Abstract
Aims
To test hypotheses about the contributions of the catecholamines epinephrine and norepinephrine (which serve as biological markers of life stress through sympathetic nervous system (SNS) activation), delay discounting, and their interaction to the prediction of drug use among young African American adults.
Design
A 1-year prospective study that involved assessment of SNS activity and collection of self-report data involving delay discounting and drug use.
Setting
Rural communities in the southeastern United States.
Participants
456 African Americans who were 19 years of age at the beginning of the study.
Measurements
At age 19, participants provided overnight urine voids that were assayed for epinephrine and norepinephrine. Participants were also assessed for hyperbolic temporal discounting functions (k) and drug use. At age 20, the participants again reported their drug use.
Findings
Linear regression analyses revealed that (a) catecholamine levels at age 19 forecast increases in drug use (B = .087, p < .01, 95% CI [.025, .148]), and (b) among young men, catecholamine levels interacted positively with delay discounting to forecast increases in drug use (simple slope = .113, p < . 001,95% CI [.074, .152]).
Conclusions
Higher urinary catecholamine concentrations in your adulthood predict higher levels of drug use a year later among young African American men in the USA who engage in high, but not low, levels of delay discounting.
Keywords: Delay discounting, drug use, stress, sympathetic nervous system, young adults
INTRODUCTION
In this study, hypotheses were tested about the contributions of the catecholamines, epinephrine and norepinephrine, which serve as biological markers of exposure to life stress through activation of the sympathetic nervous system (SNS), and delay discounting (DD), a behavioral characteristic that increases vulnerability to drug use and abuse. Particular attention was given to the interaction of catecholamines and DD in the prediction of drug use among 456 young African American adults. Using a 1-year prospective design, we tested the hypothesis that catecholamine levels assayed at age 19 would forecast drug use at age 20 among young men who engaged in high levels of DD. In the following sections, we review literature that supports these hypotheses.
Life stress is a demonstrated risk factor for youths’ high-intensity drug use and other risk behaviors because it precipitates emotional distress and perceptions of limited efficacy and control [1]. Cross-sectional surveys, prospective surveys, and experience sampling studies with youth in the United States and other countries have found initiation and escalation of drug use to be positively associated with life stress [2]. Life stress also has a demonstrated durable influence on the SNS, resulting in greater tonic release of its end products, epinephrine and norepinephrine, in children [3], adolescents [2], and adults [4]. Thus, we hypothesized that heightened levels of catecholamines, a biological embodiment of life stress, would forecast youth drug use from age 19 to age 20 years.
The second hypothesis tested in this study proposed that DD, the tendency to choose smaller immediate rewards over larger postponed rewards, will interact with catecholamine levels to predict drug use. A preference for immediate rewards is consistent with an affinity for prompt gratification and a diminished ability to resist temptations such as alcohol, tobacco, drugs, and overeating [5]. A recent meta-analysis of studies examining the relation between DD and drug use revealed significantly elevated DD with a medium effect size (d = .59) [6]. Fields et al. [7] also found that DD serves as a mediator linking stress to smoking in young adults. Research on DD to date has not focused on its relation to drug use in the context of stress indicators (i.e., a main-effect model) and has not determined whether DD processes are most relevant for youth experiencing high levels of stress (i.e., an interaction model). The interaction model comprises both risk-enhancing effects (i.e., high levels of DD increasing the impact of SNS activation on drug use) and potential protective effects (i.e., low levels of DD reducing the impact of SNS activation on drug use). This study proposed that high levels of DD would enhance the contributions of SNS activation to drug use, and that low levels of DD would buffer youth from drug use when SNS activation was high.
The third hypothesis stipulated that the postulated catecholamine level × DD interaction would be specific to young men, not emerging for young women. This hypothesis was based on epidemiological data indicating that (a) male youth tend to externalize the effects of high life stress and female youth tend to internalize such effects, with these patterns possibly linked to gender differences in drug use [8]; (b) male youth are more impulsive than are female youth [9]; and (c) male youth use drugs more frequently and at greater quantities during adolescence and young adulthood than do female youth [10].
The present research
Longitudinal data were collected across 1 year during the transition to young adulthood, a period of increased drug use for rural African Americans [2, 11]. Participants provided drug use data at ages 19 and 20 years. Overnight urine voids from which epinephrine and norepinephrine were assayed were collected at age 19, and data on DD were collected at age 20. We predicted that high catecholamine levels would forecast drug use among young men who manifested high, but not low, levels of DD.
METHODS
Participants
The sample was taken from a study of African American youth whose mean age was 11.2 years at the first assessment and 20.0 years at the last assessment. Data were collected annually. At the first assessment, 667 families were selected randomly from lists that schools provided of fifth-grade students [12]. The youth resided in nine rural counties in the state of Georgia in the United States. From a sample of 561 at the age 19 data collection (a retention rate of 84%), 500 youth were selected randomly to participate in the age 19 and 20 assessments described previously. Financial constraints associated with the overnight collection and the assay of urine necessitated the selection of a random subsample. Of the subsample of 500, 489 agreed to participate. Of the 489 participants for whom overnight urine voids were collected at age 19, 456 agreed to participate in the age 20 data collection wave. These 456 participants constituted the sample in the present study. Comparisons, using independent t tests and chi-square tests, of the 456 youth who provided data at age 20 with the 33 who did not revealed no differences on any demographic or study variable.
Participants’ mean age was 19.2 years (SD = 0.65) at the first assessment and 20.0 (SD = 0.69) years at the second assessment. Of the youth in the sample, 54% were female. The participants resided in nine rural counties in Georgia, in small towns and communities in which poverty rates are among the highest in the nation and unemployment rates are above the national average [13]. At the first assessment, 45.2% lived below federal poverty standards with a median family income per month of $1700; at the second assessment, the proportion was 46.5% with a median income of $1834.
Study procedure and outcome measures
All data were collected in participants’ homes using a standardized protocol. Written informed consent was obtained from caregivers and youth. At each wave of data collection, two African American field researchers conducted one visit to collect self-report data. The field researchers received 8 hours of training on data collection protocols. The field researchers interviewed the primary caregiver and the target youth separately and privately, with no other family members present or able to overhear the conversation. Primary caregivers suggested a place that was private and out of others’ view. Catecholamines, drug use, and socioeconomic status were assessed when youth were 19; DD and drug use were measured when they were 20.
Control variables
Six dichotomous variables measured at the age 19 data collection formed a socioeconomic status index. Primary caregivers provided this information. A score of 1 was assigned to each of the following: family poverty based on federal guidelines, caregiver unemployment, receipt of government financial assistance, caregiver single parenthood, caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. The scores were summed to form the index. Gender was dummy coded; male = 1 (n = 206) and female = 0 (n = 250). The socioeconomic status index was controlled in all analyses because this variable is associated with catecholamine levels and drug use [14]. Gender was controlled in the analysis of the main effects because it is consistently linked with drug use [2].
Young adult catecholamines
The protocol for measuring catecholamines when youth were 19 years of age was based on procedures that Evans [3] and Brody et al. [15] developed for field studies involving children and adolescents. Participants collected their overnight (8 p.m. to 8 a.m.) urine voids. All urine voided during this time was stored on ice in a container with metabisulfite as a preservative and taken to a laboratory at the University of Georgia, where total volume was recorded and four 10-ml samples were randomly extracted and deep frozen at −80° C until subsequent assays were completed. The pH of two of these samples was adjusted to 3 to inhibit oxidation of catecholamines. The frozen urine was delivered to the Emory University Hospital medical laboratory in Atlanta, Georgia, for assaying. Participants received training on the protocol that included procedures for collecting urine when they awoke in the morning. Epinephrine and norepinephrine were assayed with high-pressure liquid chromatography with electrochemical detection [16]. For epinephrine, mean intra-assay coefficients of variation (CV) for nonsequential duplicates are 27.1% (< 40 pg/ml), 13.5% (40 to 80 pg/ml), and 9.6% (> 80 pg/ml); pooled samples mean inter-assay CV for 60 to 140 pg/ml is 16.3%; and blanks read 7.0 ± 14.5 (SD) pg/ml. For norepinephrine, mean intra-assay CVs for nonsequential duplicates are 6.6% (< 400 pg/ml), 6.5% (400 to 800 pg/ml), and 7.1% (> 800 pg/ml); pooled mean inter-assay CV for 300 to 500 pg/ml is 10.3%; and blanks read 6.0 ± 10.3 (SD) pg/ml. Creatinine assay via Jaffe rate methods controlled for body size differences and incomplete urine voiding [17]. SNS catecholamine scores were calculated by summing standardized scores for overnight epinephrine and norepinephrine (r = .532, p < .001).
Delay discounting
DD was assessed at age 20 using the Monetary Choice Questionnaire (MCQ) [18], which has been widely used for examining impulsive discounting and addictive behavior [6]. This 27-item measure poses dichotomous choices between smaller immediate rewards and larger delayed rewards (e.g., $54 today vs. $80 in 30 days). Hyperbolic temporal discounting functions (k) are inferred by examining patterns of responding and determining a k value that is most consistent with the observed choices [18]. Unlike a permuted task in which a large array of possible discounted values choices are presented over several delay periods, the MCQ does not generate individual points of indifference. Nine items are used to assess DD for small, medium, and large rewards, respectively. To avoid Type I error rate inflation, however, only large reward discounting was analyzed. Small and medium reward discounting were highly intercorrelated with large reward discounting (rs = .74 – .88, p <.001). In addition, decision making for large rewards exhibited the most variability, with definitely more participants exhibiting maximum levels of discounting for small and medium magnitude rewards. The discounting functions were positively skewed, which is common [6]. The functions were transformed using a log10 transformation, which substantially improved the distribution.
Young adult drug use
When youth were 19 and 20 years of age, they reported their past month use of cigarettes, alcohol, and marijuana on a widely used instrument from the Monitoring the Future Study [19]. Youth were asked how often during the past month they had engaged in each of these forms of drug use. A 7-point response set ranging from not at all to more than two packs a day was used for cigarette smoking; a 6-point scale ranging from none to 20 or more times was used for alcohol and marijuana use. Responses to these four items were summed to form a drug use composite, a procedure that is consistent with our own and others’ prior research [20, 21]. Because drug use rates were low and skewed positively, the variables were log transformed; this improved the distribution. Alphas were .71 at age 19 and .70 at age 20.
Plan of analysis for the study hypothesis
Hierarchical regression analysis was used to test the study hypotheses because this statistical strategy conforms to best practices for testing moderational hypotheses [22]. Specifically, moderational hypotheses are evaluated through tests of an interaction term between a main effect (catecholamine levels) and a moderator (DD) to predict drug use. Three regression models were executed on drug use at age 20. In each model, family socioeconomic status and youths’ drug use at age 19 were entered first to be controlled.
The first model was designed to examine the main effects of gender, catecholamine levels, and DD. The second model examined the two-way interaction between catecholamine levels and DD. The third model estimated the contributions of the respective two-way and three-way interactions among catecholamine levels, DD, and gender. Catecholamine levels and DD scores were centered before the interaction terms were calculated. Effect sizes are reported as coefficients, and a conventional Type I error rate of p < .05 was used. In addition, a collinearity test based on a variance inflation factor (VIF) and tolerance was conducted to examine multicollinearity among the predictors. A tolerance value less than 0.1 or a VIF value greater than 10 indicates significant multicollinearity [23]. Results showed that none of the predictors used in the regression analyses, including the two-way interaction terms and the three-way interaction term, evinced any indication of multicollinearity: VIF ranged from 1.02 to 1.87 and tolerance ranged from 0.61 to 0.98. Given the group-randomized design of the study (randomization on the county level), the regression analyses also included an adjustment for clustering within counties using the COMPLEX analysis option in Mplus 7.11 [24]. This option accounts for nonindependence in the data by calculating adjusted standard errors that are robust to hierarchical sampling [25].
RESULTS
Preliminary analyses
Table 1 presents descriptive statistics along with bivariate correlations separately for men and women. We conducted 2(Time points) × 2(Gender) repeated measures ANOVAs with time as a repeated factor and gender as a between-group factor with drug use as a dependent variable. The results revealed a significant time effect (F[1, 454] = 3.961, p < .05) and a significant gender effect (F[1, 454] = 20.29, p < .001). Youth, in general, increased their drug use from age 19 (M = 0.658, 95% CI [.592, .725]) to age 20 (M = 0.723, 95% CI [.654, .793]). Young men (M = 0.828, 95% CI [.739, .917]) engaged in more drug use than did young women (M = 0.553, 95% CI [.472, .634]). No significant Time × Gender interactive effects on drug use emerged, F(1, 454) = 0.006, p = ns. As predicted, catecholamine levels at age 19 were associated with drug use at both age 19 and age 20 for young men but not for young women.
Table 1.
Descriptive Statistics and Correlations among Study Variables for Male and Female Youth a
| Variables | 1 | 2 | 3 | 4 | 5 | Male Youth
|
Female Youth
|
||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| 1. SES index (age 19) | -- | −.126 | .096 | −.045 | −.121 | 3.015 | 1.706 | 3.008 | 1.608 |
| 2. Drug use (age 19) | .015 | -- | .083 | .000 | .533*** | 0.795 | 0.821 | 0.522 | 0.621 |
| 3. Catecholamines (age 19) | −.091 | .155* | -- | −.009 | .102 | 0.134 | 1.732 | −0.113 | 1.739 |
| 4. DD (age 20) | .111 | .071 | .064 | -- | .069 | −1.474 | 0.788 | −1.580 | 0.928 |
| 5. Drug use (age 20) | .025 | .579*** | .206** | .054 | -- | 0.862 | 0.836 | 0.584 | 0.674 |
Abbreviation: DD, delay discounting.
Correlations for male youth are presented below the diagonal, and correlations for female youth are presented above the diagonal.
p < .05,
p < .01,
p < .001.
Test of the study hypotheses
The results of the linear regression models are presented in Table 2. In all models, the family socioeconomic status index and youth drug use at age 19 were controlled. The first model, presented in Table 2, Model 1, was designed to identify the main effects of catecholamine levels, DD, and gender. Results indicated that catecholamine levels at age 19 significantly predicted drug use at age 20 (B = .038, SE = .013, β = .087, p < .01, 95% CI [.025, .148]) beyond the effects of DD, gender, socioeconomic status, and drug use at age 19.
Table 2.
Catecholamines, Delay Discounting, and Gender as Predictors of Drug Use at Age 20 (N = 456)
| Predictors | Drug Use (age 20)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
Model 3
|
|||||||
| B | SE | β | B | SE | β | B | SE | β | |
| 1. SES index (age 19) | −.009 | .015 | −.019 | −.009 | .015 | −.018 | −.006 | .016 | −.013 |
| 2. Drug use (age 19) | .572 | .034 | .547*** | .572 | .033 | .547*** | .572 | .032 | .547*** |
| 3. Gender (male) | .109 | .065 | .071 | .107 | .064 | .070 | .103 | .065 | .067 |
| 4. Catecholamines (age 19) | .038 | .013 | .087** | .037 | .014 | .085** | .024 | .013 | .054 |
| 5. DD (age 20) | .036 | .041 | .055 | .037 | .049 | .042 | .047 | .038 | .053 |
| 6. Catecholamines × DD | .011 | .022 | .020 | −.028 | .019 | −.052 | |||
| 7. Catecholamines × Gender | .027 | .026 | .041 | ||||||
| 8. DD × Gender | −.028 | .092 | −.019 | ||||||
| 9. Catecholamines × DD × Gender | .102 | .022 | .115*** | ||||||
Abbreviation: DD, delay discounting.
p < .01,
p < .001.
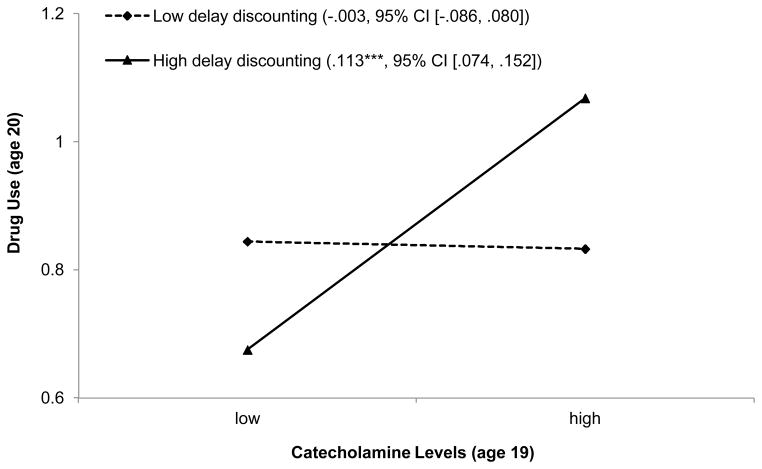
The second and third models in Table 2 were designed to determine whether catecholamine levels interacted with DD (Model 2) or whether a three-way interaction among catecholamine levels, DD, and gender would best characterize the data (Model 3). An interaction between catecholamine levels and DD did not emerge for the total sample (Table 2, Model 2), but did emerge for the three-way interaction involving gender (Table 2, Model 3, B = .102, SE = .022, β = .115, p < .001 95% CI [.057, .173]). To test further the study hypotheses, additional analyses were conducted that examined the two-way interaction between catecholamine levels and DD separately for men and women (see Table 3). The results revealed a significant two-way interaction for men (B = .074, SE = .024, β = .113, p < .01, 95% CI [.036, .191]) but not for women (B = −.028, SE = .019, β = −.063, p = ns, 95%CI [−.141, .016]). To interpret the finding for young men, we plotted estimated drug use at low (1 standard deviation below the mean; −1 SD) and high (1 standard deviation above the mean; +1 SD) levels of catecholamines and DD; the results are presented in Figure 1. Consistent with the study hypothesis, the data plots indicate that young men with high catecholamine levels at age 19 reported high levels of drug use at age 20, with drug use at age 19 controlled, when they evinced high DD (simple-slope = .113, SE = .020, p < .001, 95% CI [.074, .152]). The data also demonstrated clearly that, for young men with low DD, high levels of catecholamines at age 19 did not forecast high levels of drug use at age 20 (simple-slope = −.003, SE = .042, p = ns, 95% CI [−.086, .080]).
Table 3.
Catecholamines and Delay Discounting as Predictors of Drug Use at Age 20 for Male and Female Youth
| Predictors | Drug Use
|
|||||
|---|---|---|---|---|---|---|
| Male Youth (n = 206)
|
Female Youth (n = 250)
|
|||||
| B | SE | β | B | SE | β | |
| 1. Socioeconomic risk (age 19) | .014 | .028 | .028 | −.025 | .019 | −.059 |
| 2. Drug use (age 19) | .570 | .071 | .560*** | .568 | .053 | .523*** |
| 3. Catecholamines | .055 | .027 | .115* | .025 | .013 | .065 |
| 4. DD | .021 | .093 | .020 | .046 | .039 | .063 |
| 5. Catecholamines × DD | .074 | .024 | .113* | −.028 | .019 | −.063 |
Abbreviation: DD, delay discounting.
p < .05,
p < .001.
Figure 1.
Male youths’ drug use as a function of catecholamine levels and delay discounting, with family SES index and drug use at age 19 controlled, presented as regression lines for different levels of delay discounting (low: 1 SD below the mean; high: 1 SD above the mean). Numbers in parentheses refer to simple slopes with 95% confidence intervals.
***p < .001.
DISCUSSION
This research was designed to test the prediction that catecholamine levels would forecast drug use among young men who engaged in high levels of DD. Hierarchical multiple regression analyses showed that (a) catecholamine levels at age 19 forecast drug use at age 20, with age 19 drug use controlled; (b) a catecholamine level × DD level × gender interaction indicated that, for male youth, drug use was highest when both catecholamine levels and DD levels were high, a risk-promoting effect of DD; and (c) the catecholamine × DD × gender interaction also indicated that drug use among young men was low when catecholamine levels were high and DD levels were low, a protective effect of DD.
Our working hypothesis was that the impact of SNS activation on drug use would depend on a preference for immediate rewards as measured by DD rates, particularly for male youth. The behavioral mechanisms for the risk-enhancing effects of SNS activation on drug use are currently unknown. We conjecture that high levels of catecholamines, along with a propensity for immediate gratification, have implications for the ways in which young men with this biological and behavioral profile use drugs to cope with stress. Baumeister and Scher [26] advanced a parsimonious explanation for impulsive tendencies among persons who experience life stress. Such people desire the quickest possible escape from life stress and the negative arousal that accompanies it; therefore, they focus their attention on activities, such as drug use, that provide short-term relief.
Several mechanisms are protective for male youth who do not prefer immediate gratification; one mechanism may involve delay of responding. Low DD male youth, who are able to delay gratification, may be likely to gather information and consider alternatives before acting when they feel stressed. This orientation toward delayed gratification could effectively counteract a tendency for young men to respond impulsively and gravitate toward drug use in stressful situations. Another mode of operation could involve the ability to organize sequences of behavior and consider linkages across time [27]. During stressful periods, this approach involves anticipating events, planning responses, and considering the costs and benefits of various plans by recalling previous behavioral outcomes; this could help young men to avert the use of drugs as a coping mechanism. A preference for delayed gratification also could encourage affiliations with like-minded peers, further helping to forestall drug use during times of stress. Risk-enhancing and protective moderation effects of DD on young men may occur through a combination of cognitive, efficacy-oriented, and social modes of operation. Clearly, research is needed to test the variety of modes that contribute to the obtained moderation effects.
As expected, the interaction effect of SNS activation and DD on drug use emerged for young men but not young women. We propose two explanations for this finding. First, drug use rates were higher among young men than young women, both at age 19 and at age 20. Higher rates of drug use render interactions easier to detect. A much larger sample than the present one could include female youth who engage in higher rates of drug use than did those in this study, permitting an adequately powered test. Second, gender differences in drug use rates may reflect a tendency for young men and young women to resort to different behaviors as coping tactics. Whereas young men tend to resort to DD and drug use, young women appear to seek appetitive stimuli other than drugs, such as energy-dense food [28]. Catecholamine levels may not act as a good predictive marker for drug use in young women. Stress-related physiology may be associated more strongly with impulsivity in young men. Future research should address the possibility of such gender differences.
Several limitations of the present study should be noted. The findings’ generalizability to ethnically and socioeconomically diverse samples drawn from either rural or urban communities, either within or outside the United States, must be established empirically. Studies with larger samples are also needed to examine processes that are responsible for the DD moderation effects. The processes that we suggested could be operating should be examined in future research. Finally, the interaction of catecholamines and DD in young men demonstrates the need for prevention programs that mitigate the potential for life stress to “get under the skin.” This finding suggests that developing adaptive ways for individuals at high risk for socioeconomic and psychosocial adversity to manage stress, and thus avert SNS activation and a preference for immediate rewards, may be a key strategy for preventing drug use and abuse.
Acknowledgments
This study was supported by Award Number P30DA027827 from the National Institute on Drug Abuse to GB. All authors receive funding from the National Institutes of Health. JM receives additional funding from the Institute for Research on Gambling Disorders. GM and EC receive additional funding from the Canadian Institutes of Health Research. GM receives additional funding from the National Alliance for Research on Schizophrenia and Depression.
Footnotes
None of the authors has any conflict of interest regarding this research, financial or otherwise.
Contributor Information
Gene H. Brody, Center for Family Research, University of Georgia, 1095 College Station Road, Athens, GA 30602-4527
Tianyi Yu, Center for Family Research, University of Georgia, 1095 College Station Road, Athens, GA 30602-4527.
James MacKillop, Department of Psychology, University of Georgia, 125 Baldwin Street, Athens, GA 30602-3013.
Gregory E. Miller, Department of Psychology, Northwestern University, 2029 Sheridan Road, Evanston, IL 60208-2710
Edith Chen, Department of Psychology, Northwestern University, 2029 Sheridan Road, Evanston, IL 60208-2710.
Ezemenari M. Obasi, Department of Educational Psychology, University of Houston, 4800 Calhoun Road, Houston, TX 77204
Steven R. H. Beach, Owens Institute for Behavioral Research, University of Georgia, 200 D. W. Brooks Drive, Athens, GA 30602-7419
References
- 1.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody GH, Chen Y-f, Yu T, Beach SRH, Kogan SM, Simons RL, et al. Life stress, the dopamine receptor gene, and emerging adult drug use trajectories: a longitudinal, multilevel, mediated moderation analysis. Dev Psychopathol. 2012;24:941–51. doi: 10.1017/S0954579412000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39:924–33. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 4.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 6.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–21. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields S, Leraas K, Collins C, Reynolds B. Delay discounting as a mediator of the relationship between perceived stress and cigarette smoking status in adolescents. Behav Pharmacol. 2009;20:455–60. doi: 10.1097/FBP.0b013e328330dcff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter ML. Psychosocial resilience and protective mechanisms. In: Rolf J, Masten AS, Cicchetti D, Nuechterlein K, Weintraub S, editors. Risk and Protective Factors in the Development of Psychopathology. New York, NY: Cambridge University Press; 1990. pp. 181–215. [Google Scholar]
- 9.Ramos D, Victor T, Seidl-de-Moura ML, Daly M. Future discounting by slum-dwelling youth versus university students in Rio de Janeiro. J Res Adolescence. 2013;23:95–102. [Google Scholar]
- 10.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2003. Bethesda, MD: National Institute on Drug Abuse; 2004. (NIH Publication No. 04-5506) [Google Scholar]
- 11.Brody GH, Chen Y-f, Kogan SM. A cascade model connecting life stress to risk behavior among rural African American emerging adults. Dev Psychopathol. 2010;22:667–78. doi: 10.1017/S0954579410000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, et al. The Strong African American Families program: translating research into prevention programming. Child Dev. 2004;75:900–17. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 13.Proctor BD, Dalaker J. Poverty in the United States: 2002. Washington, DC: US Census Bureau; 2003. (Current Population Reports, P60-222) [Google Scholar]
- 14.Brody GH, Yu T, Chen Y-f, Kogan SM, Evans GW, Beach SRH, et al. Cumulative socioeconomic status risk, allostatic load, and adjustment: a prospective latent profile analysis with contextual and genetic protective factors. Dev Psychol. 2013;49:913–27. doi: 10.1037/a0028847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brody GH, Yu T, Chen Y-f, Kogan SM, Evans GW, Windle M, et al. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: a prospective analysis. J Fam Psychol. 2013;27:22–9. doi: 10.1037/a0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggin RM, Kissinger PT. Determination of catecholamines in urine by reverse-phase liquid chromatography with electrochemical detection. Anal Chem. 1977;49:2109–11. doi: 10.1021/ac50021a052. [DOI] [PubMed] [Google Scholar]
- 17.Tietz NW, editor. Fundamentals of Clinical Chemistry. 2. Philadelphia, PA: Saunders; 1976. [Google Scholar]
- 18.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2006. Volume I: Secondary School Students. Bethesda, MD: National Institute on Drug Abuse; 2007. (NIH Publication No. 07-6205) [Google Scholar]
- 20.Brody GH, Ge X. Linking parenting processes and self-regulation to psychological functioning and alcohol use during early adolescence. J Fam Psychol. 2001;15:82–94. doi: 10.1037//0893-3200.15.1.82. [DOI] [PubMed] [Google Scholar]
- 21.Newcomb MD, Bentler PM. Consequences of Adolescent Drug Use: Impact on the Lives of Young Adults. Thousand Oaks, CA: Sage; 1988. [Google Scholar]
- 22.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- 23.Kutner MH, Nachtsheim CJ, Neter J. Applied Linear Regression Models. 4. Chicago, IL: McGraw-Hill; 2004. [Google Scholar]
- 24.Muthén BO, Muthén LK. Mplus Users’ Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 25.Muthén BO, Satorra A. Technical aspects of Muthén’s LISCOMP approach to estimation of latent variable relations with a comprehensive measurement model. Psychometrika. 1995;60:489–503. [Google Scholar]
- 26.Baumeister RF, Scher SJ. Self-defeating behavior patterns among normal individuals: review and analysis of common self-destructive tendencies. Psychol Bull. 1988;104:3–22. doi: 10.1037/0033-2909.104.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Chen E, Miller GE. “Shift-and-persist” strategies: why low socioeconomic status isn’t always bad for health. Perspect Psychol Sci. 2012;7:135–58. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson EL. The psychobiology of comfort eating: implications for neuropharmacological interventions. Behav Pharmacol. 2012;23:442–60. doi: 10.1097/FBP.0b013e328357bd4e. [DOI] [PubMed] [Google Scholar]