Abstract
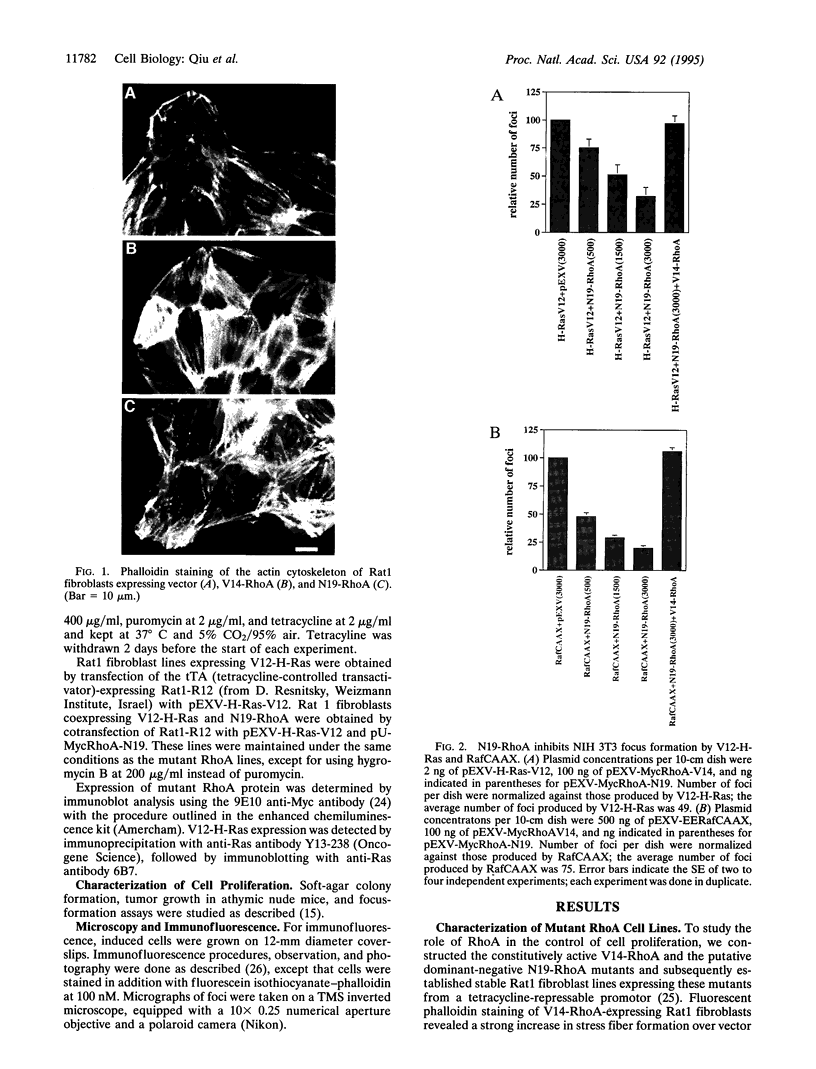
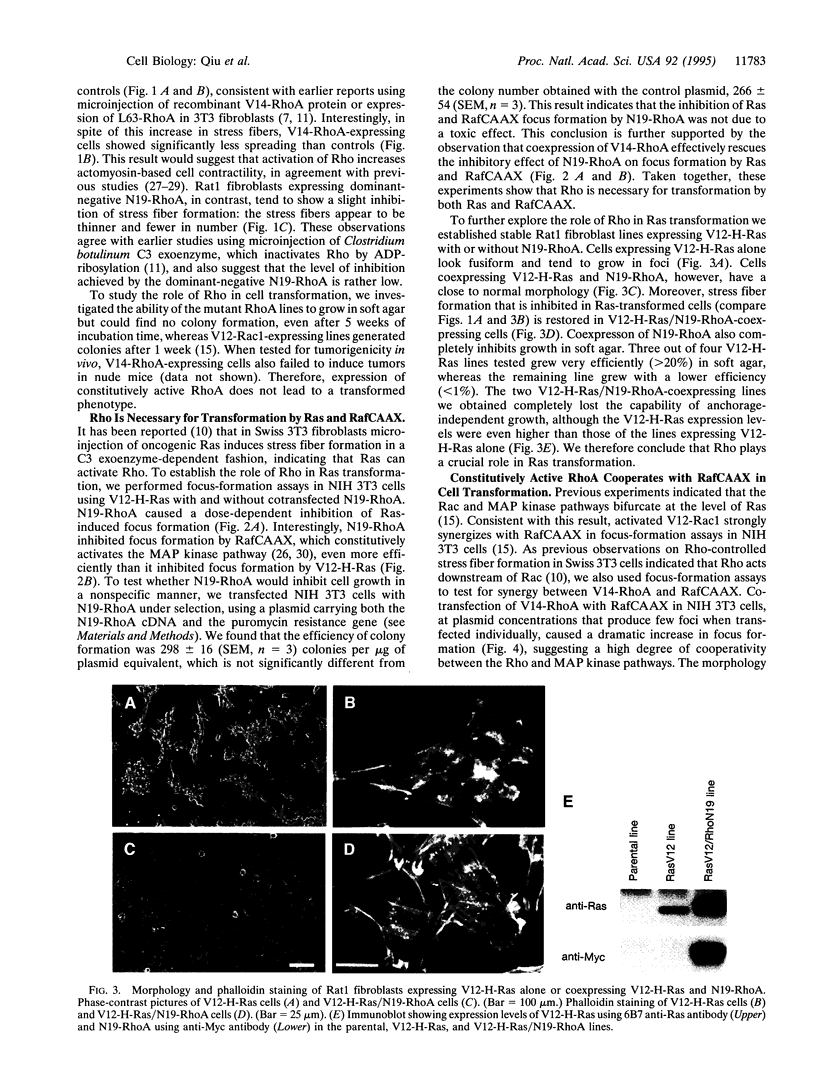
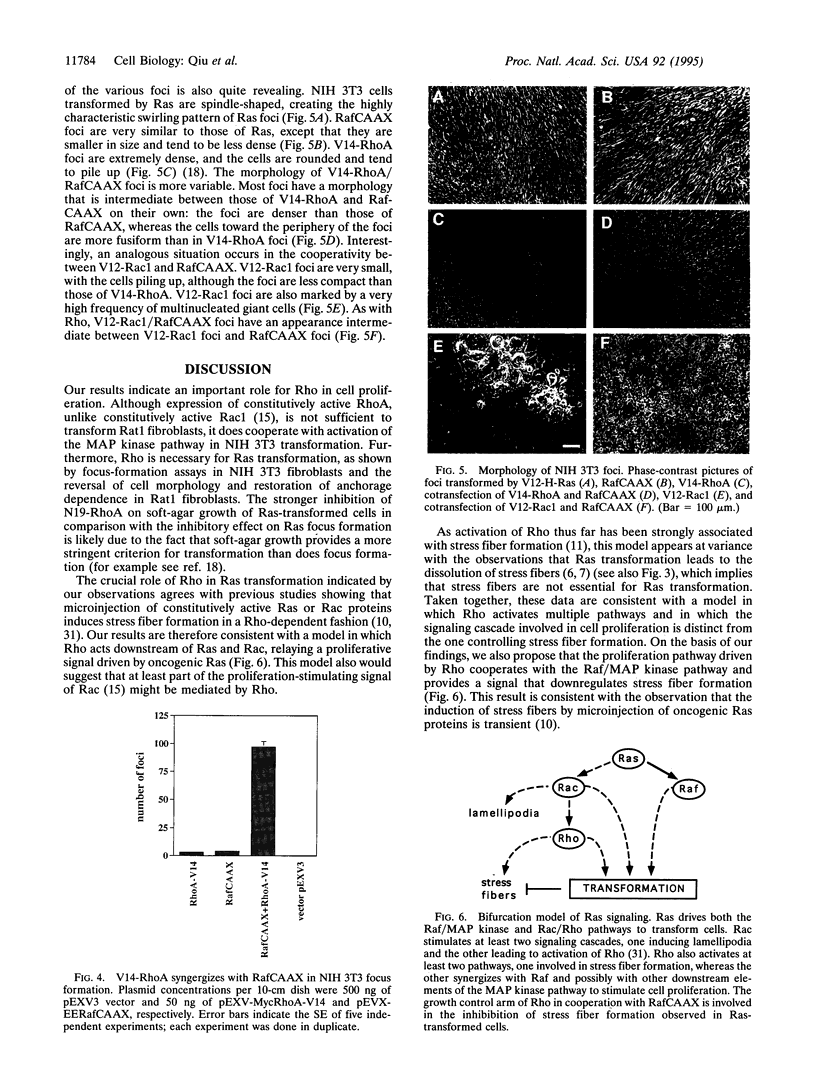
The small GTP-binding proteins Rac and Rho are key elements in the signal-transduction pathways respectively controlling the formation of lamellipodia and stress fibers induced by growth factors or oncogenic Ras. We recently reported that Rac function is necessary for Ras transformation and that expression of constitutively activated Rac1 is sufficient to cause malignant transformation. We now show that, although expression of constitutively activated V14-RhoA in Rat 1 fibroblasts does not cause transformation on its own, it strongly cooperates with constitutively active RafCAAX in focus-formation assays in NIH 3T3 cells. Furthermore, dominant-negative N19-RhoA inhibits focus formation by V12-H-Ras and RafCAAX in NIH 3T3 cells, and stable coexpression of N19-RhoA and V12-H-Ras in Rat1 fibroblasts reverts Ras transformation. Interestingly, stress fiber formation is inhibited in V12-H-Ras lines and restored by coexpression of N19-RhoA. We conclude that Rho drives at least two separate pathways, one that induces stress fiber formation and another one that is important for transformation by oncogenic Ras.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Weinberg R. A. Characterization and expression of the human rhoH12 gene product. Mol Cell Biol. 1989 May;9(5):2058–2066. doi: 10.1128/mcb.9.5.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Barr M., Wang Y., Jung V., Xu H. P., Wigler M. H. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994 Oct 7;79(1):131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chong L. D., Traynor-Kaplan A., Bokoch G. M., Schwartz M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994 Nov 4;79(3):507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Coso O. A., Chiariello M., Yu J. C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J. S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995 Jun 30;81(7):1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Dartsch P. C., Ritter M., Häussinger D., Lang F. Cytoskeletal reorganization in NIH 3T3 fibroblasts expressing the ras oncogene. Eur J Cell Biol. 1994 Apr;63(2):316–325. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Relationship among guanine nucleotide exchange, GTP hydrolysis, and transforming potential of mutated ras proteins. Mol Cell Biol. 1988 Jun;8(6):2472–2478. doi: 10.1128/mcb.8.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glück U., Kwiatkowski D. J., Ben-Ze'ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with alpha-actinin cDNA. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995 Jun 30;81(7):1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hirata K., Kikuchi A., Sasaki T., Kuroda S., Kaibuchi K., Matsuura Y., Seki H., Saida K., Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992 May 5;267(13):8719–8722. [PubMed] [Google Scholar]
- Horii Y., Beeler J. F., Sakaguchi K., Tachibana M., Miki T. A novel oncogene, ost, encodes a guanine nucleotide exchange factor that potentially links Rho and Rac signaling pathways. EMBO J. 1994 Oct 17;13(20):4776–4786. doi: 10.1002/j.1460-2075.1994.tb06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Hengeveld T., Morii N., Narumiya S., Moolenaar W. H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994 Aug;126(3):801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Chrzanowska-Wodnicka M., Solski P. A., Eva A., Burridge K., Der C. J. Dbl and Vav mediate transformation via mitogen-activated protein kinase pathways that are distinct from those activated by oncogenic Ras. Mol Cell Biol. 1994 Oct;14(10):6848–6857. doi: 10.1128/mcb.14.10.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R., Der C. J. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994 Mar;13(1):67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- Kishi K., Sasaki T., Kuroda S., Itoh T., Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J Cell Biol. 1993 Mar;120(5):1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995 Apr;15(4):1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai N., Morii N., Fujisawa K., Nemoto Y., Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993 Nov 25;268(33):24535–24538. [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Malcolm K. C., Ross A. H., Qiu R. G., Symons M., Exton J. H. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994 Oct 21;269(42):25951–25954. [PubMed] [Google Scholar]
- Michiels F., Habets G. G., Stam J. C., van der Kammen R. A., Collard J. G. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995 May 25;375(6529):338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F. X., Abo A., Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995 Jun 30;81(7):1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995 Apr;7(2):203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Qiu R. G., de Vries-Smits A. M., Tertoolen L. G., de Laat S. W., McCormick F., Hall A., Symons M. H., Bos J. L. Rac mediates growth factor-induced arachidonic acid release. Cell. 1995 Jun 16;81(6):849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Perona R., Esteve P., Jiménez B., Ballestero R. P., Ramón y Cajal S., Lacal J. C. Tumorigenic activity of rho genes from Aplysia californica. Oncogene. 1993 May;8(5):1285–1292. [PubMed] [Google Scholar]
- Prasad G. L., Fuldner R. A., Cooper H. L. Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk G. J., Bos J. L. The role of p21ras in receptor tyrosine kinase signalling. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):131–147. doi: 10.1016/0304-419x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Qiu R. G., Chen J., Kirn D., McCormick F., Symons M. An essential role for Rac in Ras transformation. Nature. 1995 Mar 30;374(6521):457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Sabanay I., Zöller M., Ben-Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992 Oct;119(2):427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self A. J., Paterson H. F., Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993 Mar;8(3):655–661. [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Zhang J., King W. G., Dillon S., Hall A., Feig L., Rittenhouse S. E. Activation of platelet phosphatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993 Oct 25;268(30):22251–22254. [PubMed] [Google Scholar]
- Zheng Y., Bender A., Cerione R. A. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J Biol Chem. 1995 Jan 13;270(2):626–630. doi: 10.1074/jbc.270.2.626. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Olson M. F., Hall A., Cerione R. A., Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J Biol Chem. 1995 Apr 21;270(16):9031–9034. doi: 10.1074/jbc.270.16.9031. [DOI] [PubMed] [Google Scholar]








