Abstract
The molecular changes that occur with cervical remodelling during pregnancy are not completely understood. This paper reviews Raman spectroscopy, an optical technique for detecting changes in the pregnant cervix, and reports preliminary studies on cervical remodelling in mice that suggest that the technique provides advantages over other methods. Conclusion: Raman spectroscopy is sensitive to biochemical changes in the pregnant cervix and has high potential as a tool for detecting premature cervical remodelling in pregnant women.
Keywords: cervix, pregnancy, parturition, preterm birth, cervical remodelling
Preterm birth (PTB), defined as delivery before 37 weeks of gestation, occurs in approximately 12% of pregnancies in the United States and is the leading cause of infant mortality and (1-3). Temporary and long-term morbidity of the infant can result, costing an estimated $26 billion each year in the US (1). Preterm birth is traditionally classified into two groups: indicated preterm birth and spontaneous preterm birth. The former is induced labour or caesarean section, initiated by the patient's healthcare provider due to complications in the mother or fetus, and the latter occurs spontaneously with over half of the cases having an unknown cause, which is the syndrome focused on in this paper (4). Poor understanding of cervical remodelling in preparation for labour and delivery is a contributory factor to the unacceptably high rates of spontaneous preterm birth over the past 30 years. However, the preterm birth rate has shown small and steady decreases since 2007 in the United States (5-8).
Current clinical practice for assessing preterm birth risk begins by completing a detailed medical history to account for risk factors such as previous preterm birth. Transvaginal ultrasound measurements of the cervix have demonstrated that a short cervix (less than 25mm prior to 24 weeks of gestation) is correlated with an increased risk for preterm birth (9). Patients with a history of preterm birth are recommended to have transvaginal ultrasound measurements every two weeks from 16 to 23 weeks of gestation (10). If a short cervix is identified, cervical cerclage (sewing the cervix closed), or prescribing progesterone may be recommended based on the patient's history and current situation (10, 11). Such interventions based upon cervical length measures have significantly reduced preterm birth and perinatal death rates. These measurements have also demonstrated that cervical shortening occurs weeks to months before subsequent preterm birth, suggesting that the cervix might be a prime source of important early information about preterm birth risk. Clinicians need tools that can quantitatively and objectively obtain biochemical information from the cervix, so that they can assess the risk of premature remodelling to have adequate time to intervene. In vivo Raman spectroscopy has the potential to provide such a tool. Preliminary findings highlighted in this paper demonstrate the ability of this technique to detect biochemical changes in the pregnant mouse cervix over the course of gestation and identify features that signify impending parturition.
Novel methods to evaluate the pregnant cervix
Clinical methods that have been explored as ways to estimate risk for preterm birth include cervical length by ultrasound, cervical Bishop score, fetal fibronectin screening, measuring salivary estriol levels and monitoring uterine contractions (9, 12-15). Of all these methods, cervical length measures have demonstrated the highest accuracy for predicting preterm delivery. However, reports vary considerably across different studies (16). Furthermore, none of these methods provide biochemical information that could be vital in characterising the microstructural and molecular processes that govern cervical remodelling and determine the aetiology of preterm labour.
In addition to exploring Raman spectroscopy as a method of monitoring cervical change, researchers have identified other technology that can evaluate the cervix to accurately predict preterm birth. These methods employ optical, ultrasonic and electrical phenomena to noninvasively interrogate state of the cervical tissue (Table 1). An in-depth review of these methods can be found in Feltovich et al (5).
Table 1.
Developing technologies for analysis of the pregnant cervix
| Technique | Collagen structure? | Tissue hydration? | Tissue elasticity/stiffness? |
|---|---|---|---|
| Acoustic Attenuation | ✓ | ||
| Electrical Impedance | ✓ | ||
| Elastography | ✓ | ||
| Light-induced fluorescence | ✓ | ||
| Second harmonic generation | ✓ | ||
| Near-infrared spectroscopy | ✓ | ||
| Backscattered power loss | ✓ | ||
| Mechanical testing | ✓ | ||
| Raman spectroscopy | ✓ | ✓ | ✓ |
Electrical Methods
Electrical methods have classically been utilised for investigating myometrial contractions, but the assessment of fundamental electrical properties are now being applied to help understand the pregnant cervix. Electrical impedance is a measure of the resistance to electrical flow and is specific to the material being evaluated. This property was measured in the cervix of pregnant women and a correlation was found between the electrical impedance and cervical hydration state (17). While this is a promising method to measure cervical hydration, it is limited in its ability to monitor other biochemical and structural changes occurring in the cervix during pregnancy.
Acoustic Methods
Ultrasound is a well established clinical technique traditionally used to track the progress of fetal development and quantify the progression of pregnancy. Multiple research groups are developing techniques that aim to capture new information using ultrasound. For example, signal attenuation in transvaginal ultrasound images of the cervix has been shown to correlate with cervical hydration level, exploiting the increasing hydration of cervical tissue in preparation for parturition (18). And backscattered power loss, a variation of ultrasound, measures acoustic scatterers, such as collagen, from different steering angles and the detected signal can provide information regarding the alignment of scatterers within the sample. This method has demonstrated that preferential alignment, presumably of collagen, is present in the cervix and that it may be able to detect slight alterations in collagen alignment associated with cervical remodeling (19).
Finally, ultrasound-based elastography probes the mechanical properties of tissue by comparing images acquired before and during deformation to estimate stiffness of the tissue (20). Studies comparing induction patients revealed that those patients who had successful induction had a significantly higher elasticity index, indicating softer tissue, than patients with unsuccessful induction (20). While ultrasound provides valuable structural information about the pregnant cervix, these methods have limited spatial resolution and cannot elucidate the biochemical and molecular dynamics that might provide insight into the parturition process.
Mechanical Methods
Direct mechanical testing of the pregnant cervix has been conducted using two main methods. Researchers have developed an aspiration device that estimates cervical stiffness by measuring the pressure required to displace cervical tissue by a preset amount and results from this work have demonstrated the ability to detect decreasing levels of stiffness over the course of pregnancy (21). Other groups have developed cervical dilators that measure cervical resistance index, which is the force required to dilate the cervix by a total of 8mm (22). Significant differences in cervical resistance index, between non-pregnant patients without abnormal obstetric history and non-pregnant patients with history of spontaneous mid-trimester abortions have been reported.
Some investigators have developed mathematical models to specifically understand the mechanical changes that occur in the in vivo cervix during pregnancy and parturition. These models could potentially use indirect measures of mechanical properties collected from other optical, acoustic, electrical and mechanical methods to model the changes that occur during pregnancy. Such models could allow individual patient parameters to be used to predict patient outcome and assess risk of preterm birth (23, 24). These methods offer important biomechanical information, but cannot be used to monitor molecular changes that may help to determine new information regarding the cervical remodelling process.
Optical Methods
Three primary methods have been explored to study preterm birth using optical techniques. The collascope, a device that excites collagen and measures light-induced fluorescence, can monitor the state of collagen in the cervix during pregnancy (25). Collagen is a natively fluorescent protein, with insoluble collagen having higher fluorescent intensity than soluble collagen. Because collagen solubility increases as the cervix ripens, the intensity of light-induced fluorescence changes with advancing gestation (25-27). This technique has been applied in mice as well as humans and results have shown that fluorescence intensity decreases as parturition approaches (25-27).
In addition to being an intrinsically fluorescent protein, collagen type 1 is capable of second harmonic generation and can produce a strong signal with very little background if excited at the appropriate wavelengths. This technique has been used to study the cervix of pregnant mice in vivo and has been demonstrated in ex vivo human cervical samples. It is capable of producing high resolution images of cervical collagen and the results have verified that the collagen networks in the cervix become increasingly disorganised with advancing gestation (28, 29).
Near-infrared spectroscopy has been used to monitor haemoglobin, water and optical scatterers in the pregnant cervix, by measuring the diffuse reflectance of light at various near-infrared wavelengths. From these measurements, the researchers calculate the optical properties that can be used to estimate concentrations of biological absorbers, such as haemoglobin and water, and determine the relative density of structural interfaces encountered such as cells and collagen (30, 31). Preliminary studies found increasing values for light scattering and haemoglobin over the course of pregnancy and observed an increase in cervical hydration during cervical ripening with misoprostol (30, 31). These methods highlight the strength of optical techniques for capturing both biochemical and structural information from the pregnant cervix.
While each method described offers important pieces of information regarding cervical remodeling, such as tissue hydration, collagen structure or tissue stiffness, none of these methods can provide information on all three components, as displayed in Table 1. Raman spectroscopy applied to the in vivo cervix has the ability to address each of these areas simultaneously.
Biomedical applications using Raman spectroscopy
Raman spectroscopy is an optical technique based on the Raman effect, defined by an exchange of energy between incident photons and scattering molecules. This technique yields sample-specific molecular fingerprints, from which the sample composition and, in some cases structure, can be ascertained. When an incident photon collides with a molecule with a specific vibrational mode, energy may be transferred from the molecule to the photon or vice versa. Energy differences resulting from the changing vibrational modes are found in the scattered photons. Therefore, a Raman spectrum consists of peaks which correspond to the different vibrational modes of scattered molecules (Figure 2a). In general, these peaks are spectrally narrow and correlate with defined molecules. The peaks of a Raman spectrum are usually associated with certain bonds in specific molecules and can be used to determine a sample's biochemical composition.
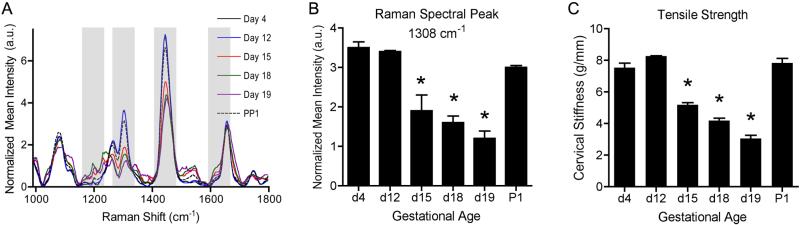
Figure 2.
(A) Raman spectra of the in vivo mouse cervix over the course of gestation (gray regions p<0.1). (B) Change in Raman spectral intensity at 1308 cm-1 (n=5 per time point). (C) Cervical stiffness, measured from stress-strain curves (*p<0.01, n=5 per time point). Raman spectroscopy for this peak and cervical compliance are in strong agreement. Adapted with permission from ref. 51.
Raman spectroscopy has been used for decades to detect disease and cancer-specific signals in various organs and soft tissues such as the cervix, as discussed in the next section, bladder (32, 33), oesophagus (34, 35), skin (36, 37), breast (38) and gastrointestinal tract (39). The spectral changes between normal and cancerous areas are usually due to an increase in cellular nucleic acid content and other molecules in the environment of the tumour, such as glycogen and collagen. Those studies interrogated both in vitro samples and in vivo animal models and human subjects with a wide variety of success.
Raman spectroscopy has been used to measure the composition and mechanical properties of healthy and diseased bone (40). When studying bone, measures such as mineral-to-matrix ratio and collagen quality are used to correlate tissue biochemistry to biomechanical properties. These calculated values are being investigated as sensitive predictors of bone fracture risk for osteoporosis patients. Raman spectroscopy has also proven useful for detecting early signs of tooth decay, due to differences in phosphate peaks within Raman spectra compared to X-rays, and will potentially lead to early intervention, decreased cavity rates and dental expenses (41).
Finally, Raman spectra can be collected and analysed in real-time. As such, Raman spectroscopy is valuable for disease detection in clinic and hospital settings to detect disease. Raman spectroscopy has been used in combination with endoscopy to diagnose abnormal or malignant areas of the gastrointestinal tract with high sensitivity and specificity (42), so that affected areas can be diagnosed and then removed during endoscopy, without the need for additional surgery. Patients undergoing routine cervical cancer screening can be given a diagnosis at the time of their visit, which could allow patients to be treated on the same day as their evaluation (43). Together, these studies demonstrate the potential use of Raman spectroscopy as a see-and-treat optical method for real-time detection of disease and guiding patient care.
Raman spectroscopy for detection of cervical disease
A number of molecules that are important for softening and remodelling the cervix during pregnancy can be distinguished by their individual Raman spectrum. Some of these biochemical components include collagen, water content, glycogen, elastin and even specific amino acids, such as phenylalanine, which can all be detected using Raman spectroscopy (44-47). The sensitivity of Raman spectroscopy has been used previously by many research groups, including ours, to detect changes in the cervix associated with dysplasia, hormonal changes, and infection with different strains of the human papilloma virus (HPV). Many groups have studied the use of Raman spectroscopy to effectively detect cervical dysplasia in both in vitro and in vivo samples (48-50).
We recently demonstrated that Raman spectroscopy can be used to detect changes in cervix biochemistry (Figure 1a) (44, 51). Raman spectroscopy has been used to detect cervical precancerous lesions in vivo, distinguishing between normal, inflammation, low-grade dysplasia and high-grade dysplasia areas with classification accuracy rates of over 97%. Such high classification rates only occurred after normal changes in hormonal levels were taken into consideration, demonstrating that Raman spectra are influenced by hormones, permanent effects of pregnancy and delivery and malignancy-associated changes (43).
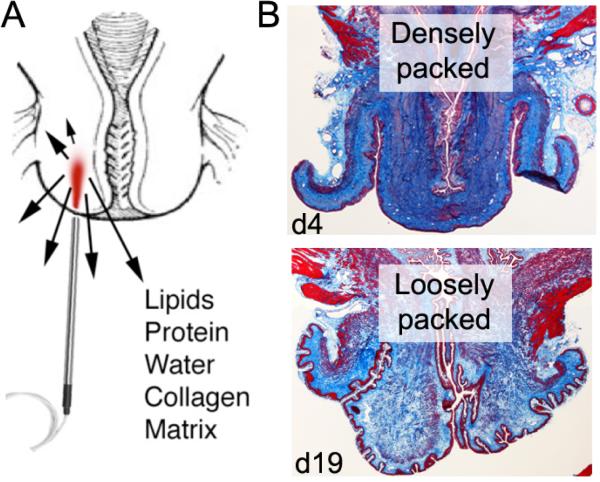
Figure 1.
(A) In vivo Raman spectroscopy of the cervix. (B) Trichrome stains of the pregnant mouse cervix at early (d4) and late gestation (d19); collagen is densely packed at the beginning and reorganizes in preparation for delivery.
Raman spectroscopy has also been used to detect strains of HPV, an infection that is correlated with nearly all cases of cervical cancer (50, 52). Spectral analysis has been performed on cultured cells, such as primary human keratinocytes, CaSki, HeLa, SiHa and C33A cells, as well as ex vivo cervical cells from patients who were screened for HPV infection at Vanderbilt University. Classification accuracies from 89% to 100% for the cultured cells and 98.5% for the patient samples were found using discrimination algorithms, such as principal component analysis and sparse multinomial logistic regression (50, 52).
The success of these studies in the cervix and other biological applications demonstrate that Raman spectroscopy is a sensitive tool that can be used to detect changes in specific biochemical components in tissues and cells. Findings from our lab and other research groups have laid the foundation for the development of Raman spectroscopy as a tool for detecting biochemical changes occurring in the cervix during pregnancy.
Raman spectroscopy as a tool to investigate cervical remodelling during pregnancy
The cervix must transform from a stiff, rigid structure into a soft, distensible passageway for successful delivery of a fetus (53). The mechanisms that lead to this transformation are not fully understood, particularly in patients who experience spontaneous preterm birth. Known biochemical constituents that undergo dramatic changes include an increase in cervical hydration, hydrophilic glycosaminoglycans, collagen solubility, prostaglandin release and inflammatory cells (Figure 1b) (54). These changes, among others, result in reduced tensile strength and increased elasticity of the cervix, which lead to the successful passage of the fetus.
Raman spectroscopy is sensitive to tissue hydration, collagen content and structure, cell density, lipids, proteins and some individual amino acids (44-47). To establish the feasibility of using this method for detecting biochemical changes in the cervix during pregnancy, a pilot study was conducted in mice (55). In vivo Raman spectra were acquired from nongravid and pregnant wild type mice at multiple time points during pregnancy (full term = 19 days), as well as post-partum one day after delivery. Measurements were made using a custom fibre optic probe that was gently inserted into the vagina and pressed against the cervix of anesthetised mice. Figure 2a illustrates the Raman spectra obtained from the in vivo mouse cervix over the 19-day mouse gestational cycle (55). In nongravid mice, subtle spectral changes were found to differ between phases of the oestrous cycle (55).
Biomechanical tests were performed on excised cervical tissues of the same mice at different gestational ages to determine whether any Raman peaks correlated with mechanical properties as a function of gestational age. One important trend observed was the decrease in intensity at 1308 cm-1, a peak indicative of lipids (Figure 2b), that correlates well with decreasing tensile strength and increasing cervical distensibility (Figure 2c) as parturition nears (55). In addition, as the cervix collagen matrix becomes solubilised with advancing gestational age (Figure 1b), the full-width at half maximum value at the 1650 cm-1 peak increases, as shown in Figure 2a (55). These changes, as measured using the Raman spectroscopy, indicate that this technique can indirectly probe the mechanical properties of the in vivo pregnant cervix.
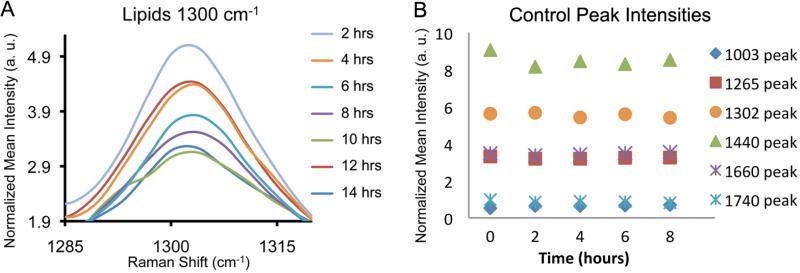
The late stages of cervical remodelling were characterised by acquisition of the Raman spectra from term gestation mice on day 19, starting approximately 12 hours before the time of expected delivery and then every two hours until delivery. As delivery approached, Raman spectra exhibited decreasing intensities in the 1300 cm -1 lipid peak depicted in Figure 3a, as well as the 1440 cm -1 lipid bond and Amide I and III bonds (1660 cm-1 and 1265 cm-1, respectively) (not shown). Such changes did not occur at earlier time points during pregnancy, as can be observed in measurements taken from day 15 mice (Figure 3b). This shift illustrates the value of Raman spectroscopy for prediction of impending delivery (Figure 3a).
Figure 3.
(A) Raman peak at 1300 cm-1 indicative of lipids from d19 mice (n=5) monitored every two hours, starting 12 hours prior to delivery. (B) Six Raman peaks plotted from d15 mice (n=5, representative plot) monitored every two hours for a total of eight hours; intensities are stable over time.
In summary, Raman spectroscopy is a powerful, non-invasive method that has the potential to quantitatively measure a number of biochemical components and properties, such as collagen cross-linking, tissue hydration and tissue elasticity, that can be correlated with cervical remodeling that occurs with pregnancy. While employing multiple methods to evaluate the pregnant cervix is advisable, the preliminary results discussed here demonstrate that Raman spectroscopy is a technique that can combine many of the previous methods and has high potential to make an important clinical impact. The main advantage of this approach is the clinical applicability of Raman spectroscopy as has been demonstrated in cervical dysplasia. Using a clinical Raman system, we have initiated studies in human subjects to both monitor the dynamic biochemical changes that occur in the cervix throughout pregnancy and determine the predictive capability of this technique for translation into clinical use.
Key Notes.
We still don't fully understand the cervical changes that take place during pregnancy.
This review looks at how Raman spectroscopy can detect those changes and reports that preliminary studies in mouse models suggest that this optical technique is sensitive to biochemical changes in the cervix and provides advantages over other methods.
We believe that Raman spectroscopy could provide a highly effective tool for detecting premature cervical changes during pregnancy.
Acknowledgments
This work was presented in part by Dr Reese at the biennial meeting of the International Perinatal Collegium, July 2013. The authors acknowledge the financial support of the National Institutes of Health (Grant No. R01-CA-095405, AMJ and HD 044741, BCP), a predoctoral fellowship (Grant No. T32-HL-7751-15) for EV and a predoctoral fellowship (NSF Graduate Research Fellowship) for CO.
References
- 1.Behrman RE, Butler AS. Preterm birth: causes, consequences, and prevention. National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Ruiz RJ, Fullerton J, Dudley DJ. The interrelationship of maternal stress, endocrine factors and inflammation on gestational length. Obstet Gynecol Surv. 2003;58:415–28. doi: 10.1097/01.OGX.0000071160.26072.DE. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JN, Regan JA, Norwitz ER, editors. The epidemiology of preterm labor. Semin Perinatol. 2001;25:204–14. doi: 10.1053/sper.2001.27548. [DOI] [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod Health. 2013;10:1. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol. 2012;207:345–54. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118:1566–73. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–58. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–73. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins-Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964–73. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 11.Society for Maternal-Fetal Medicine Publications Committee Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–86. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Park TC, Norwitz ER. Management of pregnancies with cervical shortening: a very short cervix is a very big problem. Rev Obstet Gynecol. 2009;2:107–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Catalano PM, Ashikaga T, Mann LI. Cervical change and uterine activity as predictors of preterm delivery. Am J Perinatol. 1989;6:185–90. doi: 10.1055/s-2007-999573. [DOI] [PubMed] [Google Scholar]
- 14.Moroz LA, Simhan HN. Rate of sonographic cervical shortening and the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2012;206:234e1–e5. doi: 10.1016/j.ajog.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol. 2003;101:402–12. doi: 10.1016/s0029-7844(02)02505-x. [DOI] [PubMed] [Google Scholar]
- 16.Sotiriadis A, Papatheodorou S, Kavvadias A, Makrydimas G. Transvaginal cervical length measurement for prediction of preterm birth in women with threatened preterm labor: a meta-analysis. Ultrasound Obstet Gynecol. 2010;35:54–64. doi: 10.1002/uog.7457. [DOI] [PubMed] [Google Scholar]
- 17.Jokhi R, Brown B, Anumba D. The role of cervical Electrical Impedance Spectroscopy in the prediction of the course and outcome of induced labour. BMC Pregnancy Childbirth. 2009;9:40. doi: 10.1186/1471-2393-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFarlin B, Bigelow T, Laybed Y, O'Brien W, Oelze M, Abramowicz J. Ultrasonic attenuation estimation of the pregnant cervix: a preliminary report. Ultrasound Obstet Gynecol. 2010;36:218–25. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feltovich H, Nam K, Hall TJ. Quantitative ultrasound assessment of cervical microstructure. Ultrason Imaging. 2010;32:131–42. doi: 10.1177/016173461003200302. [DOI] [PubMed] [Google Scholar]
- 20.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–6. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 21.Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn. 2013;33:737–41. doi: 10.1002/pd.4116. [DOI] [PubMed] [Google Scholar]
- 22.Anthony GS, Walker RG, Robins JB, Cameron AD, Calder AA. Management of cervical weakness based on the measurement of cervical resistance index. Eur J Obstet Gynecol Reprod Biol. 2007;134:174–8. doi: 10.1016/j.ejogrb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Myers K, Ateshian GA. Interstitial growth and remodeling of biological tissues: Tissue composition as state variables. J Mech Behav Biomed Mater. 2013;29:544–56. doi: 10.1016/j.jmbbm.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers KM, Socrate S, Paskaleva A, House M. A study of the anisotropy and tension/compression behavior of human cervical tissue. J Biomech Eng. 2010;132:021003. doi: 10.1115/1.3197847. [DOI] [PubMed] [Google Scholar]
- 25.Maul H, Saade G, Garfield RE. Prediction of term and preterm parturition and treatment monitoring by measurement of cervical cross-linked collagen using light-induced fluorescence. Acta Obstet Gynecol Scand. 2005;84:534–6. doi: 10.1111/j.0001-6349.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 26.Maul H, Olson G, Fittkow CT, Saade GR, Garfield RE. Cervical light-induced fluorescence in humans decreases throughout gestation and before delivery: preliminary observations. Am J Obstet Gynecol. 2003;188:537–41. doi: 10.1067/mob.2003.94. [DOI] [PubMed] [Google Scholar]
- 27.Schlembach D, MacKay L, Shi L, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: From biochemical and molecular studies to in vivo clinical examination. Eur J Obstet Gynecol Reprod Biol. 2009;144S:S70–S6. doi: 10.1016/j.ejogrb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Akins ML, Murari K, Xi J, Li MJ, Luby-Phelps K, et al. A compact fiber-optic SHG scanning endomicroscope and its application to visualize cervical remodeling during pregnancy. Proc Natl Acad Sci U S A. 2012;109:12878–83. doi: 10.1073/pnas.1121495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reusch LM, Feltovich H, Carlson LC, Hall G, Campagnola PJ, Eliceiri KW, et al. Nonlinear optical microscopy and ultrasound imaging of human cervical structure. J Biomed Opt. 2013;18:031110. doi: 10.1117/1.JBO.18.3.031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baños A, Wolf M, Grawe C, Stahel M, Haensse D, Fink D, et al. Frequency domain near infrared spectroscopy of the uterine cervix during cervical ripening. Lasers Surg Med. 2007;39:641–6. doi: 10.1002/lsm.20542. [DOI] [PubMed] [Google Scholar]
- 31.Hornung R, Spichtig S, Baños A, Stahel M, Zimmermann R, Wolf M. Frequency-domain near-infrared spectroscopy of the uterine cervix during regular pregnancies. Lasers Med Sci. 2011;26:205–12. doi: 10.1007/s10103-010-0832-7. [DOI] [PubMed] [Google Scholar]
- 32.Crow P, Molckovsky A, Stone N, Uff J, Wilson B, Wongkeesong L-M. Assessment of fiberoptic near-infrared Raman spectroscopy for diagnosis of bladder and prostate cancer. Urology. 2005;65:1126–30. doi: 10.1016/j.urology.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Grimbergen M, van Swol C, van Moorselaar R, Uff J, Mahadevan-Jansen A, Stone N. Raman spectroscopy of bladder tissue in the presence of 5-aminolevulinic acid. J Photochem Photobiol B. 2009;95:170–6. doi: 10.1016/j.jphotobiol.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Barr H, Kendall C, Bazant-Hegemark F, Moayyedi P, Shetty G, Stone N. Endoscopic Screening and Surveillance for Barrett's Esophagus–Clinical Implications. MedGenMed. 2006;8:88. [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty G, Kendall C, Shepherd N, Stone N, Barr H. Raman spectroscopy: elucidation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer. 2006;94:1460–4. doi: 10.1038/sj.bjc.6603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chrit L, Bastien P, Sockalingum G, Batisse D, Leroy F, Manfait M, et al. An in vivo randomized study of human skin moisturization by a new confocal Raman fiber-optic microprobe: assessment of a glycerol-based hydration cream. Skin Pharmacol Physiol. 2006;19:207–15. doi: 10.1159/000093116. [DOI] [PubMed] [Google Scholar]
- 37.Sigurdsson S, Philipsen PA, Hansen LK, Larsen J, Gniadecka M, Wulf H-C. Detection of skin cancer by classification of Raman spectra. IEEE Trans Biomed Eng. 2004;51:1784–93. doi: 10.1109/TBME.2004.831538. [DOI] [PubMed] [Google Scholar]
- 38.Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Diagnosing breast cancer by using Raman spectroscopy. Proc Natl Acad Sci U S A. 2005;102:12371–6. doi: 10.1073/pnas.0501390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, Yeoh KG, et al. Characterizing variability in in vivo Raman spectra of different anatomical locations in the upper gastrointestinal tract toward cancer detection. J Biomed Opt. 2011;16:037003. doi: 10.1117/1.3556723. [DOI] [PubMed] [Google Scholar]
- 40.Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res. 2011;469:2160–9. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko AC-T, Choo-Smith L, Hewko M, Sowa MG, Dong C, Cleghorn B. Detection of early dental caries using polarized Raman spectroscopy. Opt Express. 2006;14:203–15. doi: 10.1364/opex.14.000203. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z, Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. In vivo early diagnosis of gastric dysplasia using narrow-band image-guided Raman endoscopy. J Biomed Opt. 2010;15:037017. doi: 10.1117/1.3420115. [DOI] [PubMed] [Google Scholar]
- 43.Vargis E, Kanter EM, Majumder SK, Keller MD, Beaven RB, Rao GG, et al. Effect of normal variations on disease classification of Raman spectra from cervical tissue. Analyst. 2011;136:2981–7. doi: 10.1039/c0an01020k. [DOI] [PubMed] [Google Scholar]
- 44.Kanter EM, Vargis E, Majumder S, Keller MD, Woeste E, Rao GG, et al. Application of Raman spectroscopy for cervical dysplasia diagnosis. J Biophotonics. 2009;2:81–90. doi: 10.1002/jbio.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyng FM, Faoláin EÓ, Conroy J, Meade A, Knief P, Duffy B, et al. Vibrational spectroscopy for cervical cancer pathology, from biochemical analysis to diagnostic tool. Exp Mol Pathol. 2007;82:121–9. doi: 10.1016/j.yexmp.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Kamemoto LE, Misra AK, Sharma SK, Goodman MT, Luk H, Dykes AC, et al. Near-Infrared Micro-Raman Spectroscopy for in Vitro Detection of Cervical Cancer. Appl Spectrosc. 2010;64:255–61. doi: 10.1366/000370210790918364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone N, Kendall C, Shepherd N, Crow P, Barr H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J Raman Spectrosc. 2002;33:564–73. [Google Scholar]
- 48.Duraipandian S, Zheng W, Ng J, Low JJ, Ilancheran A, Huang Z. Simultaneous fingerprint and high-wavenumber confocal raman spectroscopy enhances early detection of cervical precancer in vivo. Anal Chem. 2012;84:5913–9. doi: 10.1021/ac300394f. [DOI] [PubMed] [Google Scholar]
- 49.Krishna CM, Prathima N, Malini R, Vadhiraja B, Bhatt RA, Fernandes DJ, et al. Raman spectroscopy studies for diagnosis of cancers in human uterine cervix. Vib Spectrosc. 2006;41:136–41. [Google Scholar]
- 50.Jess PR, Smith DD, Mazilu M, Dholakia K, Riches AC, Herrington CS. Early detection of cervical neoplasia by Raman spectroscopy. Int J Cancer. 2007;121:2723–8. doi: 10.1002/ijc.23046. [DOI] [PubMed] [Google Scholar]
- 51.Vargis E, Byrd T, Logan Q, Khabele D, Mahadevan-Jansen A. Sensitivity of Raman spectroscopy to normal patient variability. J Biomed Opt. 2011;16:117004. doi: 10.1117/1.3646210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vargis E, Tang Y-W, Khabele D, Mahadevan-Jansen A. Near-infrared Raman Microspectroscopy Detects High-risk Human Papillomaviruses. Transl Oncol. 2012;5:172. doi: 10.1593/tlo.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Word RA, Li X-H, Hnat M, Carrick K, editors. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 54.Havelock J, Keller P, Muleba N, Mayhew B, Casey B, Rainey W, et al. Human Myometrial Gene Expression Before and During Parturition. Biol Reprod. 2005;72:707–19. doi: 10.1095/biolreprod.104.032979. [DOI] [PubMed] [Google Scholar]
- 55.Vargis E, Brown N, Williams K, Al-Hendy A, Paria B, Reese J, et al. Detecting Biochemical Changes in the Rodent Cervix During Pregnancy Using Raman Spectroscopy. Ann Biomed Eng. 2012;40:1814–24. doi: 10.1007/s10439-012-0541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]