/z: 431[M-H]-, 285[M-Rha-H]-; 1H-NMR (400 MHz): 0.77 (3H, d, J = 6.0 Hz, H-6’’), 3.06-3.14 (2H, m, H-4’’,5’’), 3.46 (1H, d, J = 8.0 Hz, H-3’’), 3.97 (1H, br s, H-2’’), 5.28 (1H, br s, H-1’’), 5.78 (1H, br s, H-6), 5.92 (1H, br s, H-8), 6.86 (2H, d, J = 8.0 Hz, H-3’,5’), 7.65 (2H, d, J = 8.0 Hz, H-2’,6’), 12.53 (1H, br s, 5-OH); 13C-NMR (100 MHz): 176.3 (CO), 161.3 (C-7), 160.4 (C-5, 4’), 157.7 (C-9), 155.5 (C-2), 133.8 (C-3), 130.7 (C-2’,6’), 121.3 (C-1’), 115.8 (C-3’,5’), 102.0 (C-10), 101.8 (C-1’’), 101.0 (C-6), 95.6 (C-8), 71.6 (C-4’’), 70.8 (C-2’’,3’’), 70.6 (C-5’’), 17.9 (C-6’’).
Astragalin (3): Yellow powder (methonal), 13 mg; ESI--MS m/z: 447[M-H]-, 285[M-Glc-H]-; 1H-NMR and 13C-NMR data were similar to the reported.
Quercetin (4): Yellow crystal (ethanol), 18 mg; mp>300°C; 1H-NMR and 13C-NMR data were similar to the reported.
Kaempferol (5): Yellow crystal (ethanol), 20 mg; mp 269-271°C; 1H-NMR and 13C-NMR were similar to the reported.
Methyl gallate (6): White powder, 25 mg; ESI--MS m/z: 183[M-H]-; 1H-NMR and 13C-NMR were similar to the reported.
Ethyl gallate (7): White powder, 28 mg; ESI--MS m/z: 197[M-H]-; 1H-NMR and 13C-NMR were similar to the reported.
1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (8): Colorless powder (methanol), 14 mg; ESI-MS m/z: 939[M-H]-; 1H-NMR (400 MHz): 6.97, 6.90, 6.84, 6.81, 6.76 (2H each, s, H-2,6 on galloyl), 6.34 (1H, d, J = 8.0 Hz, H-1), 5.94 (1H, m, H-3), 5.49-5.39 (2H, m, H-2,4), 4.29 (2H, br s, H-6), 3.58 (1H, m, H-5); 13C-NMR (100 MHz): 165.9, 165.2, 165.0, 164.9, 164.4 (5 × C-7), 146.1, 146.0, 145.9, 145.8 (5 × C-3,5), 139.6, 139.4, 139.2 (5 × C-4), 119.3, 118.5, 118.3, 117.7 (5 × C-1), 109.4, 109.2 (5 × C-2,6), 93.0 (C-1), 72.7 (C-5), 72.5 (C-3), 70.2 (C-2), 68.2 (C-4), 62.0 (C-6).
The chemical structures of these eight compounds isolated from the young leaves of T. sinensis were shown as follows [Figure 1].
Figure 1.
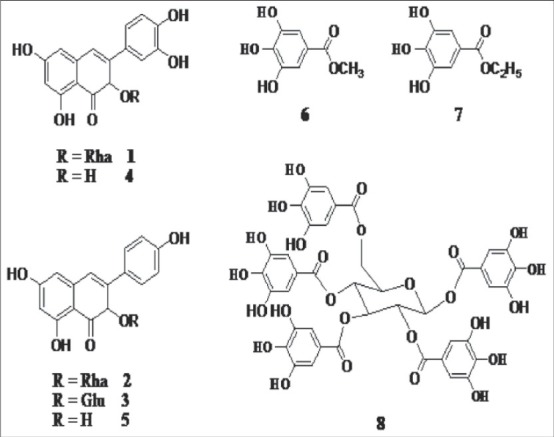
The chemical structures of five flavonols and three derivatives of gallic acid isolated from the young leaves of T. sinensis
DPPH-radical scavenging ability
DPPH-radical scavenging activity is a rapid means used to compare the anti-oxidant capacity of different natural compounds. The DPPH solution, initially deep violet in color, turns pale yellow in the presence of non-radical form of DPPH-H, induced by the isolated compounds. Table 1 shows the free-radical scavenging activity (IC50) of the compounds isolated from T. sinensis as compared to the positive control VC. Their radical scavenging ability decreased in the following decreasing order: 8 > 4 > 5 > VC > 6 > 7 > 1 > 3 > 2.
Table 1.
Antioxidant capacity of flavonols and derivatives of gallic acid isolated from Toona sinensis
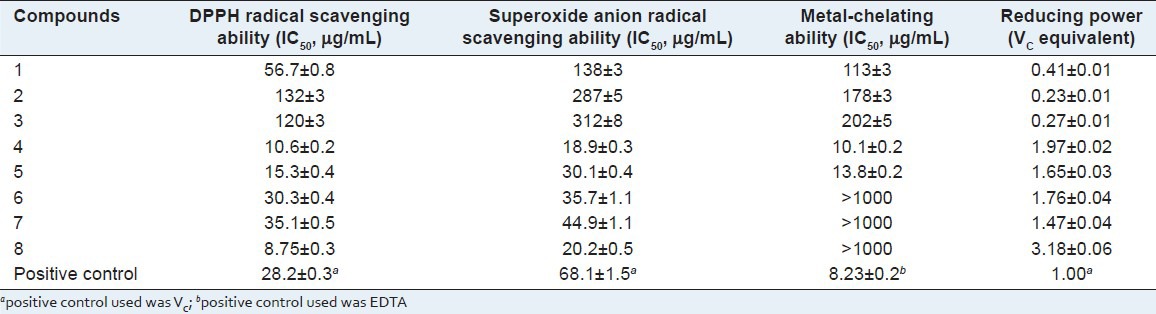
Superoxide anion-radical scavenging ability
In the PMS-NADH-NBT model, superoxide anions were generated from dissolved O2 by PMS-NADH coupling reaction, followed by reduction to NBT. The decreased in the Abs. at 560 nm with anti-oxidant indicated the consumption of the superoxide anions produced in the reaction mixture. The inhibitive efficiency of superoxide radical generation by the assayed compounds was shown in Table 1. The radical scavenging ability decreased in the order, 4 > 8 > 5 > 6 > 7 > VC > 1 > 2 > 3. The results showed that the addition of the flavonol aglycones and derivatives of gallic acid could scavenge superoxide radical generated in the system more effectively as compared to VC and three glycosides.
Metal-chelating ability
In the present study, the ability of the isolated flavonols and derivatives of gallic acid to compete with FerroZine to form complexes with Fe2+ in solution was assessed. If other chelating agents were present, the complex formation could be interrupted and lead to a decrease in red color development. As shown in Table 1, the formation of the Fe2+-FerroZine complex could not be accomplished with the five flavonols isolated, demonstrating that these compounds had metal chelating activity and could chelate to Fe2+. However, anti-oxidant activity was not exhibited by the three derivatives of gallic acid identified in terms of metal-chelating ability. The metal-chelating effect decreased in the order, EDTA > 4 > 5 > 1 > 2 > 3 > 6, 7, 8.
Reducing power
The transformation from ferric to ferrous state, in the presence of eight isolated compounds and VC, was examined to measure the reductive ability of the isolated compounds. This assay reflected the ease of a given anti-oxidant to donate electron to reactive free radicals species, thus promoting the termination of free radical chain reactions. The ability of the tested compound to reduce Fe3+ to Fe2+ was an important indicator of the ability to behave as a pro-oxidant in the reaction system. As evident in Table 1, the reducing power decreased in the order, 8 > 4 > 6 > 5 > 7 > VC > 1 > 3 > 2. The greatest reducing power was demonstrated by compound 8, which comprised of five gallic acids in its structure. Moreover, the result also showed that the five flavonol aglycones and derivatives of gallic acid performed better than VC and three glycosides in terms of reducing power.
DISCUSSION
For the first time, kaempferol-3-O-α-L-rhamopyranoside, quercetin, and kaempferol were isolated from this plant, and the anti-oxidant capacities of these isolated compounds had been evaluated using various chemical methods. The results obtained from the present study clearly indicated that the five flavonols and three derivatives of gallic acid isolated from T. sinensis demonstrated remarkable anti-oxidant ability over various in vitro oxidative models. The broad anti-oxidant properties of the compounds could have been responsible for their effectiveness as the scavengers of superoxide and free radicals, reductive capacity, and metal-chelating ability. However, three derivatives of gallic acid did not demonstrate their antioxidant activity in terms of the ability to chelate with metals. The young leaf of T. sinensis is a noteworthy material that can be utilized as a potential health supplement and an easily available resource of natural antioxidants, as well as an effective material in pharmaceutical applications.
Previously, it remained unclear as to which of the constituents in the leaves of T. sinensis were the active compounds corresponding to its antioxidant activity. The results from this antioxidant assays obtained in this study implies that the polyphenols including flavonols and derivatives of gallic acid probably were acting as preventive agents in the leaves of T. sinensis with respect to its anti-oxidant capacity.
This was also supported by the recent research in polyphenols that provided interesting insights on their bioactivities, e.g., rutin and gallic acid isolated from this plant exhibited strong antioxidant activities in vitro and in vivo.[12,13,14] In addition, the esters of gallic acid were also applied as anti-oxidant agent in food industry.
Full structure elucidation and characterization of the isolated compounds accounting for the anti-oxidant activity of T. sinensis had been conducted in this study. From the results of the in vitro assays, it was suggested that the glycosides of flavonol quercetin or kaempferol had broader spectrum of antioxidant activities than the derivatives of gallic acid. Although the results obtained from the present investigations demonstrated that the leaves of T. sinensis containing polyphenolic compounds could greatly scavenge oxygen free radicals, similar result was not reflected in the assays using cellular physiological mechanism. In addition, since bioavailability and metabolism were not being considered, the group suggests investigating further into this matter.
CONCLUSION
Five flavonols and three derivatives of gallic acid isolated from the young leaves of T. sinensis exhibited efficient anti-oxidant ability, including free radical and superoxide anion radical scavenging, metal chelation, and reducing power. The findings also suggested that T. sinensis was promising to be used as an effective and important natural source in health-related applications. Some further investigations of in vivo activities are to be carried out in order to confirm the results.
ACKNOWLEDGEMENTS
The current work was finished under the financial supports by SATCM Key Lab of New Drug Delivery System of CMM (2011NDDCM01002), Changzhou Science and Technology Research Program (CE20125042), Jiangsu Natural Science Foundation (BK2012290).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, et al. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–14. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Edmonds JM, Staniforth M. Toona sinensis. Bot Mag. 1998;15:186–96. [Google Scholar]
- 3.Yang Y, Wang J, Xing ZE, Dai YQ, Chen M. Identification of phenolics in Chinese toon and analysis of their content changes during storage. Food Chem. 2011;128:831–8. [Google Scholar]
- 4.Hseu YC, Chen SC, Lin WH, Hung DZ, Lin MK, Kuo YH, et al. Toona sinensis (leaf extracts) inhibit vascular endothelial growth factor (VEGF)-induced angiogenesis in vascular endothelial cells. J Ethnopharmcol. 2011;134:111–21. doi: 10.1016/j.jep.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 5.Poon SL, Leu SF, Hsu HK, Liu MY, Huang BM. Regulatory mechanism of Toona sinensis on mouse leydig cell steroidogenesis. Life Sci. 2005;76:1473–87. doi: 10.1016/j.lfs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Yin H, Chen B, Xia G, Yang H, Jia X. Validated reversed phase-high performance liquid chromatography-diode array detector method for the quantitation of Rutin, a natural immunostimulant for improving survival in aquaculture practice, in toonea sinensis folium. Pharmacogn Mag. 2012;8:49–53. doi: 10.4103/0973-1296.93322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang KJ, Yang CR, Zhang YJ. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis) Food Chem. 2007;101:365–71. [Google Scholar]
- 8.Yang HL, Chang WH, Chia YC, Huang CJ, Lu FJ, Hsu HK, et al. Toona sinensis extracts induces apoptosis via reactive oxygen species in human premyelocytic leukemia cells. Food Chem Toxicol. 2006;44:1978–88. doi: 10.1016/j.fct.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wu X, Huang L. Correlation between antioxidant activities and phenolic contents of Radix Angelicae Sinensis (Danggui) Molecules. 2009;14:5349–61. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohd A, Mohd M, Khan A, Ashraf K, Sharma D, Mohammad A. Phytochemical analysis and in vitro antioxidant activity of Uncaria gambir. Int J Green Pharm. 2012;6:67–72. [Google Scholar]
- 11.Lhami G, Fevzi T, Sarıkaya SB, Bursal E, Bilsel G, Gören AC. Polyphenol contents and antioxidant properties of Medlar (Mespilus germanica L.) Rec Nat Prod. 2011;5:158–75. [Google Scholar]
- 12.Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, et al. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–71. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh TJ, Wang JC, Huc CY, Li CT, Kuo CM, Hsieh SL. Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under Vibrio alginolyticus challenge. Fish Shellfish Immunol. 2008;25:581–8. doi: 10.1016/j.fsi.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Yang H, Xia G, Wang J, Cai B, Jia X. Isolation of gallic acid and methyl gallate from Folium Toonea Sinensis and validated method for their quantitation using LC based technologies. Acta Chromatogr. 2013;25:687–701. [Google Scholar]


