Abstract
HIV-1 persistence in long-lived cellular reservoirs remains a major barrier to a cure. In a recent Nature Medicine paper, Buzon et al. identify memory T cells with stem cell-like properties (TSCM) that harbor infectious provirus and that likely contribute to HIV-1 persistence.
HIV infection can be effectively suppressed and disease can be prevented with highly active antiretroviral therapy (HAART). However, HIV-infected people must remain on treatment continuously to avoid viral rebound and progression to acquired immunodeficiency syndrome. HIV persistence is thought to stem primarily from the presence of integrated copies of the proviral genome within long-lived cells. Because active viral gene expression causes cell death due to viral cytopathic effects and the immune response, long-lived cells likely harbor transcriptionally silent, latent provirus. There is great interest in identifying and characterizing the cell types that harbor latent provirus so that strategies to eliminate these ‘reservoirs’ of virus can be developed.
Resting memory CD4+ T cells are the best-characterized reservoir of latent HIV [1]. However, the mechanism by which latent infection is established within this compartment is still unclear. Activated T lymphocytes are highly susceptible to the virus but they mainly support active infection, which rapidly kills the cells. Latent reservoirs may nevertheless form if a small subset of activated cells survive long enough post-infection to return to a resting state [2]. In addition, recent studies have provided evidence that HIV-1 may rarely succeed in infecting resting cells and immediately establish latent infection [3]. Thus there may be two pathways by which HIV can establish latency in T cells.
Because different T cell subsets may require different strategies for eradication, there has been great interest in the identity of all the T cell subsets that can harbor latent virus. A number of groups have characterized a reservoir of HIV-1 within the central memory T cell compartment (TCM) and this reservoir has been extensively studied [4]. In addition, a recent study by Buzon and colleagues characterized infection of a newly described T cell subset (TSCM) [5,6] (Figure 1). These cells are of great interest because they can survive for long periods of time and potentially spread provirus vertically with self-renewal and differentiation. The authors demonstrate that TSCM can be infected in vitro by CCR5-tropic HIV-1 strains and that the relatively low frequency of infection correlates with a relatively low level of CCR5 compared with other subtypes. Interestingly, when the tropism of the virions was artificially expanded by incorporation of the vesicular stomatitis virus envelope glycoprotein, TSCM were significantly more permissive to infection than the other CD4+ T cell subtypes tested. While additional studies will be needed to understand this observation, the authors noted that the enhanced susceptibility to infection correlates with a relatively low level of the HIV-1 restriction factor TRIM5α [7].
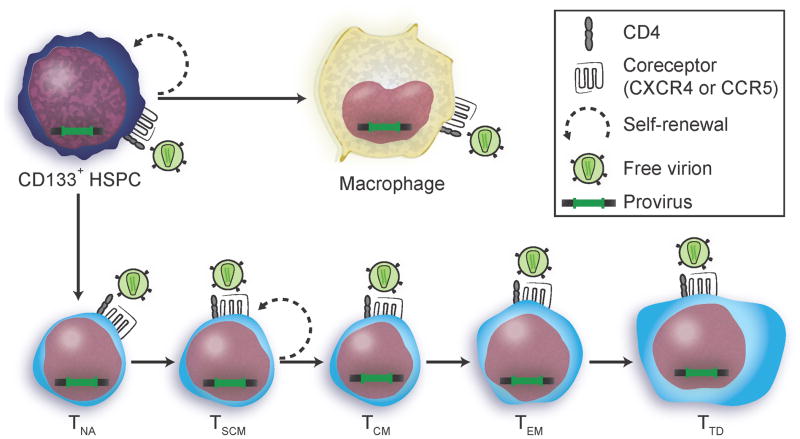
Figure 1.
HIV transmission among potential long-lived cellular reservoirs. Cartoon illustration depicting two pathways by which cells that express the HIV-1 receptors (CD4 and CXCR4 or CCR5) can acquire integrated provirus. In untreated people, virions directly infect a target cell and the error prone reverse transcriptase incorporates approximately one mutation per viral genome per replication cycle. In treated people, drugs can effectively prevent new infection. However, it has been hypothesized that integrated provirus may spread by cell division and differentiation of precursor cells. A proviral genome acquired by cellular replication will have the same sequence and integration site as the parent cell. In optimally treated people, cellular reservoirs are expected to decay over time according to the life span of the cell type that harbors them. Buzon et al. observed a significant decrease in the amount of HIV DNA associated with TEM and TTD but not TNA, TCM or TSCM over 7–11 years of continuous treatment [5]. Abbreviations: HSPC, hematopoietic stem and progenitor cells (CD133+ HSPCs are a heterogeneous population of precursor cells that include hematopoietic stem cells.); TNA, naïve CD4+ T lymphocyte; TSCM, stem cell memory CD4+ T lymphocyte; TCM, central memory CD4+ T lymphocyte; TEM, effector memory CD4+ T lymphocyte; TTD, terminally differentiated CD4+ T lymphocyte. Cell types with a greater potential to self-renew and differentiate are indicated by the dashed circular arrow.
To determine whether TSCM represent a stable reservoir of HIV-1 in vivo, the authors purified TSCM from HIV-infected people who had been optimally treated with HAART achieving long-term viral suppression. They found that provirus was present within TSCM at a comparatively high frequency. However, TSCM were present at an extremely low frequency and the total contribution of the TSCM to the cellular pool was small. Nevertheless, longitudinal evaluation of cell associated HIV-1 DNA demonstrated that the viral reservoir within TSCM and central memory T cells (TCM) was stable, while proviral DNA associated with terminally differentiated and effector memory T cell subsets (TTD and TEM) decreased over time. Moreover, the contribution of TSCM to the total HIV-1 reservoir in CD4+ T cells increased over the course of long-term HAART [5].
To provide evidence that infected TSCM are a source of virions, the authors amplified a portion of the viral genome from the pool of residual circulating plasma virus and compared it to similar amplicons from provirus associated with TSCM. Indeed, a phylogenetic analysis revealed similarities between these two populations and moreover, the data suggest that TSCM infected early in the course of disease may provide a stable and long-lived source of virus much later in the course of infection. Finally, the phylogenetic analysis revealed relationships between provirus isolated from TSCM and more differentiated T cell subtypes. While it’s tempting to speculate that identical sub-genomic fragments found within differentiated cells might indicate a common ancestry from an infected TSCM, it is also possible that highly related viruses infected different long-lived cells (Figure 1). A definitive answer to this question could be obtained by the identification of common proviral integration sites, which would uniquely identify infected daughter cells that differentiated from a precursor cell type.
Similar to TSCM, CD133+ bone marrow hematopoietic stem and progenitor cells (HSPCs) are another cellular target of HIV-1 capable of self-renewal and differentiation into terminal cell types. HIV provirus has been identified within these cells in some donors [8] and the significance of this reservoir is a subject of ongoing research. As HSPCs are even more rare than TSCM, the reservoir is likely to be even smaller. Nevertheless, all reservoirs, no matter how small, will likely need to be specifically targeted to affect a cure.
A goal of current research is to kill the latently infected cells by reactivating provirus and inducing viral cytopathic effects while preventing spread to new target cells. Therefore, the biology of viral latency and reactivation in all reservoirs is critically important to understand. For example, the mechanism of latency establishment and reactivation is different in HSPCs compared to T cells. In HSPCs, provirus appears to undergo immediate post-integration silencing that can be reversed upon activation of nuclear factor-κB (NF-κB) with tumor necrosis factor α (TNFα) treatment [9]. In contrast, TNFα is not sufficient to reactivate latently infected T lymphocytes, as quiescent resting memory T cells must additionally upregulate positive transcription elongation factor b (P-TEFb), which is needed for HIV transcription and active infection. All known cellular reservoirs can be activated by less specific strategies that reverse silencing with histone deacetylase inhibitors (HDACi). However, the viral cytopathic effects induced following reactivation by HDACi alone may be insufficient to kill infected cells [10]. A more complete basic understanding of how latency is established and how reactivation occurs will likely facilitate the development of more specific and less toxic eradication strategies.
Acknowledgments
We apologize to many whose work could not be cited due to space constraints. This work was supported by NIH RO1 AI096962 and the Burroughs Wellcome Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 2.Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swiggard WJ, et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol. 2005;79:14179–88. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzon MJ, et al. HIV-1 persistence in CD4(+) T cells with stem cell-like properties. Nat Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stremlau M, et al. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 8.McNamara LA, et al. CD133+ hematopoietic progenitor cells harbor HIV genomes in a subset of optimally treated people with long-term viral suppression. J Infect Dis. 2013;207:1807–16. doi: 10.1093/infdis/jit118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNamara LA, et al. Latent HIV-1 infection occurs in multiple subsets of hematopoietic progenitor cells and is reversed by NF-κB activation. J Virol. 2012;86:9337–50. doi: 10.1128/JVI.00895-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]