Abstract
Tobacco use is the major preventable cause of premature death in the United States. Secondhand smoke (SHS) exposure also contributes to a number of premature deaths as well as other negative health outcomes. An accurate assessment of tobacco smoke exposure is critical to understanding these disease processes. The plasma concentration of cotinine, the primary metabolite of nicotine, is widely accepted as a quantitative measure of tobacco and SHS exposure. However, it is not always feasible to collect plasma. Dried blood spots (DBS), which are collected routinely from newborns and often from young children for lead screening, provide an alternative sampling method. We have developed a quantitative high throughput liquid chromatography tandem mass spectrometry (LC/MS/MS) method for the analysis of cotinine in DBS. The limit of quantitation was 0.3 ng/g (~ 0.2 ng/ml plasma). Cotinine levels in DBS from 83 smokers and 99 nonsmokers exposed to SHS were determined. Plasma cotinine concentrations in these subjects ranged from <0.02 to 443 ng/ml. Cotinine was detected in DBS from 157 subjects, and the correlation between cotinine in plasma and DBS was excellent, 0.992 (p<0.001). We also determined the ratio of trans-3'-hydroxycotinine to cotinine, a measure of nicotine metabolism, in DBS from smokers. This ratio in DBS was well correlated with the ratio in plasma, 0.94 (p<0.001). In a small study we confirmed the feasibility of using extant DBS collected for lead screening to assess SHS exposure in children.
Keywords: Second hand smoke exposure, lead, smoking
Introduction
Tobacco use is the major preventable cause of premature death in the United States, accounting for more than 400,000 annual deaths from cancer, cardiovascular and respiratory disease.1,2 Secondhand smoke (SHS) exposure in nonsmokers increases the risk of lung cancer, cardiovascular disease, and has a number of other negative health outcomes. Health effects in children include low birth weight, asthma, increased ear and respiratory infections and sudden infant death.3 An accurate assessment of tobacco smoke exposure is critical to understanding these disease processes in tobacco users and individuals exposed to SHS.
Plasma cotinine is widely accepted as a quantitative measure of tobacco use and SHS exposure.4,5 The P450 2A6-catalyzed conversion of nicotine to cotinine is the primary pathway of nicotine metabolism.6 Cotinine has a half-life of 15–20 h compared to nicotine with a half life of 0.5 to 3 h, therefore cotinine is preferred over nicotine as a marker of tobacco exposure 4–6. Cotinine is further metabolized by P450 2A6 to trans 3'-hydroxycotinine (3'-HCOT). P450 2A6 activity varies across people and the ratio of 3'-HCOT to cotinine has been used as a measure of this variability. Cotinine has been quantified in a number of biological matrices, including urine, saliva, hair and toenails,4 and the 3'-HCOT/cotinine ratio has been routinely measured in plasma and urine and to a lesser extent in saliva.
Recently, dried blood spots (DBS), used for decades to screen for metabolic disorders in newborns, have become a popular sampling method for the quantitation of small molecules in blood.7,8 Phenylalanine was one of the first molecules analyzed in DBS, and its routine quantitation has allowed the analysis of phenylketonuria in millions of newborns.9 The recent progress quantifying biomarkers using DBS is in part driven by advances in liquid chromatography tandem mass spectrometry (LC-MS/MS). With the advent of reliable LC/MS/MS methods a single spot can be used to screen for more than 430 metabolic disorders.7,10 The analysis of small molecules in DBS has also been extended to therapeutic drug screening, pharmacokinetic and toxicological studies.8 DBS are routinely used to screen young children for lead exposure.8,11
Biospecimen collection using DBS has some notable advantages. The finger-stick procedures are technically simple and do not require extensive training or expensive equipment. The procedure is appropriate for large population-based studies, especially involving small children, since only a small volume of blood is required. Transporting specimens is relatively non-hazardous. Methods to accurately measure cotinine in DBS could extend the capacity to measure tobacco exposure to populations that have been difficult to study.
Cotinine levels in DBS have been quantified in two small studies.12,13 One study measured cotinine in DBS of newborns from 20 mothers who reported smoking during pregnancy. In that study the analysis was by GC/MS/MS and the level of cotinine detected in DBS from newborns of non-smoking mothers was surprisingly high. The second, a feasibility study, quantified cotinine in DBS prepared from collected whole blood by LC APCI MS/MS analysis and concluded that quantifying cotinine in DBS would only be possible when high levels of SHS exposure occurred. The objective of our study was to develop a sensitive high throughput method to quantify cotinine in DBS from smokers and non-smokers exposed to both high and low levels of tobacco smoke. The method we developed used electrospray LC-MS/MS, which we and others have used for the analysis of plasma cotinine levels 14–16. In anticipation of using DBS in future clinical and public health applications, we assessed the accuracy of DBS cotinine as a biomarker of tobacco smoke exposure by comparing it to plasma cotinine (considered a gold standard4). In addition, to extend studies of tobacco exposure to the characterization of nicotine metabolism the ratio of 3'-HCOT to cotinine was quantified in DBS collected from smokers.
Materials and Methods
Subject Recruitment and Sample Collection
For Study 1, the comparison of cotinine and 3'-HCOT in DBS and in plasma, participants were recruited from the Minneapolis-St. Paul, MN metropolitan area through advertisements. One cohort included current smokers (defined as smoking 1–12 cigarettes per day) and another included nonsmokers who reported exposure to SHS. SHS exposure was confirmed by a plasma cotinine level > 0.02 ng/ml. Eligibility criteria for both cohorts were age 18–80 years and English-speaking. Exclusion criteria were weight of less than 110 lbs. We recruited 83 smokers and 99 individuals with SHS exposure.
Participants were screened on the telephone and invited to a single visit. A brief current smoking history and SHS exposure questionnaire was administered, then a single blood sample (7 ml) and 4 DBS (12 mm diameter); two spots per blood spot sample card (purchased from MEDTOX Laboratories, St. Paul, MN) were collected. Medtox cards use Ahlstrom 226 filter paper, which is comparable to Whatman 906 and its performance for the analysis DBS is considered essentially equivalent 17. The protocol for blood spot collection was as described by MEDTOX.18 The DBS samples were stored at −20 °C until analysis. Storage was at −20 °C to allow future analysis of potentially less stable analytes. A subset of samples were stored at room temperature.
Study 2 was the evaluation of SHS exposure using DBS submitted for lead analysis. MEDTOX Laboratories, provided 283 de-identified DBS collected from children ≥ 4 to 10 year of age after lead analysis was complete. DBS were collected in November and December 2010, and January, February, June, and July of 2011. Children with lead levels greater than 9 µg/dL, the level established by the Center for Disease Control (CDC) as a concentration of concern in children, were excluded. MEDTOX supplied an anonymous administrative data set to accompany the DBS that included month of birth, race, sex, month of spot collection, Medicaid eligibility status, and lead level. Only 15 samples had lead levels ≥ 5 µg/dL.
Both studies were approved by the University of Minnesota Institutional Review Board.
Sample preparation and analysis
Methodology to elute cotinine and 3'-HCOT from DBS and prepare samples for LC/MS/MS analysis was developed in a 96 well plate format. DBS cotinine and 3'-HCOT levels were determined in punches from two DBS, collected from subjects at the same time (study 1). DBS cotinine and 3'-HCOT values were compared to plasma values from the same subjects (study 1). Negative controls were analyzed in all plates, and included DBS from non-tobacco exposed control subjects and blank paper punches (details are provided below). To evaluate the stability of cotinine in DBS, the analysis of punches obtained at two different times from the same DBS was carried out. The time between analyses was from 11 to 26 months, during which time DBS were stored at −20 °C in a plastic sleeve in a storage box containing desiccant. A second group of samples were analyzed from 9–11 months after storage at room temperature, in plastic sleeves in a binder.
The analysis was as follows. DBS were brought to room temperature and 1 to 3 punches (3.2 mm or 4.8 mm) were obtained, weighed and transferred to a 96-well collection plate. Filtered water (400 µl) and 50 µl of an internal standard solution containing 0.13 ng [d3-methyl]-cotinine (Toronto Research Chemicals, Toronto, Ontario, Canada) and 0.54 ng d3-methyl-3'-HCOT (Toronto Research Chemicals), were added to each well using a Quadra 4 solid phase extraction (SPE) liquid handling workstation (Tomtec, Hamden, Connecticut). The plate was shaken overnight at room temperature. The next day, samples were loaded on an Oasis MCX 96-well plate (2 mg sorbent material, particle diameter 30µm and 1ml cartridge volume, Waters Corporation, Milford, Massachusetts), that had been activated with 200µl methanol followed by 200 µl 0.5% formic acid (by volume). The columns were washed with 200 µl 0.5% formic acid and 200 µl methanol, then cotinine and 3'-HCOT were eluted with 60:40 acetonitrile: methanol (v/v) + 2% ammonium hydroxide into tapered 96-well plates. Solvent was removed under a gentle stream of nitrogen, then the residue resuspended in 16µl of 20 mM ammonium acetate (pH,6.7) and stored at −20 °C prior to LC/MS/MS analysis. All SPE steps and nitrogen drying were performed on Quadra. Each 96 well plate contained 2 positive control samples, initially a plasma sample analyzed previously15 then a single 4.8 mm punch from DBS from the same individual. The last row on the plate (H) contained negative controls: 3 wells contained blank paper punches from blood cards, 6 wells contained no sample (water blank) and 3 wells contained punches from DBS (collected from non-smokers with plasma cotinine levels < 0.02 ng/ml). The last column (12) contained additional negative controls, which were punches from blank paper adjacent to the DBS being analyzed on the plate. A stock solution containing cotinine and 3'-HCOT was prepared in water and stored at −20° C. Standards were prepared by applying a range of concentrations (0.2 to 102 ng/g) to blood spot sample cards, drying the cards and collecting a DBS from nonsmokers. Standards were also prepared by adding cotinine and/or 3'-HCOT to the water in which blank or non-smoker DBS were shaken. The recovery was the same.
Linearity of the quantitation of cotinine in DBS was determined over a range of concentrations (0.3 to 102 ng/g, n=16). The correlation coefficient for the expected to actual concentrations was 0.989. The intraday accuracy and precision were determined by analyzing replicates (n=4) of 12 concentrations (0.6 to 60 ng/g) and calculating the mean value, the standard deviation and the rel ative standard deviation (RSD). The percent accuracy (found concentration/added X 100%) ranged from 93 to 107%, (mean ± SD, 99 ± 4.9%). The mean RSD was 7.6% (ranging from 3 to 11%).
Plasma samples were analyzed for cotinine and 3'-HCOT using essentially the same 96 well plate format described above for DBS. Briefly, plasma (50 – 200 µl) was added to each well and then 400µl filtered water and 50 µL internal standard was added, the plate was shaken for 5 min, then immediately worked up as for DBS. Each 96 well plate contained 2 positive control plasma samples and water blanks in row H. Plasma samples were analyzed in triplicate. The limit of quantitation (LOQ) with 200 µL of plasma was 0.02 ng/ml for cotinine and 0.05 ng/g for 3'-HCOT.14 A relatively large volume of plasma was used to confirm little or no tobacco exposure in non-smokers when cotinine was not detected in DBS from these individuals despite their report of SHS exposure.
LC/MS/MS analysis was on an Eksigent nanoLC-Ultra and AS-2 autosampler (Eksigent, Dublin, California) interfaced to a Thermo TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific, Waltham, Massachusetts). The samples (8µl) were injected onto 150 × 0.50 mm Luna C18 (3 µm particle size), silica-based, reverse phase capillary column (Phenomenex, Torrance, California). Cotinine and 3'-HCOT were eluted isocratically with 85% 20 mM ammonium acetate: 15% acetonitrile and analyzed by selected reaction monitoring (SRM). The ESI source was operated in the positive ion mode with a collision energy of 25V for cotinine and 20V for 3'-HCOT, the s-lens set to 90V, the collision gas pressure set to 1.5mTorr and the declustering voltage set to 12V. Peak area obtained from SRM of the mass transitions for the analytes were used for quantification. The scan width was 0.30 (m/z) and the scan time 0.1s and the peak widths for both Q1 and Q3 were 0.70. The mass transitions were as follows: d0-cotinine (m/z 177 → 80 and m/z 177 → 98), d3-cotinine (m/z 180 → 80 and m/z 180 → 101), 3'-HCOT (m/z 193 → 80 and m/z 193 → 134), d3- 3'-HCOT (m/z 196 → 80 and m/z 196 → 134).
The ratio of d0:d3 cotinine and d0:d3 3'-HCOT were calculated using peak areas from the above transitions. For each plate, the mean d0:d3 ratios were calculated for the negative control samples (described above). This mean ratio was used as a measure of background peak areas due to cotinine contamination or co-eluting peaks. For 3'-HCOT analysis there was no significant background peak. However, the average d0:d3 cotinine ratios of the negative controls on 10 plates ranged from 0.007 to 0.02, which would correspond to a cotinine level of 0.9 to 2.6 pg per sample. The average negative control ratio on a plate (n= 19) was subtracted from the ratios calculated for each sample. Corrected ratios less than 1.5 times the average negative control ratio for a plate were reported as not detected. The LOQ for three 4.6mm punches was from 0.2 to 0.3 ng cotinine/g DBS. LOQ for 3'-HCOT was 0.2 ng/g.
Statistical analysis
Study one assesses the reliability and validity of cotinine and the 3'-HCOT/cotinine ratio measured from DBS. The intraclass correlation evaluated repeatability and reproducibility from two independent replications of the DBS assay and from a subset of samples where two cotinine values were measured 11 to 26 months apart. To determine validity, the DBS cotinine and 3'-HCOT/cotinine ratio were compared to the same values obtained from the plasma (the gold standard) using the Spearman correlation coefficient. These two cotinine parameters had an extremely skewed distribution with some very high values, thus a nonparametric correlation was used based on rank order. Study 2 DBS cotinine levels were summarized by subject characteristics using the median and range. These subject variables included age in months, sex, race, Medicaid status, collection month and lead level. Nonparametric methods were used for the univariate analysis of these variables on cotinine levels. Statistical procedures included the Wilcoxon rank sum test and the Spearman correlation coefficient. All statistical analyses were performed using SAS, version 9.3 (SAS institute Inc., USA). P-values less than 0.05 were considered statistically significant.
Results
Characterization of cotinine and 3'-HCOT in DBS by LC/MS/MS
To quantify cotinine in DBS, multiple punches per sample were used and cotinine was expressed as ng per g DBS. The average weights of punches of the filter paper and the DBS are listed in Table 1. For samples from smokers with plasma cotinine concentrations >10 ng/ml, 3.2 mm punches were used and for lower concentrations, typically SHS exposed subjects, 4.8 mm punches were used. The use of a 4.8 mm punch allowed three punches per spot and maximized the amount of sample analyzed. By weight the amount of blood in three 4.8mm punches was about 1.5 times that in a 6.4 mm punch, the size typically used in the analysis of small molecules in DBS.
Table 1.
Average weight of DBS and blank blood card.
| Punch | Paper | DBS |
|---|---|---|
| (grams) | ||
| One 6.4 mm | 0.0057 ± 0.0002 (n=29) | 0.0086 ± 0.0003 (n=29) |
| One 3.2 mm | 0.0016 ± 0.0001 (n=95) | 0.0023 ± 0.0001 (n=144) |
| Three 3.2 mm | 0.0044 ± 0.0001 (n=206) | 0.0065 ± 0.0001 (n=445) |
| Three 4.8 mm* | 0.0097 ± 0.0002 (n=226) | 0.0137 ± 0.0003 (n=1895) |
The majority of the cotinine data in these samples was quantified in a separate study32
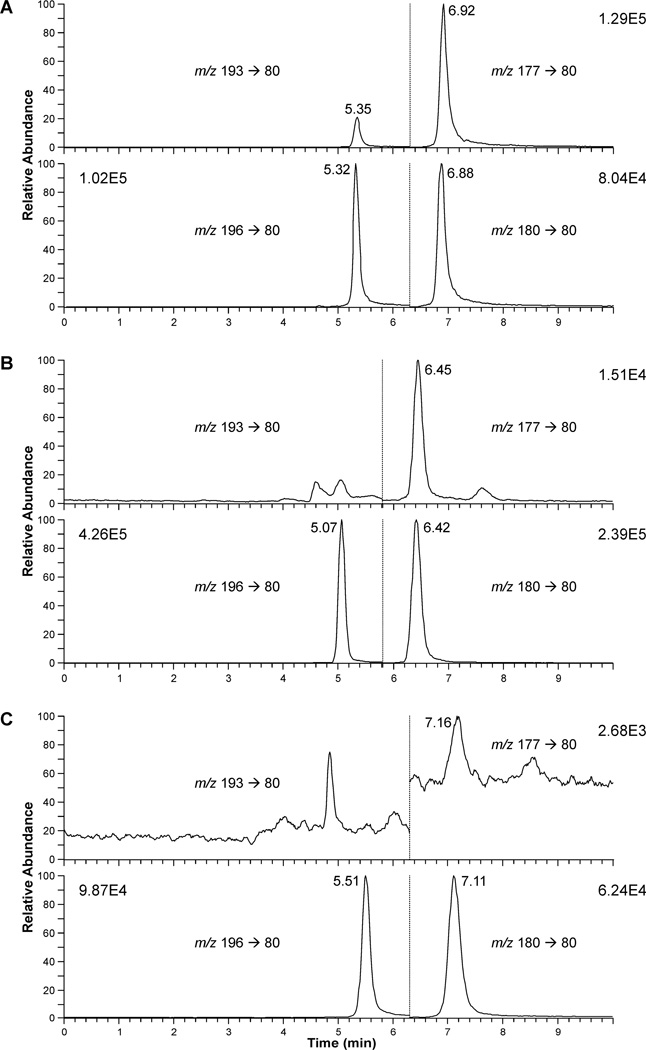
LC/MS/MS chromatograms for DBS from an active smoker (A, plasma cotinine 69 ng/ml) and an individual who was exposed to SHS (B) are presented in Figure 1. Both cotinine (6.92 min) and 3'-HCOT (5.35 min) were detected. The cotinine peak detected in the DBS from the SHS exposed individual (6.45 min) was more than 100 times the signal to noise. 3'-HCOT, which elutes 0.03 min past the d3-3'-HCOT (5.07 min) was not detected in this DBS sample with a cotinine level of 0.57 ng/g. In analyzing samples in the 96 well plate format trace amounts of cotinine and a closely eluting peak (7.16 min), were detected in the negative control samples, blank paper and DBS from individuals with no detected SHS exposure (Figure 1C, plasma cotinine < 0.02 ng/ml). The LOQ for the analysis of cotinine in DBS was set at 2.5 times the mean cotinine value determined in the negative control samples analyzed in each plate. The average of the mean negative control value for ten 96 well plates was 1.3 ± 0.8 pg per sample, resulting in an average LOQ for SHS exposed subjects (with three 4.8 mm punches) of 0.24 ng/g DBS.
Figure 1.
LC/MS/MS analysis of DBS from (A) a smoker, (B) a SHS exposed individuals, and (C) a non-smoker (plasma cotinine <0.02ng/ml). The SRM data from time 0 to the vertical dotted line is for d0 and d3-3'-HCOT and past the line is for d0 and d3- cotinine. The retention times varied based on the age/condition of the column, cotinine and 3'-HCOT elute 0.03min prior to the respective deuterated standard. In panel C the peak at 7.16, overlaps with, but does not co-elute with cotinine.
Correlation of cotinine and the 3'-HCOT:cotinine ratio in DBS with the values in plasma
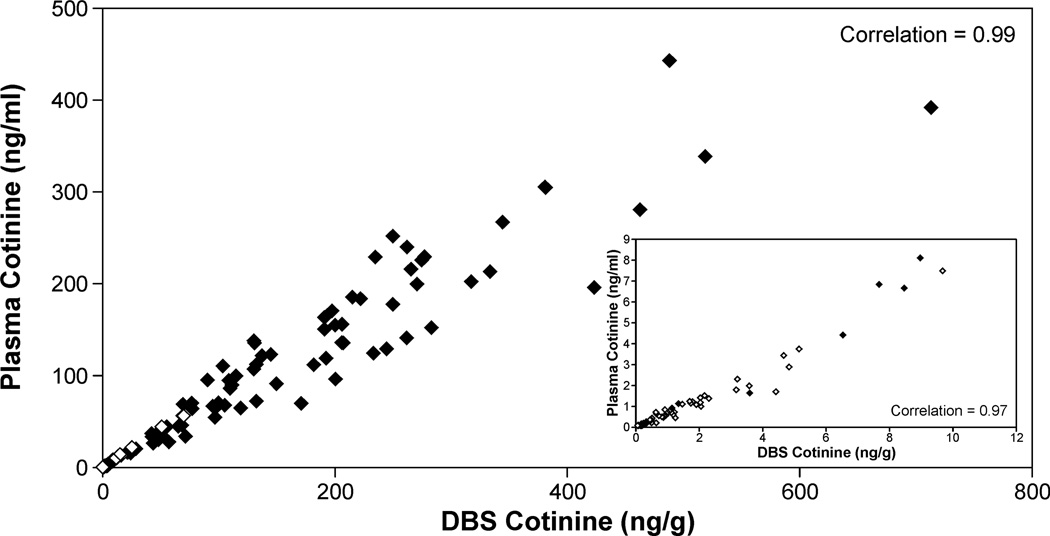
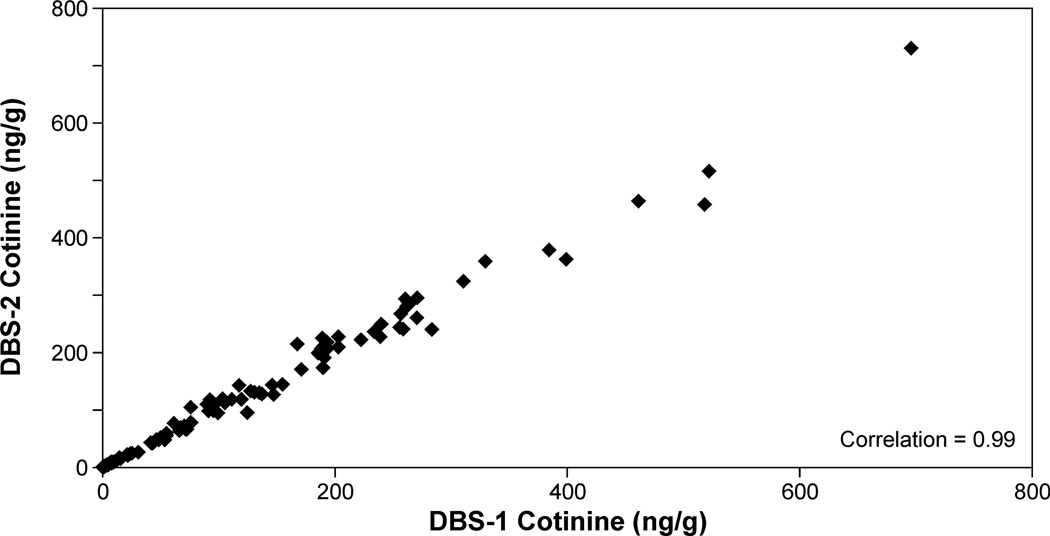
Cotinine levels were quantified in DBS collected from 83 smokers and 99 nonsmokers exposed to SHS. The nonsmokers reported living with someone who smoked and being exposed to SHS in their homes within the last 24 h. Smokers' plasma cotinine values ranged from 443 to 0.9 ng/ml; the range in individuals reporting SHS exposure was from 56 to <0.02 ng/ml. Detectable levels of cotinine in DBS were quantified in all samples with plasma cotinine concentrations greater than 0.08 ng/ml (n=157). The Spearman correlation coefficient for cotinine values in plasma and in DBS for these samples was 0.992 (p<0.001, Figure 2). The correlation coefficient in subjects with plasma cotinine concentrations < 10 ng/ml (4 smokers and 72 SHS exposed individuals) was also high, 0.973 (p< 0.001, Fig 2 insert). The mean ratio (± S.D) of cotinine in DBS (ng/g) to cotinine in plasma (ng/ml) was 1.49 ± 0.41. Replicate analyses were carried out on 139 samples with plasma cotinine concentrations from 443 to 0.08 ng/ml. The intraclass correlation between the two independent determinations was 0.995 (p< 0.001, Figure 3). In two subsets of samples, one stored at −20 °C and one stored at room temperature a second independent analysis of DBS cotinine was carried. For the samples stored at −20 °C the two analyses were carried out 11 to 26 months apart. The intraclass correlation between the two values obtained was 0.992 (p<0.001) and was not depend on the time of storage. The samples stored at room temperature were analyzed 10 months apart and the intraclass correlation was 0.986 (p <0.001). Ten samples used as positive controls (cotinine, 252 to 0.37 ng/g DBS) were analyzed independently 6 to 15 times each, the mean coefficient of variation for these samples was 0.13 ± 0.048.
Figure 2.
Correlation between DBS cotinine and plasma cotinine (♦ smokers, ◊ self reported non-smokers exposed to SHS). The insert is for plasma cotinine values <10ng/ml.
Figure 3.
Correlation between replicate analyses of punches from two DBS from the same subjects
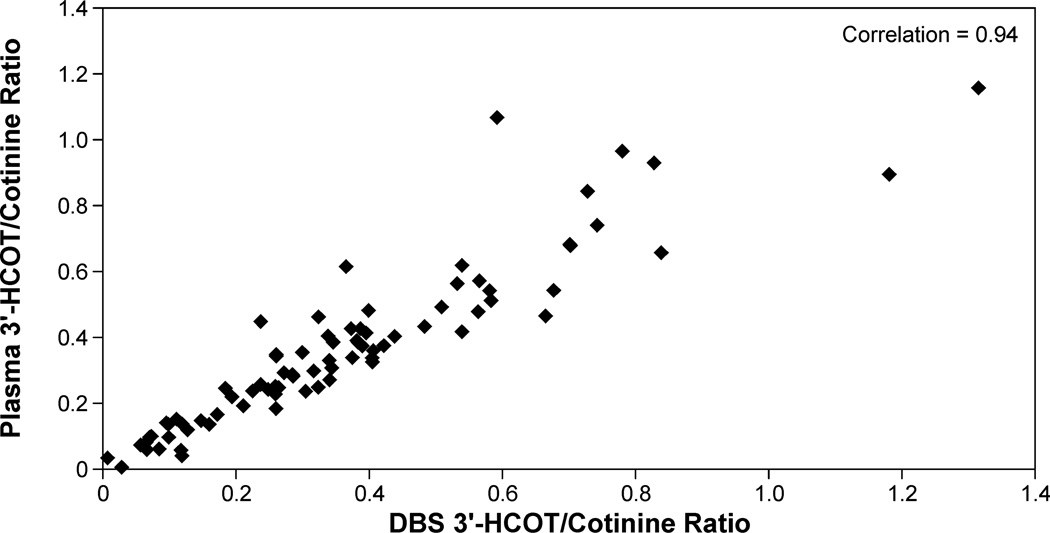
3'-HCOT levels in DBS and plasma were analyzed for all samples with mean plasma cotinine values ≥ 10ng/ml (n=81). 3'-HCOT in plasma ranged from 183 to 0.2 ng/ml and in DBS from 242 to 0.4 ng/g. The mean (± SD) 3'-HCOT to cotinine ratio in plasma was 0.36 ± 0.25 (median, 0.336; range 0.005 to 1.16), in DBS the ratio was 0.36 ± 0.25 (median, 0.324, range 0.008 to 1.3). The Spearman correlation coefficient for plasma 3'-HCOT/cotinine and DBS 3'-HCOT/Cotinine was 0.94 (p <0.001, Figure 4).
Figure 4.
Correlation between DBS 3'-HCOT/Cotinine ratio and plasma 3'-HCOT/Cotinine ratio in subjects with cotinine values ≥ 10ng/ml.
Study 2 DBS cotinine in children screened for lead exposure
To test the feasibility of analyzing cotinine in extant DBS that had been analyzed for lead exposure, we obtained de-identified samples collected from children age 4 to 10 years. Cotinine was detected in 166 of 283 samples (58.7%). The characteristics of the subjects are presented in Table 2. Cotinine level ranged from not detected (< 0.3 ng/g) to 55 ng/g (~37 ng/ml plasma based on the plasma to DBS relationship established in Study 1). There was no difference in the median DBS cotinine level by collection month, race or Medicaid status. There was a weak but significant correlation of cotinine level with age. Cotinine levels were higher in female subjects (p= 0.004) and in subjects with lead levels ≥2 µg/dL. Cotinine levels in DBS correlated with lead levels (r = 0.36, p< 0.001).
Table 2.
Analysis of Cotinine by subject characteristics.
| Cotinine | N | Median (range) (ng/g) |
P-value |
|---|---|---|---|
| Total | 283 | 0.42 (<0.3 – 55) | |
| Race | |||
| Black | 175 | 0.50 (<0.3 – 55) | 0.255† |
| White | 108 | <0.3 (<0.3 – 16) | |
| Age | |||
| ≥4 yrs up to 6 yrs | 160 | 0.54 (<0.3 – 55) | Correlation= −0.14 p=0.016†† |
| ≥6 yrs up to 8 yrs | 105 | 0.30 (<0.3 – 8.5) | |
| ≥8 yrs up to 10 yrs | 18 | <0.3 (<0.3 – 2.1) | |
| Sex | |||
| Female* | 140 | 0.61 (<0.3 – 55) | 0.004† |
| Male | 133 | <0.3 (<0.3 – 15) | |
| Collection month | |||
| June, July | 99 | 0.46 (<0.3 – 55) | 0.554† |
| Nov, Dec, Jan, Feb | 184 | 0.41 (<0.3 – 20) | |
| Medicaid | |||
| No | 160 | 0.30 (<0.3 – 18) | 0.096† |
| Yes | 123 | 0.64 (<0.3 – 55) | |
| Lead Level | |||
| < 2 | 168 | <0.3 (<0.3 – 20) | p<0.001† |
| ≥ 2 | 115 | 1.05 (<0.3 – 55) |
10 subjects were missing gender
The p-value is based on the nonparametric Wilcoxon rank sum test
The p-value is derived from the non-parametric Spearman correlation.
Discussion
An excellent correlation between plasma cotinine concentrations and cotinine levels in DBS was documented in both smokers and individuals exposed to SHS. The correlation between independent analyses from DBS collected at the same time was also excellent. Together these data confirm the accuracy of DBS cotinine as a biomarker of tobacco smoke exposure.
Sampling DBS is typically carried out using 6.4 mm or less frequently 3.2 mm punches. The volume of blood in a 6.4 mm punch on average has been determined to be 12 µL19 and this volume is used to express a concentration of analytes in DBS. However, the volume of blood contained in each punch may vary with hemocrit due to difference in viscosity of the blood, and the flow of blood to a particular region of the paper.7,20 To minimize the effect of variability in blood per punch on the quantitation of cotinine we used multiple punches per sample and expressed cotinine as ng per g DBS. This likely contributed to the reproducibility of our cotinine DBS quantitation and its close correlation with plasma cotinine concentration.
DBS are collected routinely from newborns and are often the blood collection method used to screen young children for elevated lead exposure. Lead screening of children at 1 and 2 years of age is recommended by the CDC and the American Academy of Pediatrics and has become a routine part of health care providers practice.21–23 The youngest age of children in the National Health and Nutrition Examination Survey (NHANES) for whom plasma cotinine has been measured is 3 years old.24 Therefore, the availability of DBS from infants and toddlers and the method to quantify cotinine in these samples presents an exciting opportunity to study SHS exposure in children not routinely surveyed.
A few studies have used DBS from infants to assess environmental exposures. For example, the level of perflourinated compounds in DBS from newborns was shown to decline after the year 2000, when the manufacture of perfluorooctane sulfonate related compounds was being phased out.25 The analysis was carried out by LC/MS/MS on pooled 6.4 mm DBS punches. More recently, Spector and co-workers12 reported that DBS cotinine from infants of smoking women (n=20) were significantly higher than the levels detected when the mother was a self-identified non-smoker (n=10). Quantification of cotinine in this study was by GC/MS and had a relatively high LOQ.
To our knowledge DBS collected for lead screening have not been analyzed for environmental exposures other than trace elements.26 In a small study we have confirmed the feasibility of using these extant DBS to assess SHS exposure in children. Cotinine was detected in more than 50% of the samples analyzed and the level in children with lead levels ≥ 2µg/dL was significantly higher than the level in those with lead levels < 2µg/dL. The percent of children aged 3 to 11 years, with detectable cotinine in the NHANES was 54%, comparable to what we report here. An association between cotinine and blood lead levels in children in the NHANES has been reported.27,28 However, a recent study carried out with children at a primary care clinic in San Francisco, CA found no relationship between cotinine and lead levels.29
In anticipation of collecting and using DBS in studies of tobacco related disease, not only to measure tobacco exposure, but to characterize nicotine metabolism and P450 2A6 activity we have quantified the 3'-HCOT to cotinine ratio in DBS from smokers. As was true for DBS cotinine, the correlation of the 3'-HCOT cotinine ratio in DBS with the ratio in plasma was excellent. In addition, the mean and median ratios obtained in the two matrices were almost identical and the range of values comparable to those reported previously.30,31 The use of DBS in large epidemiology studies would be a huge benefit since both the collection and storage of DBS is significantly easier than for plasma (or saliva) samples.
We have characterized DBS cotinine and the DBS 3'-HCOT:cotinine ratio as reliable biomarkers of tobacco exposure and nicotine metabolism, respectively. Both biomarkers accurately reflect the levels of these measures in plasma. Recently, we have completed two studies that use DBS cotinine as a biomarker of tobacco exposure. Both studies use extant DBS, one uses samples from children ≤ 4 years of age collected for lead screening32 and the other uses samples from newborns. In addition to future studies in these two populations, DBS cotinine can be used as a convenient biomarker of tobacco exposure in many types of clinical and epidemiology studies.
Acknowledgements
This work was supported by NHLBI 1RC2HL10140. LC/MS/MS analysis was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center supported in part by CA-77598.
References
- 1.CDC. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses --- United States, 2000--2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Washington, D.C.: U.S. Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services. Children and Secondhand Smoke Exposure. Excerpts from The Health Consequences of Involuntary Exposure to Tobacco Smoke. A Report of the Surgeon General. Washington, D.C.: U.S. Department of Health and Human Services; 2007. [Google Scholar]
- 4.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 6.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Tse FL. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 8.Stove CP, Ingels AS, De Kesel PM, Lambert WE. Dried blood spots in toxicology: from the cradle to the grave? Crit Rev Toxicol. 2012;42:230–243. doi: 10.3109/10408444.2011.650790. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketouria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 10.Wilcken B, Wiley V. Newborn screening. Pathology. 2008;40:104–115. doi: 10.1080/00313020701813743. [DOI] [PubMed] [Google Scholar]
- 11.Stanton NV, Maney JM, Jones R. Evaluation of filter paper blood lead methods: results of a pilot proficiency testing program. Clin Chem. 1999;45:2229–2235. [PubMed] [Google Scholar]
- 12.Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16:1902–1905. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 13.Sosnoff CS, Bernert JT. Analysis of cotinine in dried blood spots by LC APCI tandem mass spectrometry. Clin Chim Acta. 2008;388:228–229. doi: 10.1016/j.cca.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3',3'-d(2)]-nicotine and [3',3'-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:1–8. doi: 10.1016/j.jchromb.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom J, Hinrichs AL, Wang JC, von Weymarn LB, Bierut LJ, Goate A, et al. The Contribution of Common CYP2A6 Alleles to Variation in Nicotine Metabolism Among European Americans. Pharmacogenet Genomics. 2011;21:403–416. doi: 10.1097/FPC.0b013e328346e8c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3'-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newborn Screening Quality Assurance Program: Filter Paper Comparison Study. 2009 http://www.cdc.gov/labstandards/pdf/nsqap/nsqap_FilterPaperStudy51809.pdf.
- 18.Collection of Fingerstick Whole Blood on Filter Paper for Blood Lead Determination. 2012 http://www.medtox.com/Resources/Images/2763.pdf.
- 19.Clinical and Laboratory Standards Institute (CLSI) Blood collection on filter paper for newborn screening programs; Approved standard fifth edition. CLSI document LA4-A5. Wayne, Pennsvlvania: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 20.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics Committee on Environmental Health. Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116:1036–1046. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- 22.Recommendations for blood lead screening of young children enrolled in medicaid: targeting a group at high risk. MMWR Recomm Rep. 2000;49:1–13. [PubMed] [Google Scholar]
- 23.Wengrovitz AM, Brown MJ. Recommendations for blood lead screening of Medicaid-eligible children aged 1–5 years: an updated approach to targeting a group at high risk. MMWR Recomm Rep. 2009;58:1–11. [PubMed] [Google Scholar]
- 24.United States Environmental Protection Agency. EPA Report on the Environment: Blood Cotinine Level. 2010 http://cfpub.epa.gov/eroe/index.cfm?fuseaction=detail.viewInd&lv=list.listByAlpha&r=223968&subtop=208.
- 25.Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K, et al. Use of newborn screening program blood spots for exposure assessment: declining levels of perluorinated compounds in New York State infants. Environ Sci Technol. 2008;42:5361–5367. doi: 10.1021/es8006244. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhuri SN, Butala SJ, Ball RW, Braniff CT. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J Expo Sci Environ Epidemiol. 2009;19:298–316. doi: 10.1038/jes.2008.19. [DOI] [PubMed] [Google Scholar]
- 27.Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology. 2003;14:719–727. doi: 10.1097/01.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- 28.Apostolou A, Garcia-Esquinas E, Fadrowski JJ, McLain P, Weaver VM, Navas-Acien A. Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health. 2012;102:714–722. doi: 10.2105/AJPH.2011.300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dempsey DA, Meyers MJ, Oh SS, Nguyen EA, Fuentes-Afflick E, Wu AH, et al. Determination of Tobacco Smoke Exposure by Plasma Cotinine Levels in Infants and Children Attending Urban Public Hospital Clinics. Arch Pediatr Adolesc Med. 2012 doi: 10.1001/archpediatrics.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph AM, Spector LG, Wickham KM, Janis G, Winickoff JP, Lindgren B, et al. Biomarker evidence of tobacco smoke exposure in children participating in lead screening. Am J Public Health. 2012 doi: 10.2105/AJPH.2013.301315. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]