Abstract
Objectives
High fatigability, a dysfunctional adaption to fatigue, may lead to difficulties performing otherwise regularly encountered cognitive activities and may be related to pro-inflammatory reactivity. The purpose of the study was to investigate the effect of fatigability on cognitive processes and inflammatory response following an acute cognitive stress task in older adults.
Design
An observational laboratory stress reactivity study.
Setting
A light- and temperature-controlled laboratory.
Participants
Fifty-five community-dwelling individuals aged 75 years or older.
Measurements
We measured interleukin (IL)-6, self-reported acute fatigue, and frontally-oriented cognitive processes as part of a demanding set of cognitive tasks intended to induce stress.
Results
Subjects were classified into groups of low and high fatigability based on cluster analysis of their self-report acute fatigue before and after the cognitive tasks. The two clusters were comparable on levels of baseline IL-6 and cognitive processes; however, the high fatigability cluster had significantly higher levels of IL-6 response than the low fatigability cluster. After controlling for multiple covariates, fatigability moderated the relationship between speed of processing and IL-6 reactivity. Further exploratory analyses indicated significant adverse associations between speed of processing and attention and IL-6 reactivity in the group with low but not high fatigability.
Conclusions
While observational, these data are consistent with the notion that pro-inflammatory states in older adults might be reduced by improvements in cognitive processes. Since fatigability was associated with increased acute inflammatory response and disrupted the normal stress regulation provided by the cognitive processes, future randomized studies might examine whether fatigability alleviation reduces IL-6.
Keywords: Fatigability, aging, attention, speed of processing, executive function, interleukin-6 reactivity
Background
Stress reactivity, an individual's immediate emotional and/or physiologic response to a stressor, is a critical component of stress regulation, and independently predicts future health events (1). Immune response to stressors is an important part of stress reactivity (2). According to a meta-analysis on inflammatory reactivity to stress, the most consistently observed inducible response comes from interleukin (IL)-6 (3) and multiple mechanisms may help explain the peripheral inflammatory reactivity to acute stress (e.g., up-regulated synthesis of peripheral inflammatory markers, changes in plasma volume, and increases in the number of cytokine-synthesizing cells contributing to circulatory levels) (3). However, none of the mechanisms are related to the potential top-down regulatory role of the central nervous system in peripheral inflammatory reactivity.
Recent psychophysiological models of stress regulation suggest that the frontal-limbic network, which include several cortical regions (i.e., prefrontal cortex, anterior cingulate cortex, and insular cortex) and their communication with subcortical regions (i.e., hypothalamus and thalamus) and the hypothalamic-pituitary-adrenal (HPA) axis, plays a direct role in regulating physiological responses to stress. These cortical regions can affect the transmission of signals related to inflammatory response from two parallel pathways: vagal as well as spinal visceral and somatic sensory (4, 5). The degeneration of these cortical regions occurs early in both normal and abnormal aging processes and shifts regulation of inflammatory processes from a homeostatic balance between anti- and pro-inflammatory statuses in early life to a pro-inflammatory dominated status in late life (6, 7). Such an age-related shift may reduce psychophysiological adaptation to stressors and render older adults susceptible to exaggerated inflammatory responses to stressors, including peripheral inflammatory responses (8). Alterations in the function of these cortical regions, especially the prefrontal cortex, (e.g., applying mindfulness training) have been shown to decrease the peripheral pro-inflammatory cytokines gene expression in old age (9, 10). These cortical regions also mediate executive function (EF), speed of processing (SOP), and attention (11, 12). Williams et al. propose that these frontally-oriented cognitive processes are involved in the top-down regulation of individuals' stress responses, including peripheral inflammatory responses (13). However, this hypothesis has not been tested empirically.
Stress reactivity may not only be affected by cognitive function — conversely, assessments of cognitive function are often themselves perceived as stressors. In fact, in a meta-analysis of acute laboratory mental stress, cognitive assessments for skilled sequences, working memory, and sustained attention fall among the most commonly applied laboratory stressors in acute stress reactivity studies (14, 15). Older adults' perceptions of this stressor can be captured indirectly through assessments of fatigability, a dysfunctional adaption to fatigue (16). Fatigue is one of the most common somatic symptoms reported by older adults (17), resulting from a mismatch between task-related energy requirements and available energy resources (18). Emerging behavioral studies found that fatigability, especially in the format of self-report, is not necessarily related to the scores on cognitive tests themselves (19), but higher levels of fatigability elicit greater IL-6 reactivity (20). From a pathophysiological perspective, disturbed glucocorticoid receptor function and endocrine response, and/or unbalanced homeostasis due to bioenergic changes from the acute stress task (21, 22) may link fatigability with pro-inflammatory reactivity peripherally. From a neuro-anatomical perspective, perceived fatigability is reflected as a dysfunctional cerebral activity in the basal ganglia, involving contributions from the frontal cortex (including prefrontal cortex and anterior cingulate cortex), thalamus, and the amygdale (23). Given the overlap of fatigability-related cortical regions with frontal-limbic networks that attends to stress regulation, fatigability possibly affects peripheral inflammatory reactivity by disrupting the central regulation on peripheral inflammatory responses to acute stress (16). In the present study, we tested the hypothesis that fatigability would disrupt the effect of frontally-oriented cognitive processes on IL-6 response to acute stress.
There were three specific aims in the present study: (1) to characterize IL-6 reactivity and fatigability to the cognitive stress task; (2) to examine the association between frontally-oriented cognitive processes (i.e., EF, SOP, and attention) and IL-6 reactivity; and (3) to test fatigability as a moderator of the relationship between frontally-oriented cognitive processes and IL-6 reactivity.
Method
Design and Participants
An observational laboratory stress reactivity study was conducted. A total of 55 study participants were recruited from local community senior centers in a medium-sized Northeastern U.S. city. Inclusion criteria were: (1) English speaking, (2) aged 75 years or older, (3) self-reported adequate auditory and visual acuity for testing, and (4) community-dwelling. Exclusion criteria were: (1) Self- or clinician-reported clinically diagnosed dementia or mild cognitive impairment, (2) treatment with any cholinesterase inhibitors (e.g., donepezil, galatamine, rivastigmine) or Memantine within three years, and (3) self-reported history of stroke, clinical sleep disorders, or major depression. The study was approved by the University affiliated Institutional Review Board.
Procedure
All testing took place by trained master-prepared research assistants in the CogT Study Laboratory (PI: Lin) at the University of Rochester. During the visit to the light- and temperature-controlled laboratory, the participant was first asked to sit quietly and relax for 5 to 10 minutes in order to adapt to the environment. The participant then completed a trait fatigue questionnaire and cognitive tests. Self-report acute fatigue was assessed before and immediately after cognitive tests. The blood sample (for IL-6) was collected immediately before cognitive tests (baseline), and 20 minutes after cognitive tests (approximately 50 minutes after the baseline sample was collected). To control for the diurnal fluctuation of IL-6 level, we attempted to arrange interviews within a two hour window (8 – 10 A.M.). However, there were eleven participants (20%), a random subgroup, who completed interviews at 1 P.M. A comparison between the IL-6 data collected in the A.M. and the IL-6 data collected in the P.M. was conducted (see “Results” section). All other demographic and health data were obtained after cognitive tests.
Measurements
Frontally-oriented cognitive processes. A series of cognitive tests designed to measure specific aspects of frontally-oriented cognition known to be affected by aging (7) were administered. These tests included the Trail Making Test, parts A and B (24), The Stroop Color Word Test (25), the Wechsler Memory Scale-III (26), Digit Span Forward and Backward subtests (27), and Visual and Auditory versions of a 1-back working memory test (28). These tests are commonly used in clinical settings (12, 29), and took approximately 30 minutes to administer. Nine performance scores were calculated separately from the following tests: Trail Making Test A, Trail Making Test B, Stroop Word (total words read), Stroop Color (total colors named), Stroop Interference (total colors named), Digit Span Forward (span length), Digit Span Backward (span length), Vision-based 1-Back accuracy rate (% correct), and Audio-based 1-Back accuracy rate (% correct). To be consistent to other tests, for Trail Making Test A and B, the completion time was reversed (i.e., 300 – raw score, where 300 seconds were the maximum time limit for both test A and B) with higher scores indicating better cognitive performance. Each score was standardized to Z-scores, separately. Three composite scores were calculated to represent three cognitive domains (SOP, Attention, EF) by averaging the Z-scores as follows: SOP (Trail Making Test A and Stroop Color), Attention (Stroop Word, Digit Span Forward, Vision-based 1-Back accuracy rate, and Audio-based 1-Back accuracy rate), and EF (Trail Making Test B, Stroop Interference, and Digit Span Backward). This theoretical grouping is commonly used in the neuropsychology literature (30–32).
Fatigability was operationalized as the change between self-reported acute fatigue before and after the series of cognitive tests. Both before and after the cognitive tests, participants were presented with an acute fatigue rating scale (visual analogue scale to evaluate fatigue severity, VAS-F) consisting of 18 items measuring varying aspects of fatigue (e.g., “concentrating is a tremendous chore”, “energetic”, etc.), and they indicated their response by placing a mark on a 10-cm analogue rating line ranging from 0 cm (not at all) to 10 cm (extremely) (33). The length of line between 0 and the participant's mark indicated the level of acute fatigue and was recorded for each item. A mean score was developed for the 18 items with higher scores indicating higher levels of acute fatigue. In the present study, the Cronbach's α for the acute fatigue measure before and after the cognitive tasks were 0.88 and 0.94, respectively. This scale has been validated in adults with and without chronic illnesses across a wide range of ages (34).
IL-6 Assay. A capillary blood sample was collected in the form of a dried blood spot (DBS). The DBS technique of sample collection has been described as suitable for assaying a wide range of chemokines and cytokines, including IL-6 (e.g., (35, 36)). The blood was obtained from a finger prick made with a lancet (2.8mm depth, 21 gauge). At each time point (baseline and 50 minutes follow-up), five blood drops were absorbed, one drop at a time, onto filter paper (903 Protein Saver, Watman), and then dried at room temperature for a minimum of 4 hours. Then the paper was stored at −80°C in an air-tight plastic bag with a desiccant packet until analysis. The analysis was performed using the Quantikine HS ELISA Human IL-6 kit (R&D Systems), using a previously published method with modification (36). A calibration curve specific for a DBS sample was prepared using erythrocytes mixed with calibrator diluent containing serial dilutions of human recombinant IL-6 to create concentrations from 0 to 25 pg/ml. For elution, 6mm disks were punched from DBS standards, samples, and controls, and placed into 96-well plate. Two hundred microliters of elution buffer (Tris-buffered saline, 0.1% Tween-20) were added to each well, and the plate was sealed with adhesive tape and incubated overnight at 4°C, then at room temperature on a rotary shaker (100 rounds per minute) for 30 minutes. Then the eluate was transferred onto an ELISA plate provided with the kit, and the ELISA was performed according to manufacturer's instructions. The IL-6 concentration in patient samples was calculated from a 5-point fit standard curve. The detection limit of the assay as performed in our laboratory was 0.37 pg/ml (concentration corresponding to the optical density which was 2 standard deviations greater than the mean of 10 replicates of calibrator containing 0 pg/ml IL-6). For the samples assayed in duplicates the correlation between duplicates was high (Pearson's correlation r = 0.95).
A total of 46 participants had measurable IL-6 at both baseline and 50 minute follow-up. One participant's IL-6 level at baseline was >10 pg/ml. We considered this value an outlier indicating potential acute inflammation or infection, and this participant's IL-6 data was excluded from the analysis. We compared participants with (n = 45) and without (n = 10) IL-6 data, and they did not differ in any demographic and health characteristics.
Other demographic characteristics and health variables Age, gender, years of education, and race/ethnicity were collected via self-report. Trait fatigue was measured by a mean score of the 20-item Multidimensional Fatigue Inventory (37), which captures five domains of trait of fatigue in individuals' daily lives: mental fatigue, physical fatigue, general fatigue, reduced motivation, and reduced activities. Participants responded using a scale from 1 “Yes, that is true” to 7 “No, that is not true”. Higher scores indicated high level of trait fatigue. Internal consistency for this measure was 0.89 in this study. Depressive symptoms were measured by the 15-item Geriatric Depression Scale (GDS) (38). Participants responded to questions related to their depressive symptoms during the past week using “yes” or “no”. A total depressive symptom score was calculated as the total number of answers indicating potentially depressive symptoms. Sleepiness was measured by the 8-item Epworth scale (39). Participants responded to questions related to their sleepiness (in contrast to feeling just tired) under different situations (e.g., sitting and reading) using a scale ranging from 0 “would never doze” to 3 “high chance of dozing”. A mean score was computed with higher scores indicating more sleepiness. Internal consistency of the scale was 0.68 in this study. Participants' health conditions (hypertension, high cholesterol, diabetes, obesity) were obtained by self report. Their medications, specifically anti-inflammatory (e.g., aspirin, ibuprofen, and naproxen) and beta-blockers (e.g., Atenolol, Propranolol, and Metoprolol) were extracted from the medication list participants brought to the study.
Data analysis
Analyses were conducted using IBM SPSS 19.0. Descriptive statistics were first computed. Change of IL-6 from baseline to 50 minutes follow-up was analyzed using a paired t test. To classify the level of fatigability in response to the cognitive tests, a cluster analysis using both self-report acute fatigue rating before and after the cognitive tests was performed in two steps as suggested by Clatworthy and colleagues (40), who showed the method was viable in small samples (i.e., as low as the low 40s). First, a Hierarchical Cluster Analysis using Ward's Method identified the number of homogenous clusters. The dendrogram plot was examined to determine the number of clusters (2 clusters in this study). Second, using the number of clusters identified in step 1, a K-means Cluster Analysis of the two fatigue variables was performed. These variables had relatively normal distributions (kurtosis: 1.44 and −0.06, respectively; skewness: 0.80 and 0.63, respectively). After the two steps, the 55 participants were classified into one of the two fatigability clusters.
To compare the main variables and covariates by fatigability cluster, independent t-tests and χ2 tests were used for continuous and categorical variables, respectively. Analysis of covariance (ANCOVA) was used if any confounding factors needed to be controlled. To examine the association of IL-6 response with demographic and health variables, Pearson's r was used for continuous variables and Spearman's ρ for categorical variables.
To examine the association of frontally-oriented cognitive processes and fatigability, as well as their relationships with IL-6 response, Generalized Linear Models (GLM) were applied, setting low fatigability cluster as a reference group. The equation was: YIL-6 response = β0 + β1confoudning factor 1… + βn confounding factor n + β(n+1) domain of cognitive processes + β(n+2) fatigability + β(n+3) domain of cognitive processes× fatigability + εdomain of cognitive processes. The interaction term in this model provided the statistical test of whether the two fatigability groups differed in IL-6 response. After performing the formal test of interaction, we then examined the association between cognitive processes and IL-6 response within the two fatigability clusters separately to estimate the associations in each cluster. Equations were similar to the ones described above without the term of fatigability or fatigability × cognitive processes. In GLM analyses, age, gender, anti-inflammation medication, beta-blocker, depression, sleepiness, trait fatigue, and baseline IL-6 were confounding factors.
Unless specifically defined as raw data, IL-6 data at baseline and 50 minutes follow-up were log-transformed. IL-6 response was computed as discrepancy score of IL-6 raw data at 50 minutes follow-up and baseline. Statistical significance for all analyses was set at P < 0.05.
Results
Sample Characteristics
The average age of participants was 82.95 years and 43.6% were male. The level of education for the sample was equivalent to a college degree. All participants were White. With respect to health characteristics, hypertension was the most prevalent vascular risk factor, and participants reported low levels of sleepiness, trait fatigue, and few depressive symptoms.
IL-6 Reactivity
For the entire sample, there was a small change between baseline IL-6 (raw data: Mean = 1.75 pg/ml, SD = 1.34) and IL-6 at 50 minutes follow-up (raw data: Mean = 2.08 pg/ml, SD = 1.86) (paired t = −1.88, df = 44, p = .066). We also examined whether IL-6 level was different by the participation time (A.M. vs. P.M.); there was no significant difference in IL-6 at baseline (AM: Mean = 0.39, SD = 0.70; PM: Mean = 0.19, SD = 0.78; t = 0.80, p = 0.43), 50 minutes follow-up (AM: Mean = 0.47, SD = 0.85; PM: Mean = 0.08, SD = 1.13; t = 1.23, p = 0.22), or IL-6 response (AM: Mean = 0.32, SD = 0.66; PM: Mean = 0.36, SD = 2.17; t = −0.10, p = 0.92).
Fatigability Clusters
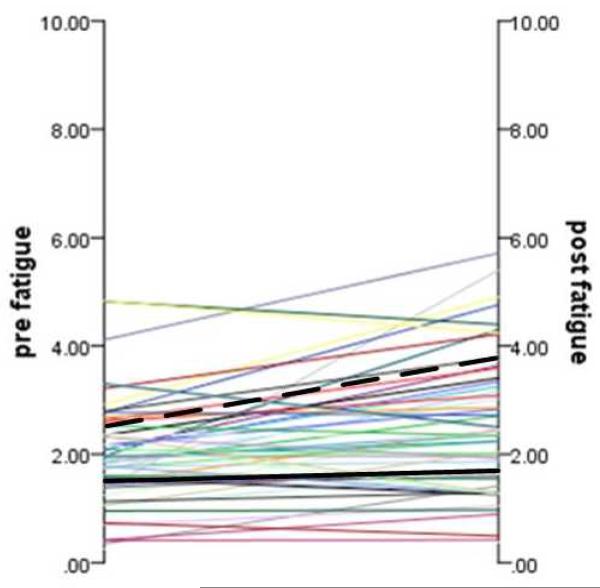
Two clusters were identified (Figure 1). One group (n = 33) had low levels of fatigue before the cognitive tests (Mean = 1.49, SD = 0.62), which remained low after the cognitive tests (Mean = 1.65, SD = 0.62). The change in acute fatigue level before and after the cognitive tests was non-significant for this group (paired t test = −1.79, df = 32, p = 0.08). This cluster was labeled “low fatigability”. The other group (n = 22) had moderate levels of acute fatigue before the cognitive tests (Mean = 2.75, SD = 0.91), which increased after cognitive tests (Mean = 3.71, SD = 0.92). The change in acute fatigue level before and after the cognitive tests was significant for this group (paired t test = −3.94, df = 21, p = 0.001). This cluster was labeled as “high fatigability”.
Figure 1.
Individual and Cluster Trajectory of Fatigability before and after Fatigue-Manipulation Tasks. Black solid line represents the “low fatigability” cluster, and the black dash line represents the “high fatigability” cluster. Other lines are individual trajectories.
There were significantly more male participants in the low fatigability cluster than in the high fatigability cluster (χ2 = 6.52, p = .014). Participants in the low fatigability cluster had significantly lower levels of trait fatigue (t = −2.52, p = .015) and depressive symptoms (t = −3.22, p = .002) compared to those in the high fatigability cluster. The two clusters did not differ in other demographic parameters, health characteristics, or any domains of cognitive processes (see Table 1).
Table 1.
Demographic and Health Characteristics as a Total Sample and By Fatigability Cluster
| Total (n = 55) | By Cluster | ||||
|---|---|---|---|---|---|
| Low fatigability (n = 33) | High fatigability (n = 22) | t or χ2 or F test value (p value) | df | ||
| Age, Mean (SD) | 82.95 (3.17) | 82.30 (2.85) | 83.91 (3.45) | −1.88 (0.07) | 53 |
| Years of education, Mean (SD) | 15.80 (2.15) | 15.94 (2.15) | 15.59 (2.18) | 0.59 (0.56) | 53 |
| Male, n (%) | 24 (43.6%) | 19 (57.6%) | 5 (22.7%) | 6.52 (0.011) | 1 |
| White, n (%) | 55 (100%) | 33 (100%) | 22 (100%) | n/a | n/a |
| Diabetes, n (%) | 8 (14.5%) | 6 (18.2%) | 2 (9.1%) | 0.88 (0.35) | 1 |
| Hypertension, n (%) | 49 (89.1%) | 30 (90.9%) | 19 (86.4%) | 0.28 (0.60) | 1 |
| High cholesterol, n (%) | 43 (79.6%) | 28 (84.8%) | 15 (71.4%) | 1.43 (0.23) | 1 |
| Obesity, n (%) | 6 (10.9%) | 2 (6.1%) | 4 (18.2%) | 2.00 (0.20) | 1 |
| Anti-inflammatory medication, n (%) | 32 (58.2%) | 22 (66.7%) | 10 (45.5%) | 2.44 (0.12) | 1 |
| Beta-blocker, n (%) | 31 (56.4%) | 19 (57.6%) | 12 (54.5%) | 0.05 (0.82) | 1 |
| Sleepiness, Mean (SD) | 0.81 (0.40) | 0.77 (0.40) | 0.87 (0.42) | −0.87 (0.39) | 53 |
| Depressive symptoms, Mean (SD) | 0.85 (1.22) | 0.71 (0.12) | 1.57 (0.33) | −2.81 (0.009) # | 27 |
| Chronic fatigue | 2.58 (0.87) | 2.35 (0.86) | 2.93 (0.80) | −2.52 (0.015) | 53 |
| EF ‡ | 0 (0.63) | 0.18 (0.51) | −0.26 (0.72) | 3.26 (0.08) b | 1, 54 |
| Attention ‡ | 0 (0.73) | 0.10 (0.41) | −0.19 (0.72) | 3.33 (0.07) b | 1, 54 |
| SOP ‡ | 0 (0.78) | 0.02 (0.68) | −0.05 (0.93) | 0.02 (0.88) b | 1, 54 |
| Baseline IL-6 †, a | 0.34 (0.71) | 0.27 (0.77) | 0.44 (0.63) | 2.42 (0.13) c, # | 1, 44 |
| IL-6 response a | 0.33 (1.18) | 0.13 (0.72) | 0.64 (1.63) | 4.17 (0.048) d, # | 1, 44 |
Note.
controlled for age, gender, and education;
log-transformed;
45 participants' data were included;
controlled for age, gender, and years of education;
controlled for age, gender, anti-inflammatory medication;
controlled for age, gender, anti-inflammatory medication and baseline IL-6;
Levene's test significance > .05.
EF = executive function, comprised by Trail Making Test B, Stroop Interference, and Digit Span Backward;
Attention: comprised by Stroop Word, Digit Span Forward, Vision-based 1-Back accuracy rate, and Audio-based 1-Back accuracy rate;
SOP = speed of processing, comprised by Trail Making Test A and Stroop Color.
Difference of IL-6 at baseline and IL-6 response by Fatigability Cluster
There were 23 participants (60%) who showed increased IL-6 in response to cognitive tests. We examined the correlations between IL-6 response and demographic and health variables, and the only significant association was with anti-inflammatory medication (Spearman's ρ = 0.41, p = 0.005).
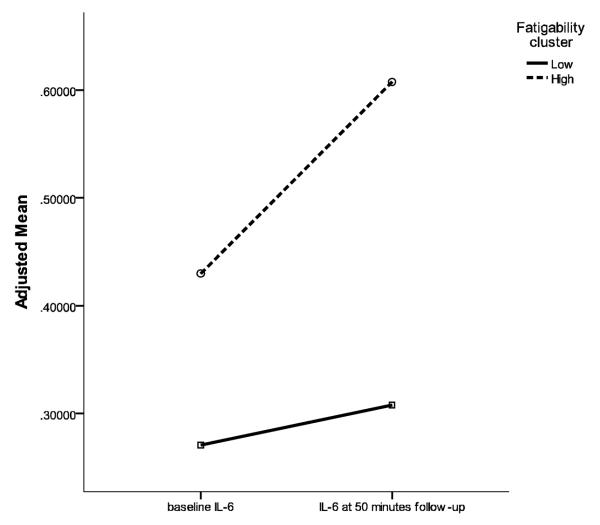
Table 1 displays the IL-6 plasma concentrations in the two fatigability clusters, which did not differ in baseline IL-6. We examined the cognitive test-related change of IL-6 response by fatigability cluster. Controlling for age, gender, anti-inflammatory medication, depression, and baseline IL-6, the high fatigability cluster had a significantly greater change in IL-6 response (M = 0.64, SD = 1.63) than the low fatigability cluster (M = 0.13, SD = 0.72) (F(1, 42) = 5.32, p = 0.027). Figure 2 displays the change of IL-6 at baseline and 50 minute follow-up by fatigability cluster.
Figure 2.
Relationship between Fatigue and IL-6 before and after Cognitive Tests. Note. IL-6 data were log transformed. Adjusted Mean indicates IL-6 data presented were controlled for age, gender, anti-inflammatory medication, and depressive symptoms.
Frontally-oriented Cognitive Processes and IL-6 Response
After controlling for age, gender, anti-inflammation medication, beta-blocker, depression, sleepiness, trait fatigue, and baseline IL-6, the main effects of the cognitive processes (i.e., EF, SOP and attention) on IL-6 response were non-significant (Model a, Table 2).
Table 2.
Interactions between Cognitive Processes and Fatigability Cluster on IL-6 Response (N = 44). †
| Model a (df1 = 1, df2 = 33) | Model b (df1 = 1, df2 = 31) | |||||
|---|---|---|---|---|---|---|
| B (SE) | 95% CI | p value | B (SE) | 95% CI | p value | |
| EF | −0.08 (0.12) | −0.32, 0.15 | .49 | −0.21 (0.19) | −0.57, 0.16 | .27 |
| Fatigability ‡ | 0.33 (0.17) | −0.001, 0.66 | .05 | |||
| EF × Fatigability ‡ | 0.28 (0.24) | −0.19, 0.75 | .25 | |||
| SOP | −0.11 (0.10) | −0.31, 0.10 | .30 | −0.34 (0.13) | −0.60, −0.08 | .010 |
| Fatigability ‡ | 0.42 (0.16) | 0.11, 0.73 | .009 | |||
| SOP × Fatigability ‡ | 0.39 (0.17) | 0.05, 0.74 | .027 | |||
| Attention | −0.16 (0.10) | −0.36, 0.04 | .11 | −0.25 (0.12) | −0.50, −0.01 | .041 |
| Fatigability ‡ | 0.32 (0.16) | −0.002, 0.64 | .05 | |||
| Attention × Fatigability ‡ | 0.29 (0.19) | −0.07, 0.66 | .12 | |||
Note. p value was generated using F test.
controlled for age, gender, anti-inflammation medication, beta-blocker, depressive symptoms, sleepiness, chronic fatigue, and baseline IL-6.
Low fatigability cluster was the reference.
CI = confidential interval; EF = executive function; SOP = speed of processing
Interaction of Cognitive Processes and Fatigability on IL-6 Response
There was a significant interaction effect of SOP (not EF or attention) and fatigability on IL-6 response (Model b, Table 2). To clarify the interaction effect, we examined the association between SOP and IL-6 response within the two fatigability clusters, controlling for age, gender, anti-inflammation medication, beta-blocker, depression, sleepiness, chronic fatigue, and baseline IL-6 (see Model b, Table 3). As seen in Figure 3b, there was a significant association between lower SOP and higher IL-6 response in the low fatigability cluster; there was no association between SOP and IL-6 response in the high fatigability cluster.
Table 3.
Relationship between Cognitive Processes and IL-6 Responses by Fatigability Cluster †
| Low Fatigability (n = 26) (df1 = 1, df2 = 16) | High Fatigability (n = 18) (df1 = 1, df2 = 8) | |||||
|---|---|---|---|---|---|---|
| B (SE) | 95% CI | p value | B (SE) | 95% CI | p value | |
| Model a: EF | −0.14 (0.21) | −0.55, 0.27 | .51 | 0.06 (0.12) | −0.18, 0.30 | .63 |
| Model b: SOP | −0.35 (0.15) | −0.64, −0.07 | .016 | −0.05 (0.13) | −0.30, 0.20 | .69 |
| Model c: Attention | −0.31 (0.12) | −0.56, −0.07 | .013 | −0.01 (0.14) | −0.28, 0.26 | .97 |
Note. p value was generated using F test.
controlled for age, gender, anti-inflammation medication, beta-blocker, depressive symptoms, sleepiness, chronic fatigue, and baseline IL-6.
CI = confidential interval; EF = executive function; SOP = speed of processing
Figure 3.
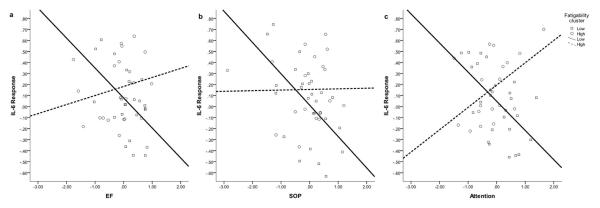
Relationship between Cognitive Processes and IL-6 Responses by Fatigability Cluster. Note. IL-6 Response in the figures was adjusted for age, gender, anti-inflammation medication, beta-blocker, depression, sleepiness, and baseline IL-6. EF = executive function, SOP = speed of processing.
We also further examined the association between attention and EF and IL-6 response by fatigability cluster (see Model a and Model c, Table 3). As seen in Figure 3a, there was no association between EF and IL-6 response in low or high fatigability cluster. As seen in Figure 3c, there was a significant association between lower attention and higher IL-6 reactivity in the low, but not high, fatigability cluster.
Discussion
In the present study, we found a small, but not statistically significant (p = 0.066), change of IL-6 from baseline to 20 minutes after a set of acute cognitively stressful tasks (i.e., 30 minutes of cognitive testing). We were able to identify two clusters of fatigability based on individuals' self-reported fatigue at baseline and after the series of cognitive tests. One group had low levels of fatigue at baseline which did not increase significantly over time, and the other had relatively high levels of fatigue at baseline which increased significantly over time. The two fatigability clusters had comparable frontally-oriented cognitive performance (i.e., EF, SOP, attention) and IL-6 levels at baseline; however, they significantly differed in their IL-6 reactivity with the high fatigability group showing greater IL-6 reactivity. There was no association between frontally-oriented cognitive processes and IL-6 reactivity as a total sample. However, we found that fatigability moderated the relationship between cognitive processes, especially SOP, and IL-6 reactivity. When further analyzing the associations between cognitive processes and IL-6 reactivity by fatigability cluster, in addition to SOP, attention was also significantly negatively associated with IL-6 reactivity in lower, but not higher, fatigability cluster. All these effects were independent from the influence of age, gender, sleepiness, depressive symptoms, vascular risk factors, trait fatigue, and baseline IL-6.
Most of the literature on IL-6 response to acute stress suggests that the increase of IL-6 is delayed and may be most apparent two hours following exposure to an acute stressor (41). In this study, the average increase of IL-6 from baseline to 50 minute follow-up was approximately 0.33 pg/mL for the total sample, and although not statistically significant at our small sample size, the change was comparable to that of previous studies with a similar interval between stressor and IL-6 measurement (41). We then identified two fatigability clusters which distinguish the IL-6 response at 50 minutes follow-up (or 20 minutes after the acute stress). These findings suggest that perceived fatigability may predict inflammatory reactivity to stress, adding new information to support the notion that fatigue may signal pro-inflammatory processes (42). Importantly, although vascular risks were prevalent in this sample, they did not significantly differ between the two fatigability clusters and were even controlled in analyses, indicating that vascular risk factors are probably not the cause of the difference in IL-6 reactivity. However, such assumption may need further testing, given the lack of objective measures of these vascular risks (e.g., body mass index, glucose, blood pressure). Since fatigability is a dynamic adaptation to an acute stressor, it is not surprising that IL-6 at baseline, which reflects more chronic states of stress, did not differ between the two fatigability clusters.
An important finding of the present study is that fatigability was in particular found to be a significant moderator of SOP's effect on IL-6 response. Of note, SOP is not a simple psychomotor ability, but a fundamental brain process related to temporary information manipulation (43, 44). According to a recent review, SOP reflects the integrity of multiple neural networks involved in other levels or domains of cognitive processes (e.g., attention, EF) and most higher-order cognitive functions (e.g., memory, reasoning, and language) (11). Previous studies found that, increased fatigability can specifically affect medial and lateral frontal cortex independent of basal ganglia inputs (23), while atrophy of these frontal regions often occurs early in individuals with decreased SOP, making these regions particularly vulnerable to adverse impact of fatigability (11). It is possible that fatigability will first and primarily influence this most fundamental and vulnerable cognitive process by disrupting the control of SOP on different formats of stress responses.
The other interesting exploratory finding here is the dissociable patterns of association between frontally-oriented cognitive processes and inflammatory reactivity by fatigability cluster. That is, the link between cognitive processes and IL-6 response exists only in individuals who perceive little conflict between intrinsic energy resources and the apparent demands of the stressor. Specifically, among persons who do not easily fatigue, greater SOP and attention were associated with lower IL-6 reactivity. In this group, persons who scored higher on these cognitive tests may have better relevant brain functions on stress regulation. By contrasts, no association between cognitive scores and IL-6 reactivity was observed among easily fatigable individuals. Among these already fatigued persons, other processes may “overpower” the role cognitive performance plays in predicting IL-6 reactivity. For instance, other factors implicated in elevating IL-6 include poor physical (i.e., number of chronic conditions, physical frailty) and psychological health (i.e., low levels of well-being, negative affect) (45–47). Although our study had strict selection criteria to exclude individuals with poor health on some indices, this explanation cannot be ruled out. Variation among non-exclusionary criteria might trump the impact of cognitive processes on IL-6 response among fatigued persons. A second possibility is that there may be a high rate of neuropathological deficits in how basal ganglia work with the limbic system in the easily fatigable group. When self appraised fatigability is high, the functional connectivity of the brain regions that adapt to acute stress and diminish acute inflammatory response may be disrupted. The potential regulatory effect of cognitive processes on acute inflammatory processes can thus be affected (23). In contrast, the lack of significant finding in the domain of EF may be related to the tests we used. Although Trail Making Test B, Stroop Interference, and Digit Span Backward are all commonly applied tests for different aspects of EF (e.g., inhibition, sequencing skills, working memory) (12), some other more time-consuming tests (e.g., Wisconsin Card Sorting test), may have potential to induce greater fatigability that interfere with EF, a relatively upper level cognitive organization.
In addition to the limitations discussed above, some other limitations of the study design may also affect our interpretations. First, a small proportion of participants were interviewed beyond the 2 hour window (8 – 10 am). We did not find a statistically significant difference in IL-6 levels between different data collection points (A.M. vs. P.M.) among participants, and time of day was random with respect to cognitive function and fatigability, and previous literature suggests the IL-6 may exhibit a diurnal rhythm that follows the sleep/wake cycle, which is not necessarily related or reflected in a morning-early afternoon difference (48). Regardless, future studies may still consider holding to a strict two-hour window for consistency. Second, as a study using cognitive testing as an acute stressor, we were not able to completely disentangle fatigability from IL-6 response since they were measured contemporaneously rather than serially. That is, the relations between IL-6 reactivity and fatigability as well as IL-6 reactivity and cognitive capacity may be bi-directional (49). Future studies may temporally separate the stressor induce fatigability and IL-6 response from the cognitive tests. Third, we found sexual differences in fatigability. However, the existing studies report inconsistent findings regarding the relationships (50–52). Future studies with larger sample sizes might separate the examination of fatigability, or the effect of fatigability, by sex instead of simply utilizing it as a covariate. Finally, we excluded individuals with major depression or clinical sleep disorders from the study to isolate fatigability by design. However, given the theoretically high correlations between fatigue, sleepiness, and depression (16), it will be worthwhile to explore whether these three phenomenon are jointly associated with the cognition-inflammatory response link.
Inflammation is recognized as a potent risk factor for psychiatric and physical morbidity and mortality in older adults. It is therefore important to understand the potentially modifiable contributors to not only chronic, but also stress-induced levels of pro-inflammatory cytokines. Poor performance on cognitive tasks supported by the frontal lobe may be one potential contributor, or at least predictive sign, in some individuals. Should future work establish a causal connection, attention and SOP may be plausible targets for interventions designed to ameliorate inflammation, among individuals with low fatigue. For older adults who easily feel fatigable, scrutinizing potential neuropathological changes in relevant brain networks may be valuable. Finally, because fatigability was associated with increased acute inflammatory response and disrupted stress regulation provided by cognitive processes, future studies should also clarify the causal role of fatigability, if any, and consider fatigue interventions as appropriate.
Acknowledgement
Data collection of the present project was supported by Sigma Theta Tau International Small Grants to F. Lin. The manuscript preparation of the project was supported by the University of Rochester CTSA award number KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F. Lin, and K08AG031328 and R01AG044588 to B. Chapman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, U.S. government, or the Department of Veterans Affairs.
Footnotes
The authors report no personal or financial disclosures.
References
- 1.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AG, Goehler LE, Galper DI, et al. Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 2010;6:29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 7.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci. 2013;8:15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suchy Y. Executive functioning: overview, assessment, and research issues for non-neuropsychologists. Ann Behav Med. 2009;37:106–116. doi: 10.1007/s12160-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 13.Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- 14.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 15.Heffner KL, Ng HM, Suhr JA, et al. Sleep Disturbance and Older Adults' Inflammatory Responses to Acute Stress. Am J Geriatr Psychiatry. 2012 doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avlund K. Fatigue in older adults: an early indicator of the aging process? Aging Clin Exp Res. 2010;22:100–115. doi: 10.1007/BF03324782. [DOI] [PubMed] [Google Scholar]
- 18.Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2011;58:967–975. doi: 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leavitt VM, DeLuca J. Central fatigue: issues related to cognition, mood and behavior, and psychiatric diagnoses. PM R. 2011;2:332–337. doi: 10.1016/j.pmrj.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Geiss A, Rohleder N, Anton F. Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology. 2012;37:671–684. doi: 10.1016/j.psyneuen.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 22.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2011;2:406–413. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca J, Genova HM, Capili EJ, et al. Functional neuroimaging of fatigue. Phys Med Rehabil Clin N Am. 2009;20:325–337. doi: 10.1016/j.pmr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–276. [Google Scholar]
- 25.Golden CJ, Freshwater SM. Stroop color and word test. 1978. [Google Scholar]
- 26.Wechsler D. Manual for the Wechsler adult intelligence scale-revised (WAIS-R) The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 27.Guarch J, Marcos T, Salamero M, et al. Neuropsychological markers of dementia in patients with memory complaints. Int J Geriatr Psychiatry. 2004;19:352–358. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- 28.Pashler H. Dual-task interference in simple tasks: Data and theory. Psychological Bulletin. 1994;116:220. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 29.Bossers WJ, van der Woude LH, Boersma F, et al. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: a systematic review. Dement Geriatr Cogn Dis Extra. 2012;2:589–609. doi: 10.1159/000345038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin F, Heffner KL, Mapstone M, et al. Frequency of Mentally Stimulating Activities Modifies Cardiac Reactivity to Acute Mental Stress in Old Age. The American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachman ME, Agrigoroaei S, Murphy C, et al. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2011;18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 34.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: A practical guide for clinicians and researchers. Journal of Psychosomatic Research. 2004;56:157–170. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 35.Skogstrand K, Ekelund CK, Thorsen P, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Miller EM, McDade TW. A highly sensitive immunoassay for interleukin-6 in dried blood spots. Am J Hum Biol. 2012;24:863–865. doi: 10.1002/ajhb.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smets EMA, Garssen B, Bonke B, et al. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical gerontologist. 1986;5:165–173. [Google Scholar]
- 39.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 40.Clatworthy J, Hankins M, Buick D, et al. Cluster analysis in illness perception research: A Monte Carlo study to identify the most appropriate method. Psychology & Health. 2007;22:123–142. [Google Scholar]
- 41.Brydon L, Edwards S, Mohamed-Ali V, et al. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96. doi: 10.1111/j.1749-6632.2012.06634.x. [DOI] [PubMed] [Google Scholar]
- 43.Lin F, Chen D, Vance DE, et al. Trajectories of Combined Laboratory- and Real World-based Speed of Processing in Community-Dwelling Older adults: Predictors and Functional Outcomes Journal of Gerontology: Psychological Sciences and Social Sciences. 2012 doi: 10.1093/geronb/gbs075. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 45.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 47.Friedman EM, Hayney M, Love GD, et al. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Bixler EO, Lin HM, et al. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 49.Poon DC, Ho YS, Chiu K, et al. Cytokines: how important are they in mediating sickness? Neurosci Biobehav Rev. 2013;37:1–10. doi: 10.1016/j.neubiorev.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Allman BL, Rice CL. Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve. 2002;25:785–796. doi: 10.1002/mus.10116. [DOI] [PubMed] [Google Scholar]
- 51.Edwards KM, Burns VE, Ring C, et al. Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol. 2006;71:236–239. doi: 10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Steptoe A, Owen N, Kunz-Ebrecht S, et al. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]