Abstract
A metabolic health crisis is evident as cardiovascular diseases (CVD) remain the leading cause of mortality in the US. Effects of resistant starch type 4 (RS4), a prebiotic fiber, in comprehensive management of metabolic syndrome (MetS) remain unknown. This study examined the effects of a blinded exchange of resistant starch type-4 (RS4)-enriched flour (30% v/v) with regular/control flour (CF) diet on multiple MetS comorbidities. In a double-blind (participants-investigators), placebo-controlled, cluster crossover intervention (n=86, age ≥18, 2–12week interventions, 2week washout) in the US, individuals were classified as having MetS (With-MetS) or not (No-MetS) following International Diabetes Federation (IDF)-criteria. RS4 consumption compared with CF resulted in 7.2% (p=0.002) lower mean total cholesterol (TC), 5.5% (p=0.04) lower non-HDL, and a 12.8% (p<0.001) lower HDL cholesterol in the With-MetS group. No-MetS individuals had a 2.6% (p=0.02) smaller waist circumference and 1.5% (p=0.03) lower percent body fat following RS4 intervention compared to CF. A small but significant 1% increase in fat-free mass was observed in all participants combined (p=0.02). No significant effect of RS4 was observed for glycemic variables and blood pressures. RS4 consumption improved dyslipidemia and body composition. Incorporation of RS4 in routine diets could offer an effective strategy for public metabolic-CVD health promotion.
The clinicaltrials.gov-reference NCT01887964.
Keywords: cardiovascular diseases, dietary intervention, metabolic syndrome, resistant starch type 4
The MetS affects 34% of adults in the US, creating a severe burden on the health care system. The IDF defines MetS as a cluster of heart attack and CVD risk factors: diabetes/prediabetes, abdominal obesity, dyslipidemia, and hypertension [1]. Human studies indicate benefits of dietary fiber in MetS [2]. Resistant starch (RS) is a naturally low-calorie, prebiotic dietary fiber derived from starchy food grains, which escapes enzymatic digestion in the small intestine but is subjected to microbial fermentation in the large intestine. RS is classified into four types (RS1, RS2, RS3, and RS4), based on the properties that render them indigestible [3]. Clinical studies reported potential benefits of RS2 and RS3 on blood lipids, glycemic index and colon cancer risk reduction [2, 4].
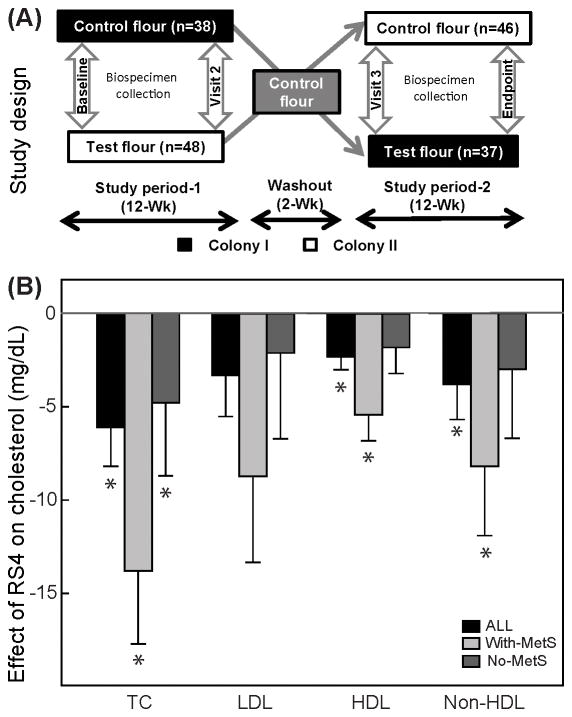
The RS4 is the crosslinked type, minimally alters the physicochemical and organoleptic properties of the final food product and has minimal gastrointestinal side effects such as bloating [5–6]. These features make RS4 highly adaptable and, hence, an ideal fiber candidate for incorporation into routine diet for proposed long-term management of MetS [7]. RS4 consumption decreased postprandial glucose and insulin levels [5] and altered the gut microbial profile in humans [6]. In animals, RS4 attenuated obesity/body-weight and dyslipidemia [8]. Most of these investigations were short-term, involving small number of healthy humans or animal models. Here we report a 26-week long double-blind (participants-investigators), placebo-controlled, cluster crossover intervention study (Figure 1A) comprehensively evaluating effects of dietary RS4 consumption in healthy participants as well as in participants with MetS.
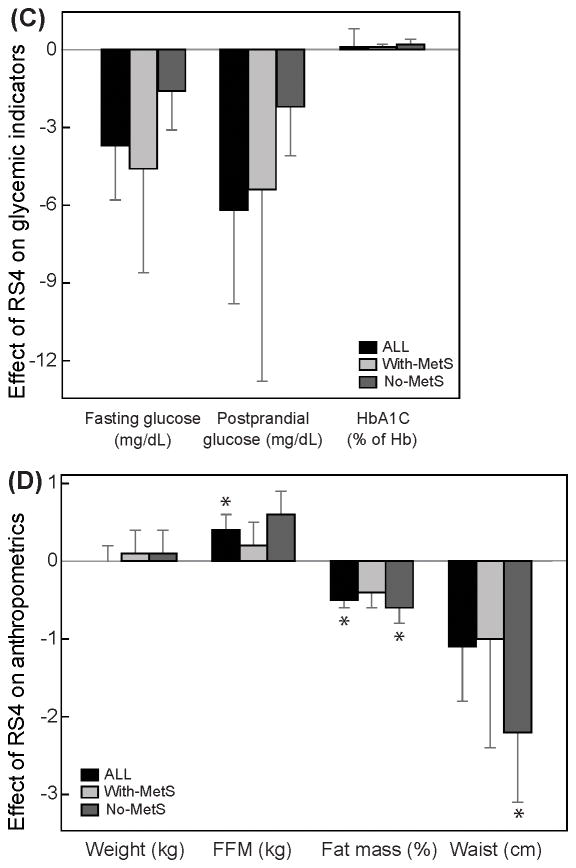
Figure 1. A placebo (CF)-controlled, crossover, dietary intervention with RS4.
Study design (A), Effects of RS4 flour on lipids (B), glucose (C) and anthropometric variables (D). Differences between means (RS4-CF) following the intervention periods were obtained from a linear mixed model adjusting for age, gender, season, and colony (± standard error of the mean). Participants were stratified into three groups based on the presence of metabolic syndrome during the study: With-MetS, had metabolic syndrome throughout the study; No-MetS, never had metabolic syndrome during the study; Change, changed status during the study (data not shown); ALL= With-MetS + No-MetS + Change. Asterisks above the difference in the means indicates that RS4 consumption had a significant effect (p≤0.05).
The study was conducted in two Hutterite communities in Eastern South-Dakota and included adult men and women. The Hutterites are a culturally homogeneous Caucasian population of Central European ancestry. Published MetS prevalence rates specific to Hutterites was not found but 46.5% of the recruited Hutterites in the study had MetS at baseline as compared to 34% national average [1]. Approval for research was obtained from the Institutional Review Board for Human Subjects Research of South-Dakota State University and are in accordance with the Declaration of Helsinki. Individual written informed consent for participation was obtained before enrollment. Exclusion criteria included pregnancy, lactation, long-term antibiotic therapy, immune compromised, cancer and other conditions that would affect the ability to provide informed consent or comply with the protocol.
RS4-flour had a 30% (v/v) substitution of RS4 (Fibersym, MGP Ingredients, Atchison, KS) in the CF (Table 1). Participants consumed RS4-flour and CF ad libitum in a free-living, domestic environment in form of any flour-based recipes that they would normally have prepared, to match realistic conditions. Bread, noodles, maultaschen, and dumplings are the most frequent flour-based foods consumed in Hutterite colonies. Typical Hutterite diets contain high- protein, fat, cholesterol, salt, and low fiber compared with the RDA levels [9]. Hutterites have relatively small interpersonal differences in diet, although a large number of food options are offered at each meal, in a common dining hall. The sequence of flour consumption was randomly assigned to the colonies.
Table 1.
Nutrient composition of intervention flours
| Nutrient (g/100 g) | CF (control flour) | RS4 flour |
|---|---|---|
|
| ||
| Water | 13.4 | 12.5 |
| Proteins | 11.0 | 7.9 |
| Carbohydrates | 73.5 | 77.8 |
| Total fat | 1.7 | 1.3 |
| Saturated | 0.2 | 0.2 |
| Monounsaturated | 0.1 | 0.1 |
| Polyunsaturated | 0.7 | 0.6 |
| Trans-fat | 0.0 | 0.0 |
| Fiber (RS4) | 2.4 (0.0) | 25.7 (24.0) |
| Sugars | 0.3 | 0.2 |
| Ash | 0.5 | 0.6 |
| Calcium (mg/100 g) | 24.0 | 50.4 |
| Sodium (mg/100 g) | 2.0 | 91.4 |
| Vitamin C (mg/100 g) | 0.0 | 0.4 |
| Calories (kcal) | 361.0 | 266.8 |
All data and biospecimens were collected onsite from both colonies at baseline, 12, 14 and 26 weeks except body composition analyses, which was omitted at 14 week. Dietary intake was assessed using a checklist that each participant used to check off servings of various food items common to the Hutterite diet that were categorized under dairy products; beverages; breads, cereals and grains; eggs, fish, poultry and meats; fruits, vegetables; and, sweets, crackers, pickles and soups. To assess physical activity a seven-day physical-activity recall-questionnaire administered by trained interviewers [10]. Medication use, medical histories and stool characteristics were collected. Further methodological details are provided as supplementary information.
Data analysis was performed in two ways: first by considering all participants together (the ALL group) and then after stratifying participants based on MetS status during the control and intervention periods following the IDF criteria for MetS (Table 2). The With-MetS group had MetS during the entire study (both treatment periods), the No-MetS group never had MetS during the study, and the Change group had a change in MetS status from one study period to the next.
Table 2.
International Diabetes Federation criteria for metabolic syndrome (MetS)
| Risk factor criteria | Defining level | |
|---|---|---|
|
| ||
| Men | Women | |
|
| ||
| I. Set a | ||
| Waist circumference (cm) | >94 | >80 |
| BMI (kg/m2) | >30 | >30 |
| II. Set b | ||
| HDL cholesterol (mg/dL)a | <40 | <50 |
| Triglycerides (mg/dL)a | ≥150 | ≥150 |
| Blood pressure (mm Hg)a | systolic > 130 diastolic > 85 |
systolic > 130 diastolic > 85 |
| Fasting glucose (mg/dL)a | >100 | >100 |
Participants meeting any one criterion from set a, and any two criteria from set b (or if they were on medications for markeda abnormalities) were considered as having MetS.
The effect of RS4 flour on anthropometric measurements (weight, body mass index [BMI], waist circumference, systolic and diastolic blood pressure, fat-mass [FM], and fat-free mass [FFM]), lipid profile (TC, LDL, HDL, non-HDL, and triglycerides), and glucose profile (fasting glucose, postprandial glucose, and HbA1C) was investigated. Triglyceride concentrations were converted to a normal distribution by log transformation. The effect of the RS4 flour was evaluated using a linear mixed effects model (SAS MIXED procedure) with subject designated as a random effect. The final models included season, colony, gender, age, and initial baseline value of the response variable as fixed effects. The effect of RS4 flour was based on the difference of least square means for response variables at the end of treatment periods and statistically evaluated as different from zero with a t-statistic and degrees of freedom calculated using the Kenward-Roger’s method. Interactions were considered to be significant at p≤0.10 following Bakris et al. [11]. Baseline differences in outcome variables between MetS subpopulations were investigated using a two-tailed Student’s t-test or chi-square test. Changes in stool frequency, constipation, diarrhea, as well as bloating/gas and abdominal pain were assessed in participants using McNemar’s test, with a continuity correction used for n≤5. Data analyses were performed using SAS 9.1.3 (SAS, Cary, NC). All differences were considered to be significantly different at p≤0.05. Details of statistical analyses are provided as supplementary information.
Baseline characteristics of the participants by MetS group are shown in Table S1. There were 127 individuals aged 18 years or older on two colonies. Of these 127 individuals, 3 were pregnant, 5 were ineligible or unable to participate, and 33 declined to participate. A total of 86 individuals received the RS4-flour and CF. Three participants withdrew before completion for reasons unrelated to the study. The prevalence of MetS at enrollment was 46.5%, with 40.7% taking medication for cholesterol, diabetes, or blood pressure among ALL participants. Medication usage was 70.4% in the With-MetS group. All outcomes of the study are presented in Table S2. The effects of RS4 on blood lipids, glycemic parameters and anthropometric parameters are summarized in Figure 1 B–D. RS4 consumption compared with CF resulted in 7.2% (p=0.002) lower mean total cholesterol (TC), 5.5% (p=0.04) lower non-HDL, and a 12.8% (p<0.001) lower HDL cholesterol in the With-MetS group (Figure 1B). Individuals in the No-MetS group had a 2.6% (p=0.02) smaller waist circumference and 1.5% (p=0.03) lower percent body fat following RS4 intervention compared to CF. A small but significant 1% increase in fat-free mass also was observed in all participants combined (p=0.02) (Figure 1D). No significant effect of RS4 was observed for glycemic variables (Figure 1C) and blood pressure (data not shown). Stool frequency increased from 1/day (considered normal) to >1/day (considered increased) in eight and 17 individuals while consuming CF and RS4 flour, respectively, but the outcome was statistically non-significant (p=0.11, McNemar’s test). Also, significant changes were not observed for other gastrointestinal side effects, such as constipation (p=0.62), diarrhea (p=0.18), bloating/gas (p=0.10), and abdominal pain (p=0.31) (data not shown). No other adverse effects of the intervention were reported. Further details of results are provided as supplementary information.
This is the first report of dietary intervention for MetS with a prebiotic fiber in a domestic community setting. Our data indicate, that consumption of wheat-based, RS4-enriched flour (30% v/v) in place of regular wheat flour as part of a routine diet has significant cholesterol-lowering effects (Figure 1B). Effects of RS4 on dyslipidemia in humans were previously unknown. Our findings are in line with a meta-analysis of 67 clinical trials controlled for fiber (RS4-data was not available and was not included) and energy density associated fiber consumption with a significant decrease in TC, HDL, and LDL but there was no association with triglycerides [12]. Likewise, RS4-flour significantly lowered all forms of cholesterols (TC, HDL, and non-HDL that includes LDL) except no changes in triglyceride levels. A differential effect of RS4 on TC was observed, depending on the MetS status of the volunteers. This could be due to an increased response by individuals with dyslipidemia to RS4, which may be desirable for consumers, food scientists, and clinicians. It is to note here that lowering of TC and non-HLD cholesterols that includes LDL remain key for cutting CVD risks and that lower HDL levels did not always associate with increased heart disease risk [13].
The effect of RS4 on glycemic control (Figure 1C) was assessed by measuring fasting-glucose, postprandial-glucose, and HbA1C. Mean blood glucose levels were lower with RS4 consumption but not statistically significance. The large variability in this measure, may explain why these results are not exactly in line with previous reports where RS4 improved glucose tolerance during serial postprandial measurements [8]. Due to the larger number of participants and the logistics of communal living (same dining time for all participants), serial collection of postprandial blood was not possible in our study.
A prospective cohort study on the effect of total dietary fiber (from all dietary sources) showed that an increase in total fiber intake by 10 g/d is associated with a decrease of 0.08 cm/y waist circumference [14]. In our study, just 12 weeks of RS4-intervention resulted in a 2.3 cm (2.6%, p=0.02) lower mean waist circumference compared with CF in No-Mets participants. The No-MetS group was predominantly females and although we included gender as a covariate in all of our analyses, some uncontrolled confounding, although less likely, cannot be ruled out. We also observed albeit small but significant desirable changes in fat-free mass (ALL group) and body fat percent (ALL, No-MetS groups), but no significant overall changes in bodyweight.
Strengths of the current study include a decent sample size, a longer duration, and low attrition rates, increasing the validity of the findings. Although the non-uniform calorie and macronutrients intakes may have potentially undermined some of the hypothesized health benefits of RS4 such as improving glycemic parameters, we believe that such variations in macronutrient intake are a fair representation of studying food-based dietary recommendations under realistic conditions as opposed to in tightly controlled experimental environments.
The present study also has limitations. Because the crossover design used only two clusters (colonies), any carryover effect is confounded by cluster sampling effect (colony effects), and thus a carryover effect could not be independently evaluated in the mixed models. The unrestricted design although produced several statistically significant outcomes may have contributed to greater variation in certain outcome variables such as postprandial blood glucose, and conclusive data were not obtained for blood pressure (data not shown). However, to minimize such variation, we adjusted for multiple covariates (age, gender, season, and colony) in the data analysis. Also, the study design allowed each participant to serve as his/her own control, minimizing the influence of confounding variables on treatment effects.
Several mechanisms have been proposed by which RS exerts beneficial effects. A major portion of RS is insoluble and is fermented by gut microbes, which release short-chain fatty acids, such as butyrate, propionate, and acetate. An increased consumption of RS produces more butyrate, which is associated with higher insulin sensitivity and improved glycemic control [15]. Modulation of the gut microbial profile [6] could be another way that RS promotes health. While all types of RS may share some common mechanisms in their biological activities, the presence of distinct factors is also likely. RS4 was more effective in lowering postprandial glucose compared with RS2 [5]. Also, changes in the microbial profiles induced by RS2 and RS4 were distinct [6]. Follow-up mechanistic studies that will provide a better understanding of how RS4 modulates risk factors for MetS in the Hutterites are underway and will be reported elsewhere.
Finally, fiber is an important class of dietary constituent implicated in the prevention of MetS [2]. Currently, fewer than 3% of Americans, including the Hutterites, meet the daily recommended intake of dietary fiber of 25–38 g/day in age 50-plus adults. Wheat-based menus are routine in a western-style diet, and a simple exchange of regular wheat flour in a daily domestic or commercial kitchen (such as in a restaurant) with RS4-enriched wheat flour might be an effective way to increase fiber intake and promote a healthier dietary lifestyle. Further long-term (multi-year) studies considering larger segments of the general population and with longer washout periods will be necessary to confirm the long-term advantages and benefits of RS4. Future studies should also elucidate the interaction between host genotype, gut microbial profiles, and RS4 consumption to tailor preventive strategies for various chronic diseases that arise from dysbiosis in the gut.
Therefore, we conclude that we have presented results suggesting that RS4-enriched diet may be effective for reducing pathophysiological consequences in individuals with MetS. Synergistic risk reduction of multiple comorbidities for CVD by RS4 may potentially contribute to improved management of major public health issues. Our results also indicate that unrestricted dietary interventions with potentially better adaptability over long-term, could be an effective strategy for health promotion. However specific validation of such potential is warranted in future.
Supplementary Material
Acknowledgments
We are thankful to all the study participants for their time on the study. Major support for this work came from MGP Ingredients, Atchison, KS (# 3P2662 to MD). MGP Ingredients also generously provided the test flours used in this study. Additional support came from the National Institutes of Health grant R00AT4245 (to MD), SD-Agriculture Experiment Station grant 3AH360 (to MD) and EA Martin Endowment at SDSU (to BLS). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ contributions:
Study conception: MD; Designed research: MD, BLS, SNN; Conducted Research: SNN, MD, BLS, LAW, TMB; Provided reagents/materials/equipment: MD, BLS; Performed statistical analyses: BLS, SNN, HEW; Major contribution to manuscript writing: MD, BLS; All authors read, provided comments and approved the final manuscript.
Competing interest statement:
The authors declare no competing interests or no conflict of interest.
Contributor Information
Sailendra N. Nichenametla, Email: Sailendra.Nichenametla@sdstate.edu.
Lee A. Weidauer, Email: Lee.Weidauer@sdstate.edu.
Howard E. Wey, Email: Howard.Wey@SDSTATE.EDU.
Tianna M. Beare, Email: Tianna.Beare@sdstate.edu.
Bonny L. Specker, Email: Bonny.Specker@SDSTATE.EDU.
Bibliography
- 1.Yadav D, Mahajan S, Subramanian SK, Bisen PS, Chung CH, Prasad G. Prevalence of Metabolic Syndrome in Type 2 Diabetes Mellitus Using NCEP-ATPIII, IDF and WHO Definition and Its Agreement in Gwalior Chambal Region of Central India. Diabetes care. 2011;34(1):216–219. doi: 10.5539/gjhs.v5n6p142. [DOI] [PMC free article] [PubMed] [Google Scholar]; Glob J Health Sci. 2013;5(6):142–155. doi: 10.5539/gjhs.v5n6p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson JJ, Eisenmann JC, Norman GJ, Ortiz KA, Young PC. Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents. J Am Diet Assoc. 2011;111(11):1688–1695. doi: 10.1016/j.jada.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46 (Suppl 2):S33–50. [PubMed] [Google Scholar]
- 4.Heijnen ML, van Amelsvoort JM, Deurenberg P, Beynen AC. Limited effect of consumption of uncooked (RS2) or retrograded (RS3) resistant starch on putative risk factors for colon cancer in healthy men. Am J Clin Nutr. 1998;67(2):322–331. doi: 10.1093/ajcn/67.2.322. [DOI] [PubMed] [Google Scholar]
- 5.Haub MD, Hubach KL, Al-tamimi EK, Ornelas S, Seib PA. Different Types of Resistant Starch Elicit Different Glucose Reponses in Humans. Journal of Nutrition and Metabolism. 2010;2010:1–4. doi: 10.1155/2010/230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. Plos One. 2010;5(11):e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maziarz M, Sherrard M, Juma S, Prasad C, Imrhan V, Vijayagopal P. Sensory characteristics of high-amylose maize-resistant starch in three food products. Food Science & Nutrition. 2012:n/a–n/a. doi: 10.1002/fsn3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KY, Yoo S-H, Lee HG. The effect of chemically-modified resistant starch, RS type-4, on body weight and blood lipid profiles of high fat diet-induced obese mice. Starch - Stärke. 2012;64(1):78–85. [Google Scholar]
- 9.Rokusek-Kennedy C, Parry RE, Schienker EH. The nutritional status of Hutterites in South Dakota. Fed Proc. 1987;46:1157. [Google Scholar]
- 10.Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011;96(1):106–114. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292(18):2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 12.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 13.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du H, van der AD, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, Tjonneland A, Overvad K, Jakobsen MU, Boeing H, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91(2):329–336. doi: 10.3945/ajcn.2009.28191. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.