Abstract
Chlamydia trachomatis is the most common cause of human bacterial sexually transmitted infections and is the world’s leading cause of infectious preventable blindness. The chlamydial 7.5 kb plasmid and chromosomal gene CT135 have been shown to be important virulence factors in both nonhuman primate and mouse infection models. C. trachomatis plasmid-deficient urogenital isolates and a predicted CT135 null mutant have been evaluated independently in the female mouse genital tract model and both have been shown to reduce infectivity and virulence. However, these attenuating phenotypes have not been evaluated collectively in the murine model. Here, we test the infectivity of C. trachomatis serovar D strains in the mouse model that are plasmid-deficient, CT135 disrupted, or possess a combination of these attenuating genotypes. We find that the presence of the plasmid results in infections with higher infectious burdens whereas CT135 facilitates a more protracted or chronic infection. Not unexpectedly, a combination of these genetic deficiencies resulted in a strain with enhanced infection attenuation characteristics.
Keywords: Chlamydiae, plasmid, CT135, mouse infectivity
Manuscript
Chlamydia trachomatis is an obligate intracellular human pathogen with a unique biphasic developmental growth cycle (Moulder, 1966). It is the etiological agent of trachoma, the world’s leading cause of preventable blindness and the most common cause of bacterial sexually transmitted disease (Schachter, 1978; Whitcher, 2001). Vaccines capable of controlling or preventing these diseases are needed (Brunham, 2013). Strategies for vaccine development have focused on subunit vaccines (Hafner, 2008; Rockey, 2009) and, more recently, live-attenuated vaccines using plasmid-deficient organisms (Kari, 2011). The 7.5 kb chlamydial plasmid encodes eight highly conserved genes (Palmer, 1986), two of which (pgp3 and pgp4) have been associated with virulence. Pgp3 is a secreted protein (Li, 2008) of unknown function, and Pgp4 functions as a transcriptional regulator of multiple chromosomal genes (Song, 2013) that are uncharacterized virulence factors. C. trachomatis CT135 is a plasmid independent regulated chromosomal gene expressed very early in the chlamydial developmental cycle (Belland, 2003) and a predicted inclusion membrane protein (Lutter, 2013). CT135 is known to enhance the infectivity of a urogenital C. trachomatis serovar D strain in the female mouse genital tract (Sturdevant, 2010). It has also been recently reported that plasmid-deficient urogenital C. trachomatis strains have a reduced infectivity and virulence in the female mouse genital tract (Sigar, 2013). These findings implicate both the plasmid and CT135 as virulence determinants that attenuate in vivo C. trachomatis infection in mice; however they fail to define the collective roles of these mutations on the attenuation of a single strain. In this report we directly compare the infectivity of isogenic human C. trachomatis serovar D strains in a female mouse infection model that are (i) plasmid-deficient, (ii) CT135 disrupted, or (iii) both plasmid-deficient and CT135 disrupted.
Plasmid-deficient strains were generated using C. trachomatis strain D/UW-3/Cx previously designated as late (D-LC) and early clearance (D-EC) phenotypes (Sturdevant, 2010). D-LC and D-EC are isogenic with the exception of CT135; D-EC has a single base insertion at nt position 152686 that is predicted to centrally disrupt the protein’s ORF. D-LC also has a single nucleotide deletion at 152276 compared to the original D/UW-3 annotation (Stephens, 1998), although this N-terminal deletion leaves the majority of the CT135 ORF intact. The mutation in D-EC results in the strain’s attenuation for C3H/HeJ female mice compared to D-LC. Infection with D-EC, compared to D-LC, produces genital tract infections with lower chlamydial burdens of a much shorter duration (Sturdevant, 2010). Based on this correlation of a single gene change resulting in in vivo attenuation, we concluded that strain D-EC can be considered a predicted null mutant. Plasmid free strains of D-LC and D-EC were isolated employing novobiocin curing as previously described (Kari, 2011). The plasmid and CT135 genotype designation of the strains are: DP+CT135+, DP+CT135−, DP−CT135+ and DP−CT135−, respectively. All strains were propagated in McCoy cells and elementary bodies (EB) purified by density gradient centrifugation (Caldwell, 1981). Plasmid deficient organisms exhibited characteristic atypical late-inclusion morphology with a donut appearance that failed to stain glycogen (O'Connell, 2006; Carlson, 2008; Wang, 2013). Plasmid cured strains were PCR negative for all eight plasmid genes when compared directly to plasmid containing positive controls.
Progesterone treated female eight-week old inbred C3H/HeJ mice were infected intravaginally with 1 × 105 inclusion forming units (IFU). Six to eight mice were infected for each of the different chlamydial genotypes studied (n=8 for strains DP+CT135− and DP−CT135−; n=6 for DP+CT135+ and DP−CT135+). Chlamydial burdens (IFU) and duration of infection of individual mice were monitored biweekly for two weeks and then weekly thereafter by culturing cervico-vaginal swabs for Chlamydiae on monolayers of McCoy cells. Two-way ANOVA statistical analyses were calculated comparing strain infection course curves. All animal procedures used throughout this study were conducted in accordance with Animal Care and Use Guidelines, and were reviewed and approved by the Animal Care and Use Committee at RML.
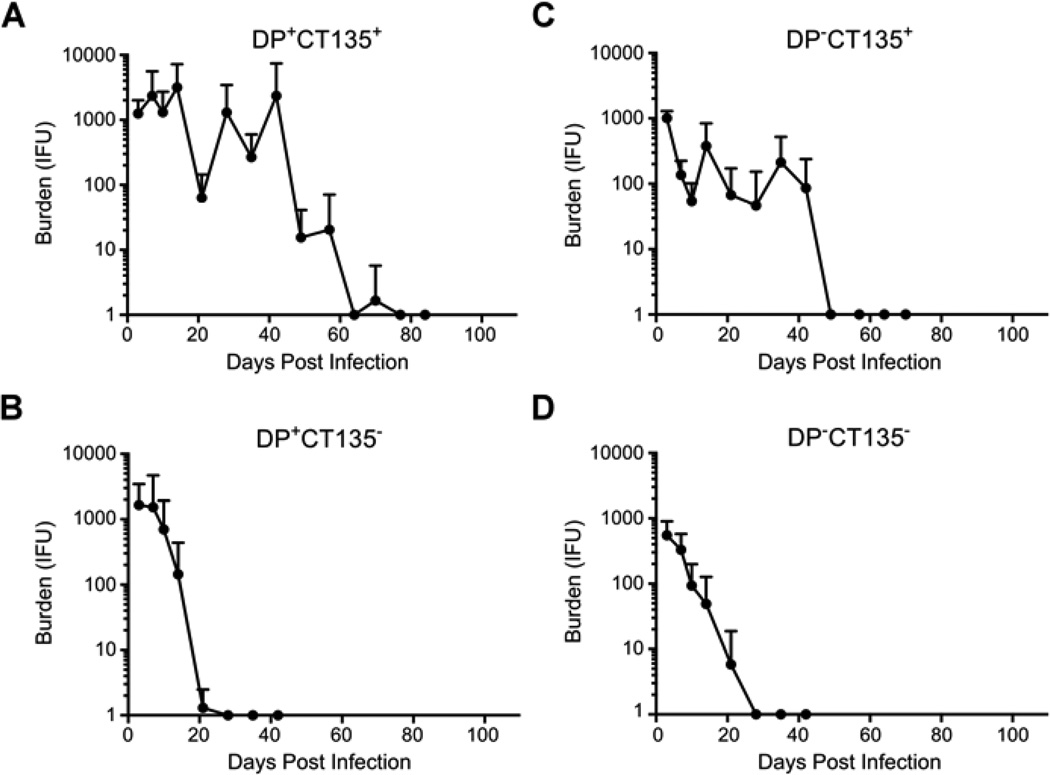
Figure 1 shows the results of this study. Infection of mice with wild type virulent DP+CT135+ organisms resulted in infections with significantly greater chlamydial burden post-infection (PI) than all other strains at 14 and 21 days PI (P ≤ 0.046) and were of longer duration that resolved spontaneously by 77 days PI (Fig. 1A). Mice infected with DP+CT135− exhibited infectious burdens similar to DP+CT135+ in the first week PI but in contrast eradicated infection by day 28 PI (Fig. 1B). DP−CT135+ infected mice exhibited significantly lower infectious burdens than the DP+CT135+ strain (P ≤ 0.046 at days 10, 14, and 28 PI) with infections resolving at 42 days PI, a duration in between that of the two plasmid bearing strains (Fig. 1C). Lastly, mice infected with DP−CT135− exhibited the most attenuated infection kinetics shedding significantly lower levels of Chlamydiae at four time points compared to the wild type virulent DP+CT135+ strain (P ≤ 0.043 at days 3, 10, 14, and 28 PI), and resolving their infection by day 28 PI (Fig. 1D). The absence of plasmid in the CT135− background resulted in significantly lower IFU at the earliest time point (P = 0.016 at day 3 PI). We conclude from these in vivo results that the chlamydial plasmid functions to enhance chlamydial growth, particularly at early time points PI; whereas CT135 functions in sustaining a more persistent or chronic infection. The mechanisms by which the plasmid and CT135 function to enhance these virulence characteristics are unknown but it is intriguing to speculate that it may involve different aspects of innate and adaptive immunity. Based on our findings, a logical future strategy for making a live-attenuated vaccine to prevent human chlamydial sexually transmitted infections would be to generate vaccine strains that are both plasmid and CT135 deficient.
Fig. 1. Infectivity of C. trachomatis serovar D strains deficient in plasmid, CT135 or both in C3H/HeJ female mice.
Six to eight female C3H/HeJ mice were infected cervico-vaginally with 1 × 105 IFU of the following C. trachomatis serovar D strains: (A) DP+CT135+, n=6; (B) DP+CT135−, n=8; (C) DP−CT135+, n=6; and (D) DP−CT135−, n=8. At days 3, 7, 10, 14, and weekly intervals thereafter cervico-vaginal specimens were collected and recoverable IFU enumerated by titration on monolayers of McCoy cells. The results show the mean chlamydial burden and duration of infection for each group over the entire period with standard deviations for each time point indicated.
ACKNOWLEDGEMENTS
We thank Kelly Matteson, Anita Mora, and Dan Sturdevant for editorial assistance, graphics preparation, and statistical analyses, respectively. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
In this paper a role for the Chlamydia trachomatis plasmid and CT135 of a urogenital strain, both known virulence factors, were evaluated independently and together in a female mouse genital tract infection model. We report that the plasmid and CT135 play distinct roles in the kinetics of infection and a strain possessing both genetic deficiencies is highly attenuated.
REFERENCES
- 1.Belland RJ, Nelson DE, Virok D, Crane DD, Hogan D, Sturdevant D, Beatty WL, Caldwell HD. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc Natl Acad Sci U S A. 2003;100:15971–15976. doi: 10.1073/pnas.2535394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31:1892–1897. doi: 10.1016/j.vaccine.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson JH, Whitmire WM, Crane DD, et al. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafner LM, McNeilly C. Vaccines for Chlamydia infections of the female genital tract. Future Microbiol. 2008;3:67–77. doi: 10.2217/17460913.3.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Kari L, Whitmire WM, Olivares-Zavaleta N, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. 2011;208:2217–2223. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–3428. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutter EI, Barger AC, Nair V, Hackstadt T. Chlamydia trachomatis inclusion membrane protein CT228 recruits elements of the myosin phosphatase pathway to regulate release mechanisms. Cell Rep. 2013;3:1921–1931. doi: 10.1016/j.celrep.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder JW. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- 10.O'Connell CM, Nicks KM. A plasmid-cured Chlamydia muridarium strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology. 2006;152:1601–1607. doi: 10.1099/mic.0.28658-0. [DOI] [PubMed] [Google Scholar]
- 11.Palmer L, Falkow S. A common plasmid of Chlamydia trachomatis. Plasmid. 1986;16:52–62. doi: 10.1016/0147-619x(86)90079-x. [DOI] [PubMed] [Google Scholar]
- 12.Rockey DD, Wang J, Lei L, Zhong G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines. 2009;8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 13.Schachter J. Chlamydial infections (First of three parts) N Engl J Med. 1978;298:428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 14.Sigar IM, Schripsema JH, Wang Y, et al. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis. 2013 doi: 10.1111/2049-632X.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L, Carlson JH, Whitmire WM, et al. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect Immun. 2013;81:636–644. doi: 10.1128/IAI.01305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens RS, Kalman S, Lammel C, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 17.Sturdevant GL, Kari L, Gardner DJ, et al. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun. 2010;78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Persson K, Bjartling C, Clarke IN. Genetic transformation of a clinical (genital tract), plasmid-free isolate of Chlamydia trachomatis: engineering the plasmid as a cloning vector. Plos One. 2013;8:e59195. doi: 10.1371/journal.pone.0059195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]