Abstract
Background
Some dominant bacterial divisions of the intestines have been linked to metabolic diseases such as overweight and diabetes.
Objective
A pilot study aimed to evaluate the relations between the culturable intestinal bacteria with body mass index (BMI) and some principal cellular and metabolic markers of blood in people older than 65.
Design
Altogether 38 generally healthy elderly people were recruited: ambulatory (n=19) and orthopedic surgery (n=19). Questionnaires on general health, anthropometric measurements, routine clinical and laboratory data, and quantitative composition of cultivable gut microbiota were performed.
Results
Blood glucose level was positively correlated with BMI (r=0.402; p=0.014). Higher blood glucose level had negative correlation with relative share of intestinal anaerobic bacteria such as bacteroides (r=−0.434; p=0.0076) and gram-positive anaerobic cocci (r=−0.364; p=0.027). In contrast, the relative share of bifidobacteria (r=0.383; p=0.019) and staphylococci (r=0.433; p=0.008) was positively correlated to blood glucose level. In elderly people, a higher blood glucose concentration was predicted by the reduction of the anaerobes’ proportion (adj. sex, age, and BMI R2=0.192, p=0.028) and that of Bacteroides sp. (adj. R2=0.309, p=0.016).
Conclusion
A tight interplay between increased BMI, level of blood glucose, and the reduced proportion of cultivable bacteroides is taking place in the gut microbiota of elderly people.
Keywords: lactobacilli, bacteroides, intestine, quantitative composition, elderly people, BMI, glucose level
Gut microbiota forms an essential part of the complex ecosystem of the host and is involved in nutrition and health. Sequencing 16S rRNA genes from human stool samples and further biochemical investigations have shown that some dominant bacterial divisions are associated with host nutrient uptake, metabolism, and body weight. In obese children, adults, and experimental animals, a higher proportion of gram-positive Firmicutes was found compared with gram-negative Bacteroidetes (1–5). However, it has not been fully explored among different age groups which particular groups of microbes from the Firmicutes division could be the main players in the collective metabolic influence on human health.
Elderly persons (>65 years) are the fastest growing subpopulation in the world (6). During aging, degenerative alterations in gut morphology and physiology mainly occur (7–10). The disturbances in some metabolic pathways and decreased energy expenditure commonly result in excessive weight and obesity, driving elderly people to several medical complications, such as cardiovascular disease, hypertension, diabetes, and osteoarthritis (11–13).
A wide variety of host, dietary, and environmental factors affect bacterial colonization in the intestinal tract. In the elderly, the bacteriological and non-culture-dependent molecular studies have indicated a reduction in numbers and species diversity of anaerobes, such as bacteroides and bifidobacteria, and an increase in the abundance of aerobes, such as enterobacteria (14–19). Still, it has not been established whether the old-age-related shifts in the well-balanced microbial ecosystem of the gastrointestinal tract may impair the health of the host. In our previous study of the elderly people, the Lactobacillus sp. counts and composition were positively related to weight, blood cell count, blood glucose level, and content of ox-LDL (20, 21). However, other important aerobic and anaerobic live intestinal bacteria were not tested for their impact on health biomarkers. Our current hypothesis is that, in generally healthy elderly people, the intestinal cultivable microbiota could predict some clinically valuable indices of human blood, which are related to host metabolic and defense reactions.
The purpose of the pilot study was to evaluate the relations between culturable intestinal bacteria with body mass index (BMI) and laboratory indices of blood (cellular erythrocytes, hemoglobin, thrombocytes; metabolic glucose, and cholesterol) in healthy elderly people (ambulatory volunteers and patients hospitalized for orthopedic surgery).
Subjects and methods
A total of 42 elderly persons from an area in southern Estonia, described in our previous pilot study, were recruited (20). The inclusion criteria for volunteers were persons aged 65 years or older, those who considered themselves generally healthy, and those who were willing to participate in the study (confirmed by written informed consent).
For this study, 38 elderly persons were selected, whose complete data by questionnaire, clinical investigation, blood biochemistry, and microbiological analyses of feces were available. In two drop-outs, the blood sample was clotted and from two other persons the fecal samples were not obtained. These four subjects did not differ by questionnaire data or BMI from subjects who were included in the study. The healthy elderly were divided into two groups: ambulatory individuals from the local community (Group HE, n=19, recruited in 2005) and hospitalized patients (Group PE, n=19; recruited in 2006). Group HE volunteers were selected from the registry of family doctors, having no clinical signs of osteoarthritis; Group PE was recruited from the Department of Orthopaedics of Tartu University Hospital scheduled for elective orthopedic surgery (knee or hip replacement due to osteoarthritis). Hospitalized persons were recruited before their operation, when they had not been on a standard hospital diet. The study was approved by the Ethics Committee of Tartu University Medical Faculty (No. 139/16 20.06.2005).
Methods
Information on general health and smoking habits was obtained from a self-administered questionnaire. The self-reported questionnaire contained questions on education, welfare, nutritional habits, and habitual gastrointestinal symptoms (abdominal pain, flatulence, bloating, and stool frequency). Also medications used during the past 6 months were registered (22).
Clinical investigation
All subjects completed a comprehensive medical evaluation, including medical history and physical examination, to evaluate the function of the cardiovascular system and to determine the state of skin and mucosa. Each participant was clinically checked for anthropometrical indices, and their BMI was calculated as weight (kg) divided by height squared (m2). Blood pressure (mmHg) was measured manually after the subjects had remained in the supine position for 15 minutes and was recorded to the nearest 2 mmHg.
Blood samples
Blood samples were obtained early in the morning, after 8 hours of fasting. Samples (6 ml) were drawn from antecubital vein with vacutainer. Hematological–clinical indices (hemoglobin, red blood cells, and platelets) and routine biochemical–clinical indices like plasma glucose and serum total cholesterol were determined by standard laboratory methods using certified assays in the local clinical laboratory of Tartu University Hospital.
Fecal samples for bacteriological evaluation
Approximately 2 g of fresh stool samples were obtained and put into sterile plastic cups. The samples were collected and delivered to the laboratory within 1 hour after defecation, where they were stored at −20°C before being processed.
For bacteriological analyses, the weighed samples of feces were serially diluted (10−2–10−9) in pre-reduced phosphate buffer (pH 7.2) inside the anaerobic glove box (Sheldon Manufacturing Inc., USA) with a gas mixture of 5% CO2, 5% H2, and 90% N2. A quantitative analysis of gut bacteria was performed using duplicate samples of 0.05 ml of each dilution on non-selective and selective media (23).
The de Man–Rogosa–Sharpe agar (MRS; Oxoid) for microaerobic lactobacilli and streptococci, Endo agar for enterobacteria, Wilkins–Chalgren (W–C) agar (Oxoid) for total anaerobes, W–C agar with vancomycin and nalidixic acid supplement (Oxoid) for gram-negative anaerobes as bacteroides, W–C agar with colistin and nalidixic acid supplement (BBL) for gram-positive anaerobes as bifidobacteria, anaerobic gram-positive cocci, and clostridia were used. The inoculated W–C media were incubated in the anaerobic glove box for 5–6 days and MRS medium in a microaerobic atmosphere (CO2 incubator ‘Jouan’ IG 150, France) with gas mixture (10% CO2) at 37°C for 48 hours. Endo medium was incubated aerobically at 37°C and inspected after 24 and 48 hours (23).
The colony counts of the different fecal dilutions on different media were recorded, and from the highest dilutions with growth all colonies of different morphology were isolated for identification. The microorganisms were identified on genus level. Anaerobes were identified by growth on selective media, colonial and cellular morphology, and gram-stain reaction (gram-negative rods as bacteroides; gram-positive rods as bifidobacteria, eubacteria, and clostridia; and gram-positive cocci as anaerobic cocci) after checking their inability to grow in aerobic and microaerobic environments. Clostridia were assessed after ethanol treatment. Lactobacilli were identified after growth in selective MRS media, gram-positive rod-shaped morphology, and negative catalase test (24, 25). For identification of enterobacteria, the standard methods were used (26).
The composition of the gut microbiota was expressed as counts colony forming unit (CFU/g expressed in log10) of the various bacteria, the relative proportion (%) of different bacteria of the total isolated microbiota, and prevalence (%) of particular bacteria in the groups of investigated persons. The detection level of the various microorganisms was 3 log CFU/g.
Statistical analysis
The statistical analyses were performed using ‘SigmaStat’ (Jandel Scientific, USA) and SPSS 11.0 (SPSS Inc., Chicago, IL, USA) and PAST (27) statistical software packages. Data were presented as the mean value±S.D. or median in the case of non-parametric distribution; the prevalence of the subjects was described as the proportions (%) of those investigated. The Student's t-test or Mann–Whitney U test (non-parametric distribution) was applied to compare the differences in the clinical, biochemical, and microbiological indices of the two subgroups. The χ2-test or Fisher exact test was used to determine the between-group differences in categorical variables.
For analyzing general bacterial diversity, Shannon diversity index was calculated. The Shannon diversity index and the Spearman rank correlation test were used to test the associations between the microbiological, clinical, and biochemical indices of the elderly. The multiple linear regression models were developed by entering the data of microbiological investigations as predictors for the dependent variables, such as cholesterol and glucose. The models were adjusted for age, sex, and BMI. All differences were considered statistically significant if the p value was <0.05.
Results
The two groups of elderly persons were similar in terms of age and gender (HE: range 66–80 years, 6 male and 13 female; PE: range 65–84 years, 9 male and 10 female).
Questionnaire data
According to the self-reported questionnaires, there were no substantial differences between the ambulatory volunteers and hospitalized patients concerning their education, diets, medical history, or any of the habitual gastrointestinal symptoms (abdominal pain, flatulence, bloating, and stool frequency; p>0.05; data not shown). The number of current smokers was very low in both groups (1 and 2, respectively; p>0.05).
Clinical and blood indices
No substantial differences in the function of cardiovascular systems and the state of skin and mucosa (data not shown) were observed between the two study groups (HE and PE). The distribution of normal, overweight, and obese persons was different in both groups with more normal values found in HE group (p=0.008; Table 1). The BMI and fasting plasma glucose values were significantly higher (p=0.002 and 0.001, respectively) in PE as compared to HE volunteers. No significant differences were found between the two groups in blood pressure, erythrocytes and platelets counts, hemoglobin, and total cholesterol level (Table 1).
Table 1.
Clinical–biochemical data of healthy elderly volunteers (HE) and hospitalized patients (PE)
| Mean±SD, prevalence N (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | Total N=38 Age 72±5.0 |
Group HE N=19 Age 72±4.0 |
Group PE N=19 Age 72±5.9 |
p values1–2
HE vs. PE |
| Body mass index (kg/m2) | 27.1±4.0 | 25.3±3.2 | 28.9±4 | 10.002 |
| Normal 18.5–24.9 kg/m2 (N%) | 15 (39%) | 12 (63%) | 3 (16%) | |
| Overweight >25 kg/m2 (N%) | 14 (37%) | 5 (26%) | 9 (47%) | 20.008 |
| Obese >30 kg/m2 (N%) | 9 (24%) | 2 (11%) | 7 (37%) | |
| Systolic blood pressure (mmHg) | 134±16.1 | 134±19 | 134±13 | NS |
| >130 mmHg (N%) | 13 (34%) | 9 (47%) | 7 (37%) | NS |
| Diastolic blood pressure (mmHg) | 79±8.0 | 79±8 | 79±8 | NS |
| >85 mmHg (N%) | 6 (16%) | 2 (11%) | 4 (21%) | NS |
| Fasting plasma glucose mmol/L | 5.2±0.6 | 4,9±0,5 | 5.5±0.5 | 10.001 |
| Erythrocytes×1012/L | 4.6±0.4 | 4.7±0.4 | 4.6±0.3 | NS |
| Hemoglobin | 139±11 | 142±8 | 137±14 | NS |
| Platelets×109 L | 227.4±41 | 232±41 | 222±41 | NS |
| Total cholesterol (mmol/L) | 5.8±1.1 | 5.8±1.1 | 5.8±1.1 | NS |
| >5.0 mmol/L (N%) | 29 (76%) | 13 (68%) | 16 (84%) | NS |
NS, no statistically significant differences.
Student t-test or Mann–Whitney test according to parametric vs. non-parametric distribution of data.
Fisher exact test.
We compared clinical and blood markers in elderly people with normal BMI (n=13; range 67–80 years, male 5 and female 8) versus with overweight/obese BMI (>25 kg/m2, n=25; range 65–84 years, male 10 and female 15) and people with normal blood glucose level (n=15; range 66–84 years, male 6 and female 9) with enhanced blood glucose level (>5 mmol/L; n=22; range 65–84 years, male 9 and female 13). No significant differences were found between blood pressure, erythrocytes and platelet counts, hemoglobin, and total cholesterol level in the two groups mentioned above (data not shown).
For further association analysis, to increase the study power, the elderly groups were linked (n=38). Cholesterol level had a positive correlation with coffee (r=0.499; p=0.002) and soft drinks (r=0.499; p=0.002) consumption and at the same time a negative correlation with tea (r=−0.555; p<0.001) and food additives (r=−0.324; p=0.04) such as cod-liver oil, ginseng, sea buckthorn, honey, bio-glucosamine, ginkgo extract, flaxseed oil, whitethorn berry tea, and bee glue consumption.
Quantitative composition of fecal microbiota
In hospitalized patients, as compared to ambulatory persons, a higher total count of intestinal bacteria (p=0.004) and a higher count of lactobacilli (p=0.021) were found (Table 2). Lactobacilli were present in all investigated elderly persons. However, the relative share of gut bacteria and the Shannon diversity index of intestinal microbiota (mean 1.589±0.143 vs. mean 1.690±0.154) was not statistically different in ambulatory and in hospitalized persons.
Table 2.
Intestinal microbiota in healthy volunteers (HE) versus hospitalized patients (PE) and in normal weight versus overweight elderly people (prevalence, %; count log cfu/g median—25%; 75%)1
| Volunteers (HE) and hospitalized (PE) | Normal (BMI <25) and overweight (BMI >25) | ||||
|---|---|---|---|---|---|
| Bacteria | Total N=38 |
Group HE N=19 |
Group PE N=19 |
BMI <25 N=13 |
BMI >25 N=25 |
| Total count | 100% | 100% | 100% | 100% | 100% |
| 10.2 | 9.9 | 10.7 | 10.3 | 8.3 | |
| (9.9; 10.7) | (9.8; 10.1)a | (10.2; 11.2)a | (9.5; 11)c | (6; 9.3)c | |
| Anaerobes | 100% | 100% | 100% | 100% | 100% |
| Prevalence | 9.8 | 9.8 | 10.0 | 9.8 | 6.4 |
| Count | (9.6; 10.4) | (9.6; 9.9) | (9.3; 10.9) | (9.6; 10.3)d | (5.5; 8.6)d |
| Bacteroides | 97% | 100% | 95% | 92% | 100% |
| Prevalence | 9.6 | 9.6 | 9.6 | 9.6 | 9.5 |
| Count | (9.3; 10.3) | (9.4; 9.8) | (9.2; 10.9) | (9.6; 9.9) | (9.2; 10.4) |
| Bifidobacteria | 50% | 47% | 53% | 31% | 60% |
| Prevalence | 9.3 | 9.3 | 9.3 | 8.8 | 9.3 |
| Count | (8.6; 9.6) | (8.5; 9.4) | (8.6; 9.8) | (8.2; 9.3) | (8.8; 9.8) |
| Anaerobic cocci | 73% | 79% | 68% | 62% | 74% |
| Prevalence | 8.8 | 8.9 | 8.3 | 8.45 | 8.9 |
| Count | (8.1; 9.3) | (8.6; 9.3) | (7; 9.1) | (7.8; 8.9) | (8.1; 9.3) |
| Clostridia | 39% | 53% | 26% | 46% | 36% |
| Prevalence | 0 | 7.3 | 0 | 9.1 | 9.0 |
| Count | (0–8.8) | (0–9.25) | (0–3.6) | (7.8; 9.5) | (7.3; 9.5) |
| Lactobacilli | 100% | 100% | 100% | 100% | 100% |
| Prevalence | 7.2 | 6.3 | 7.8 | 7.3 | 6.8 |
| Count | (5.5; 8.6) | (4.5; 7.3)b | (6.3; 9.3)b | (4.6; 8.4) | (5.9; 8.6) |
| Coliforms | 94% | 100% | 89% | 100% | 96% |
| Prevalence | 8.3 | 7.3 | 9.3 | 8.3 | 8.3 |
| Count | (6.3; 9.3) | (6.3; 8.3) | (7.7; 9.8) | (6.3; 9.2) | (6.2; 9.3) |
| Staphylococci | 84% | 88% | 88% | 85% | 76% |
| Prevalence | 9.2 | 7.3 | 9.7 | 8.3 | 9.6 |
| Count | (7.3; 9.8) | (5.3; 8.5) | (9.2; 10.2) | (6.8; 8.9)e | (7.2; 10.1)e |
| Streptococci | 60% | 50% | 77% | 62% | 60% |
| Prevalence | 9.4 | 9.3 | 10.3 | 9.4 | 10.0 |
| Count | (8.7; 10.5) | (8.5; 9.4) | (9.6; 10.8) | (9; 9.5) | (8.5; 10.89) |
Data were analyzed using Student's t-test for unpaired samples for continuous variables or Mann–Whitney rank sum test (non-parametric distribution) and Chi-square test for categorical variables.
p=0.004;
p=0.02;
p<0.001;
p<0.001;
p=0.037.
Comparing microbiota data with health indices
In elderly with normal BMI, the total counts of gut bacteria and anaerobic bacteria (p<0.001 for both) were higher and the counts of staphylococci (p=0.037) were lower than in overweight and/or obese elderly people (Table 2). In overweight/obese and normal weight elderly people, the relative share of gut bacteria and Shannon diversity index of gut microbiota (mean 1.679±0.142 vs. mean 1.563±0.156) were not statistically different.
The count of clostridia was higher in people with low blood glucose level than in people with higher glucose level (median 4.8 mmol/L, range 0–9.6 vs. median 0 mmol/L, range 0–10.3; p=0.017). In people with enhanced blood glucose level, higher relative share of staphylococci (mean 11.7±14.1% vs. mean 2.4±7.1%; p=0.005) and bifidobacteria (mean 12.9±16.4% vs. mean 4.5±12.0%; p=0.008) were found compared to people with normal blood glucose level. The relative share of bacteroides was higher in elderly people with normal glucose level (mean 54.8±21.5% vs. mean 31±29.5%; p=0.048). The Shannon diversity index was similar in both groups (mean 1.629±0.154 vs. mean 1.659±0.150).
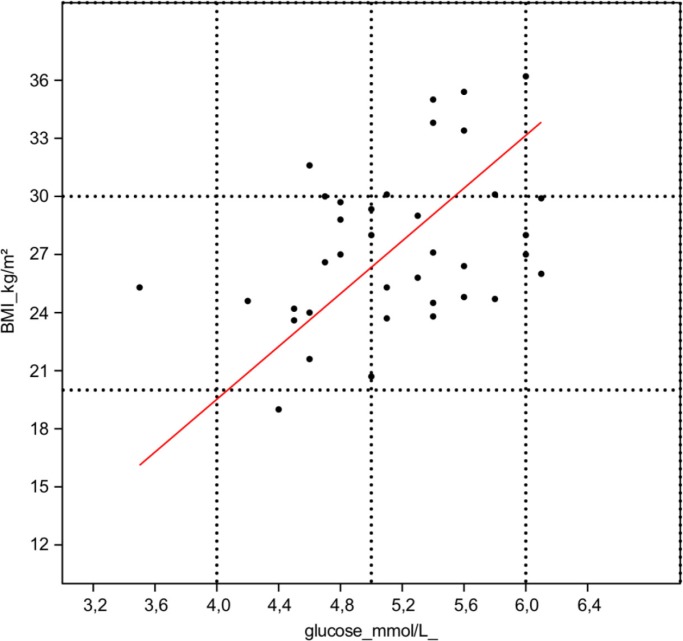
In linked groups of elderly (n=38), blood glucose level was positively correlated with BMI (r=0.402; p=0.014; Fig. 1). The diversity of gut microbiota was in positive correlation with anaerobic bacteria as gram-positive cocci (r=0.449; p=0.005) and clostridia (r=0.489; p=0.002). At the same time, blood glucose level had negative correlation with total relative share of anaerobic bacteria in gut (r=−0.329; p=0.047). The enhanced blood glucose level had negative correlation with relative share of intestinal anaerobic bacteria-like bacteroides (r=−0.434; p=0.0076) and with counts and relative share of gram-positive anaerobic cocci (r=−0.365; p=0.024; and r=−0.364; p=0.027). In contrast, the counts and relative share of bifidobacteria (r=0.325; p=0.046 and r=0.383; p=0.019) and staphylococci (r=0.382; p=0.018 and r=0.433; p=0.008) had positive correlation with blood glucose level.
Fig. 1.
The correlation of BMI and blood glucose level.
After adjustment by sex, age, and BMI, blood glucose level correlated negatively to the relative share of anaerobic gut bacteria. Higher blood glucose level was predicted by the reduction of the proportion of anaerobes (adj. R2=0.192; p=0.028) and particularly that of bacteroides (adj. R2=0.309; p=0.016).
Discussion
Advancements in medicine as well as improved living standards have contributed to the marked increase in the number of people older than 65, which clearly is bound to emerging multiple risk factors for cardiovascular diseases and metabolic syndrome.
Cardiovascular diseases are related to enhanced blood cholesterol level. In our study, blood cholesterol level was positively correlated to coffee and negatively to tea consumption. Tea and coffee drinking have been associated with the risk of cardiovascular diseases, both positively and negatively. In meta-analysis of tea consumption, the incidence rate of myocardial infarction is estimated to decrease by 11%. The polyphenol flavonoids with antioxidative properties in tea are thought to have a protective effect on cardiovascular diseases (28). Coffee consumption on one hand is strongly and positively related to higher serum cholesterol level, and thus increases the risk of coronary heart diseases. On the other hand, coffee has antioxidant activity and reduces plasma glucose concentration and therefore may reduce the risk of type 2 diabetes mellitus (29).
It has been shown recently that the composition of intestinal microbiota is related to cardiovascular and metabolic diseases (4, 30–33). The intestinal microbiota comprises at least 1013 microbes and over 1000 species of bacteria reside in the colons of healthy adults. The great majority (up to about 80%) of these bacterial species have not been cultured. These numbers have mainly been assessed by molecular methods, counting both dead and live cells (34, 35). The advantage of our study is the possibility to explore the potential interplay between intestinal microbiota and human metabolism. Our study results show that the living predominating anaerobic populations and facultative anaerobic were associated with BMI and blood glucose level.
In our study of healthy elderly persons, we included ambulatory subjects and patients hospitalized for orthopedic surgery. BMI was elevated in more than 80% of hospitalized patients with osteoarthrosis. The etiology and pathogenesis of osteoarthritis (whether of inflammatory origin or due to the degeneration of cartilage) is still an issue of debate (36, 37). The increase of lactobacilli count was one of the remarkable findings in patients with osteoarthritis (20). Hospitalized patients had higher blood glucose level and counts of lactobacilli in their gut similar to a study where lactobacilli correlated positively with fasting glucose level (33).
A positive correlation between BMI and fasting blood glucose was found. Overweight and higher blood glucose levels were associated with the lower number of total anaerobes found in the gut. Blood glucose level had a negative correlation to the relative share of anaerobes such as gram-positive anaerobic cocci and bacteroides. Further, the high content of glucose was predicted from a low abundance of anaerobes, particularly bacteroides. This is in accordance with the data of J. Gordon laboratory, where a lower proportion of anaerobic gram-negative Bacteroidetes phyla in obese adults and experimental animals has recently been shown (1, 2, 4, 30–32). Aging brings along reduced prevalence and diversity of bacteroides in elderly people compared to young volunteers (17, 38). Bacteroides species are nutritionally versatile and are able to use different carbon sources. They are the main producers of short fatty acids as propionate from fermentation of unabsorbed carbohydrates and thus important to colonic physiology (31, 39).
Elderly people have a lower number of anaerobes and less diversity of microbiota when compared to adults (18, 40–42). Less diversity of the dominating bacterial community has been found in overweight people in contrast to normal weight people (32, 43). In our study, gut microbiota diversity was bound to colonization of anaerobes like gram-positive anaerobic cocci and clostridia. The proportion of anaerobic cocci and counts of clostridia were higher in people with lower blood glucose level. Karlsson with co-workers found that abundance of clostridium correlated negatively with fasting glucose, but not with BMI (33).
At the same time, the counts and proportions of bifidobacteria and staphylococci were associated with higher glucose level. A Finnish study has compared the early colonization profile of 7-year-old children during their first year of life with their body weight, showing that less prevalence of Staphylococcus aureus and high amounts of intestinal bifidobacteria at infancy might lower the risk of obesity later in life (44). This seems to contradict previous data where the higher proportion of Bifidobacterium was associated with higher body weight and BMI in 5-year-old children (45). This discrepancy can be explained by the fact that in pre-school children gut microbiota has developed to a degree where it is similar to that of adults, where the overall proportion of Bifidobacterium has decreased and Bacteroides have become predominant (39). Similar to our study, the proportion of phyla Actinobacteria, including bifidobacteria and staphylococci, was higher among overweight subjects (32, 46, 47). In pregnant women, Staphylococcus aureus counts correlated with excessive weight gain (46).
The metabolic syndrome is associated with the level of blood glucose. Khanam and co-workers found that in the presence of higher blood glucose level, metabolic syndrome has a significant negative effect on survival (69% vs. 95%) (13). In our pilot study of elderly persons, blood glucose level was closely associated with BMI and intestinal anaerobes. The higher counts and/or proportion of anaerobes as bacteroides, gram-positive cocci, and clostridia were associated with lower blood glucose level and a higher blood glucose concentration was predicted by the reduction of bacteroides. Thus, we have revealed the tight interplay among increased BMI, level of blood glucose, and the reduced proportion of cultivable bacteroides in gut microbiota of elderly persons.
Acknowledgements
This study was supported by grants from the Estonian Science Foundation grant no. 6782 and the Ministry of Higher Education and Research grant no. SF018255. We are grateful to Piret Soovares and Liina Salusaar for their invaluable help in organizing the study. We appreciate the excellent statistical evaluation by Heti Pisarev and the technical assistance from Agnes Laasimer.
References
- 1.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Nat Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis E, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 4.Nadal I, Santacruz A, Marcos A, Warnberg J, Garogorri M, Moreno LA, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes. 2008;33:758–67. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 5.Xu P, Li M, Zhang J, Zhang T. Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol. 2012;12:283. doi: 10.1186/1471-2180-12-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel RP. Health care – associated infections. Major issues in early years of 21st century. Clin Inf Dis. 2007;45:S85–S8. doi: 10.1086/518136. [DOI] [PubMed] [Google Scholar]
- 7.Timiras PS. Physiology of aging: standards for age-related functional competence. In: Greger R, Windhorst U, editors. Comprehensive human physiology. Vol. 2. Berlin: Springer-Verlag; 1996. pp. 2391–405. [Google Scholar]
- 8.Gill HS, Darragh AJ, Cross M. Optimizing immunity and gut function in the elderly. J Nutr Health Aging. 2001;5:60–91. [PubMed] [Google Scholar]
- 9.D'Souza AL. Ageing and the gut. Postgrad Med J. 2007;83:44–53. doi: 10.1136/pgmj.2006.049361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rayner CK, Horowitz M. Physiology of the ageing gut. Curr Opin Clin Metab Care. 2013;16:33–8. doi: 10.1097/MCO.0b013e32835acaf4. [DOI] [PubMed] [Google Scholar]
- 11.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 12.McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity. 2006;14:1485–97. doi: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 13.Khanam MA, Qiu C, Lindeboom W, Streatfield PK, Kabir ZN, Wahlin Å. The metabolic syndrome: prevalence, associated factors, and impact on survival among older persons in rural Bangladesh. PLoS One. 2011;6:e20259. doi: 10.1371/journal.pone.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbach SL, Nahas PI, Lerner PI, Weinstein L. Studies of intestinal differences in the distribution of bifidobacterial and enterobacterial species in human microflora. I Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967;53:845–55. [PubMed] [Google Scholar]
- 15.Mitsuoka T, Hayakawa K, Kimura N. Die Faekalflora bei Menschen. II. Mitteilung. Die Zusammensetzung der Bifidobakterienflora der verschiedener Altersgruppen. Zentbl Bakteriol Hyg Abt I. 1974;226:469–78. [PubMed] [Google Scholar]
- 16.Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profile. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodmansey EJ, McMurdo MET, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–22. doi: 10.1128/AEM.70.10.6113-6122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodmansey EJ. Intestinal bacteria and ageing. J Appl Microbiol. 2007;102:1178–86. doi: 10.1111/j.1365-2672.2007.03400.x. [DOI] [PubMed] [Google Scholar]
- 19.Tiihonen K, Tynkkynen S, Ouwehand A, Ahlroos T, Rautonen N. The effect of aging with and without non-steroidal anti-inflammatory drugs on gastrointestinal microbiology and immunology. Br J Nutr. 2008;18:1–18. doi: 10.1017/S000711450888871X. [DOI] [PubMed] [Google Scholar]
- 20.Mikelsaar M, Štšepetova J, Hütt P, Kolk H, Sepp E, Lõivukene K, et al. Intestinal Lactobacillus sp is associated with some cellular metabolic characteristics of blood in elderly people. Anaerobe. 2010;16:240–6. doi: 10.1016/j.anaerobe.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Štšepetova J, Sepp E, Kolk H, Lõivukene K, Songisepp E, Mikelsaar M. Diversity and metabolic impact of intestinal Lactobacillus species in healthy adults and the elderly. Br J Nutr. 2011;105:1235–44. doi: 10.1017/S0007114510004770. [DOI] [PubMed] [Google Scholar]
- 22.Svedlund J, Sjodin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 23.Sepp E, Julge K, Mikelsaar M, Björkstén B. Intestinal microbiota and immunoglobulin E responses in 5-year-old Estonian children. Clin Exp Allergy. 2005;35:1141–6. doi: 10.1111/j.1365-2222.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 24.Kandler O, Weiss N. Genus Lactobacillus Beijerinck. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, MD: Williams and Wilkins; 1986. pp. 1209–34. [Google Scholar]
- 25.Mikelsaar M, Annuk H, Stsepetova J, Mändar R, Sepp E, Björksten B. Intestinal Lactobacilli of Estonian and Swedish children. Microb Ecol Health Dis. 2002;14:75–80. [Google Scholar]
- 26.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Washington, DC: ASM Press; 1995. Manual of clinical microbiology. [Google Scholar]
- 27.Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:9. [Google Scholar]
- 28.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 29.Ranheim T, Halvorsen B. Coffee consumption and human health—beneficial or detrimental?—mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49:274–84. doi: 10.1002/mnfr.200400109. [DOI] [PubMed] [Google Scholar]
- 30.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLos One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2009;18:190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Hanady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson FH, Tremaroli T, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 34.Eckburg PB, Bik EM, Bernstein C, Purdom E, Dethlefsen L, Sargent M, et al. Diversity in the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaughan EE, Heilig HG, Ben-Amor K, de Vos MW. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev. 2005;29:477–99. doi: 10.1016/j.femsre.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Haq I, Murphy E, Dacre I. Osteoarthritis. Postgrad Med J. 2003;79:377–83. doi: 10.1136/pmj.79.933.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonaghan PG, Vanharanta H, Dieppe PA. Is progressive osteoarthritis an atheromatous vascular disease? Ann Rheum Dis. 2005;64:1539–41. doi: 10.1136/ard.2005.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He T, Harmsen HMJ, Raagns GC, Welling GV. Composition of faecal microbiota of elderly people. Microbiol Ecol Health Dis. 2003;15:153–9. [Google Scholar]
- 39.Balamurugan R, Janardhan HP, George S, Chittaranjan SP, Ramakrishna BS. Bacterial succession in the colon during childhood and adolescence: molecular studies in a southern Indian village. Am J Clin Nutr. 2008;88:1643–7. doi: 10.3945/ajcn.2008.26511. [DOI] [PubMed] [Google Scholar]
- 40.Hébuterne X. Gut changes attributed to ageing: effects on intestinal microflora. Curr Opin Clin Nutr Metab Care. 2003;6:49–54. doi: 10.1097/00075197-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Calesson MJ, Cusack S, O'Sullivan O, Green-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108:4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippe B, Zwielehner J, Liszt K, Lassl C, Unger F, Haslberger AG. Quantification of butyryl CoA: acetate CoA-transferase genes reveals different butyrate production capacity in individuals according to diet and age. EMS Microbiol Lett. 2011;316:130–5. doi: 10.1111/j.1574-6968.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrne S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–61. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 44.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 45.Sepp E, Lõivukene K, Julge K, Voor T, Mikelsaar M. The association of gut microbiota with body weight and body mass index in preschool children of Estonia. Microb Ecol Health Dis. 2013;24:19231. doi: 10.3402/mehd.v24i0.19231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 47.Santacruz A, Marcos A, Wärnberg J, Marti A, Martin-Matillas M, Campoy C, et al. Interplay between weight loss and gut microbiota. Composition in overweight adolescents. Obesity. 2009;17:1906–15. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]