Abstract
Purpose of review
Renal disease remains an important cause of morbidity and mortality in scleroderma. The spectrum of renal complications in systemic sclerosis includes scleroderma renal crisis (SRC), normotensive renal crisis, antineutrophil cytoplasmic antibodies-associated glomerulonephritis, penacillamine-associated renal disease, and reduced renal functional reserves manifested by proteinuria, microalbuminuria, or isolated reduction in glomerular filtration rate. The purpose of this review is to provide a concise and up-to-date review of the evaluation, risk stratification, pathogenesis, and management of scleroderma-associated renal disease.
Recent findings
Although SRC survival has significantly improved, mortality of this complication remains high outside of specialized centers. Recent data demonstrate strong associations between anti-RNA polymerase III antibodies and SRC. Subclinical renal impairment affects approximately 50% of scleroderma patients and may be associated with other vascular manifestations. Subclinical renal involvement rarely progresses to end-stage renal failure; however, recent studies suggest it may predict mortality in patients with other vasculopathic manifestations.
Summary
Testing for anti-RNA polymerase III antibodies should be incorporated into clinical care to identify patients at high risk for SRC. Recommendations from European League Against Rheumatism (EULAR), EULAR Scleroderma Trials and Research, and the Scleroderma Clinical Trials Consortium confirm angiotensin-converting enzyme inhibitors as first-line therapy for SRC, and give recommendations for second-line agents.
Keywords: autoantibodies, kidney, renal, scleroderma, scleroderma renal crisis
INTRODUCTION
Scleroderma is a rare multisystem immune disease affecting one to two patients per 100 000 in the United States. It is characterized by immune activation, autoantibody production, fibroblast dysfunction, extracellular matrix deposition, and vasculopathy. Clinically patients with scleroderma are categorized into limited and diffuse subsets on the basis of the extent of skin involvement [1,2]. These clinical phenotypes along with autoantibody profiles are helpful in predicting clinical outcomes [3,4]. Renal disease, particularly scleroderma renal crisis (SRC), adversely correlates with mortality in scleroderma [5–7].
The purpose of this review is to discuss the subtypes of renal involvement in scleroderma (Table 1) and to review the evaluation, risk stratification, pathogenesis, and management of scleroderma-associated renal disease.
Table 1.
Spectrum and associations of renal complications in scleroderma
| Renal complication | Associations |
|---|---|
| Scleroderma renal crisis | Rapidly progressive skin disease |
| Tendon friction rubs | |
| Corticosteroid exposure (≥15 mg per day in preceding 6 months) | |
| Anti-RNA polymerase III antibodies | |
| HLA-DRB1*0407 | |
| HLA-DRB1*1304 | |
| Normotensive renal crisis | Prior use of ACEi and calcium channel blockers |
| Myocardial involvement | |
| ANCA-associated glomerulonephritis | MPO-ANCA |
| Longstanding limited scleroderma | |
| Penicillamine-associated renal disease | 20% of patients treated with D-Penicillamine |
| Proteinuria and microalbuminuria | Reported in 25% of scleroderma patients |
| Isolated reduction in glomerular filtration rate | Correlates with vasculopathic manifestations and prognosis in scleroderma |
ACEi, angiotensin-converting enzyme inhibitors; MPO-ANCA, myeloperoxidase-specific antineutrophil cytoplasmic antibodies.
SCLERODERMA RENAL CRISIS
SRC remains one of the most feared complications of scleroderma.
Prevalence
Prevalence of SRC is decreasing, perhaps related to aggressive early therapy with angiotensin-converting enzyme inhibitors (ACEi). However, in the United States, SRC affects approximately 10% of patients with diffuse scleroderma [8] and 2% of patients with limited disease. Studies from the European League Against Rheumatism (EULAR) Scleroderma Trials and Research (EUSTAR) database suggest a lower prevalence (5% of diffuse scleroderma and 2% of limited) [9] and a retrospective cohort study [10] from Japan reported a prevalence of 3.2%. Geographic differences in autoantibody profiles, particularly anti-RNA polymerase III anti-bodies likely contribute to these prevalence variations.
Clinical presentation
SRC presents early, with a mean of 3.2 years since onset of disease [8] and, in up to 25% of cases, the diagnosis of scleroderma is made at the time of SRC presentation [11,12]. SRC usually manifests with acute onset of moderate-to-severe ‘accelerated’ hypertension and oliguric renal failure [7,8]. Hyperreninemia, thrombotic microangiopathy, anemia, and congestive heart failure are common, and some patients exhibit hypertensive encephalopathy and retinopathy [7].
In up to 10% of cases, SRC occurs without hypertension, a condition referred to as normotensive SRC. This presentation is associated with prior use of ACEi, calcium channel blockers, and myocardial involvement [7,13]. In some cases, these patients may have actually been relatively hypotensive at baseline such that their blood pressure (BP) elevation, although significant for them as an individual, still falls within the normotensive range.
Pathologic findings and pathogenesis
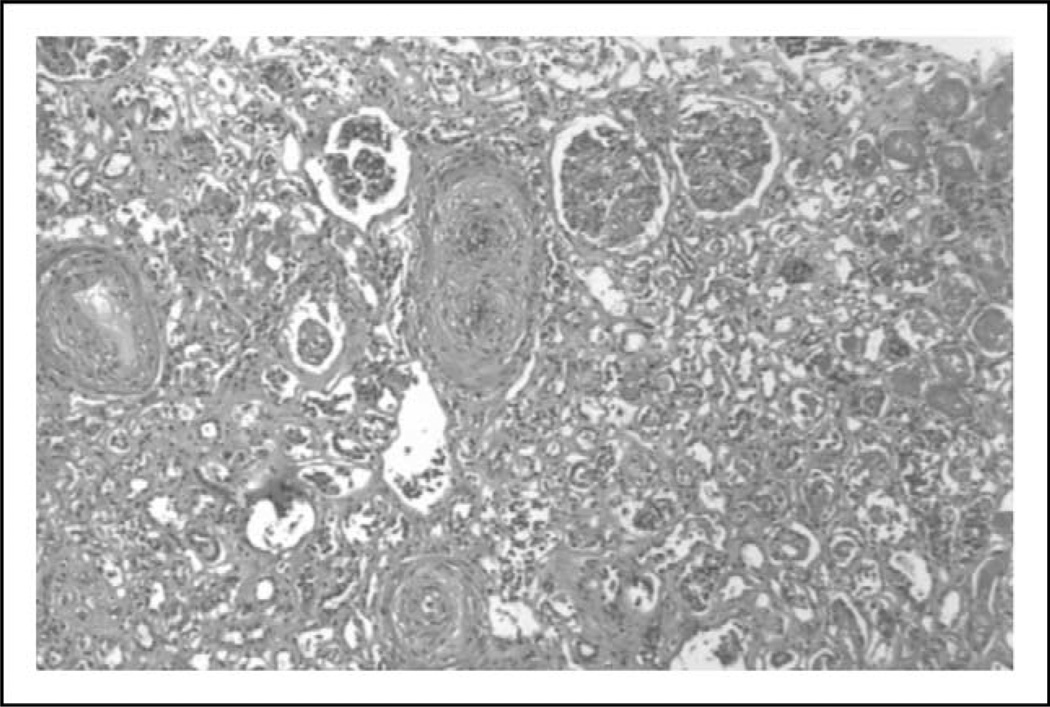
Pathologically SRC causes a proliferative obliterative vasculopathy with concentric ‘onion skin’ narrowing of arterioles and glomerular ischemia (Fig. 1) [14]. The glomeruli do not show inflammatory changes or immune deposits. These pathologic findings, along with the presence of marked hyperreninemia, suggest that altered perfusion of the juxtaglomerular apparatus triggering renin-driven hypertension may be a driver of SRC. However, autopsy studies demonstrate renal vascular changes in some patients without clinical evidence of SRC. Additionally, some patients have physiologic evidence of episodic renal vasospasm (‘renal Raynaud’s phenomenon’) and, although these changes correlate with baseline and cold-induced renin levels, they do not predict development of SRC [15].
FIGURE 1.
Renal biopsy from a patient with scleroderma renal crisis. Hematoxylin and eosin stain demonstrates onion skinning concentric narrowing of arterioles and glomerular ischemic changes.
Risk stratification in scleroderma renal crisis
Recent studies suggest several factors that can be helpful to stratify and identify patients at high risk for SRC.
Autoantibody profile
Anti-RNA polymerase III antibodies are seen in 9.4–59% of SRC patients [16, 17▪▪,18, 19▪, 20▪, 21, 22]. Although anti-RNA polymerase III antibodies are associated with diffuse cutaneous disease, tendon friction rubs, and joint contractures, there is also a strong independent association with SRC [17▪▪,22]. In the Australian Scleroderma Cohort Study, independent of corticosteroid exposure, anti-RNA polymerase III antibodies carried a 25% risk of developing SRC, compared with a 2% risk in the absence of this antibody. Development of an ELISA to detect anti-RNA polymerase III antibodies has helped translate these associations into clinical practice [21].
Other autoantibody profiles are not associated with SRC. Antitopoisomerase I (anti-Scl70) antibody affects 30% of patients with diffuse scleroderma but does not predict SRC [22]. Anticentromere antibodies are negatively associated with SRC, [23▪▪] and anti-U3RNP antibodies are not a risk factor for SRC [24].
Skin involvement and tendon friction rubs
The highest risk group for SRC remains patients with early diffuse scleroderma, with 75–86% of cases occurring during the first 4 years of disease [12,25]. SRC is especially associated with rapidly progressive skin disease [8,11] and tendon friction rubs [26,27].
Corticosteroid exposure
Exposure to corticosteroids (equivalent to ≥15 mg per day in the preceding 6 months) is well recognized as a risk factor for SRC [28, 29▪▪]. A recent meta-analysis identified 10 patients from 26 studies involving 500 patients exposed to corticosteroids through scleroderma clinical trials [30▪]. All patients had either received pulse dose corticosteroids (n=8) or medium-dose corticosteroids (15–30 mg per day, n=2), equivalent to a rate of SRC of 2% of steroid-exposed patients. This was higher than expected but due to differing inclusion and exclusion criteria for the individual studies, high-risk patients for SRC may have been disproportionately represented, skewing the prevalence data.
Human leukocyte antigen types associated with scleroderma renal crisis
Nguyen et al. [23▪▪] studied 90 SRC patients from a cohort of 1519 scleroderma cases. Although the population under study had a high prevalence of anti-RNA polymerase III antibodies, this study identified human leukocyte antigen (HLA) DRB1*0407 and HLA-DRB1*1304 as independent risk factors for SRC.
Endothelin pathways in scleroderma renal crisis
Endothelin B receptor polymorphisms are associated with diffuse scleroderma [31] and endothelin-1 and endothelin B receptors are upregulated in renal tissues from SRC cases [32, 33▪,34]. A pilot study to investigate the safety of adding a nonselective endothelin-1 receptor antagonist (Bosentan) to ACEi in SRC found that this combination was well tolerated, but there were no significant differences in mortality, rates of dialysis, or renal functional improvement, compared with historical controls. This open-label study [32] was not blinded or randomized, and only six patients were enrolled.
Soluble CD147 in scleroderma renal crisis
CD147 is a glycosylated membrane protein that stimulates matrix metalloproteinase production by stromal cells. In a cohort of 61 Japanese scleroderma patients, serum CD147 levels were significantly higher in SRC patients ( p<0.05), suggesting promise as a biomarker for SRC [35▪▪]. However, these findings need to be validated in a larger independent scleroderma population before translation into clinical use.
Magnitude of hypertension
Normotensive SRC is associated with worse outcomes than hypertensive SRC. Multivariate analyses of the SRC population show normotensive renal crisis is an independent predictor of reduced dialysis-free survival [12,13].
Hyperreninemia
Although significant elevations of plasma renin are characteristic of SRC, with levels sometimes reaching 100 times normal [36], the degree of hyper-reninemia does not correlate with outcome in SRC. Lack of timely availability of renin assays limits the usefulness of plasma renin levels in the clinical setting.
Factors not associated with scleroderma renal crisis
Baseline BP, serum creatinine, and presence of proteinuria or hematuria do not predict SRC [8]. There is no association between sex and SRC [11].
Management of scleroderma renal crisis
Evidence-based recommendations from EULAR and EUSTAR included two recommendations pertaining to renal disease in scleroderma: ACEi should be used in the treatment of scleroderma renal crisis and patients on steroids should be carefully monitored for BP and renal function. Several studies [37▪▪,38▪▪] have shown strong agreement amongst experts with these recommendations.
ACEi have significantly reduced SRC mortality from 76% at 1 year to less than 15% [39]. Captopril (D3-mercapto-2-methylpropionyl-L-proline) competitively inhibits peptidyl dipeptide hydrolase, blocking conversion of angiotensin I to angiotensin II. It is optimum as first-line therapy due to its short half-life that allows it to be readily titrated. The goal is to bring the SBP down by 20 mmHg per 24 h and the DBP down by 10 mmHg per 24 h until the BP is within normal limits, while avoiding hypotension. Careful titration of BP is of utmost importance, and many clinicians recommend monitoring in ICU or intermediate care units.
Since the introduction of ACEi, approximately 60% of SRC patients now avoid permanent dialysis. Of patients requiring dialysis, up to 30% have renal functional recovery if ACEi therapy continues concurrently with dialysis [7]. Efficacy of ACEi is dependent on the degree of renal damage at initiation, with greatest likelihood of renal function improvement if therapy is initiated when serum creatinine is less than 4 mg/dl [7,40].
In patients with persistent hypertension despite maximization of ACEi dose, there are no formal studies investigating the next-appropriate agent. Angiotensin II receptor blockers (ARBs) can safely be added once ACEi dose has been maximized, but ARBs alone are insufficient to control SRC without ACEi [41,42]. Recent guidelines from the Canadian Scleroderma Research Group and the Scleroderma Clinical Trials Consortium recommend addition of calcium channel and a antagonists in refractory SRC [37▪▪]. Case reports and small uncontrolled clinical studies [32,43] suggest that endothelin antagonists and direct renin inhibitors may also have a role.
Prognosis and outcome in scleroderma renal crisis
In specialized centers, ACEi have dramatically reduced mortality due to SRC, with data from the Pittsburgh database showing a reduction in mortality from 42 to 4% over a 30-year period [6,44]. However, the overall 5-year mortality from SRC reported in several studies [11,12] in North America, Australia, and Europe remains between 30 and 50%. Recent data from France suggest SRC mortality remains high outside of specialized centers with 1-year mortality rates reported at 29.1% in this cohort [29▪▪].
Retrospective studies [45▪▪] of patients with SRC who require dialysis show that although scleroderma is an independent predictor of mortality in the dialysis population, it is also an independent predictor of renal recovery (hazard ratio 11.1, 95% confidence interval 6.37–19.4). Rates of renal functional recovery reported for the scleroderma population differ geographically, with rates of 10% reported from Australia compared with 34–38% in the United States. Differences in reporting likely contribute to this discrepancy as the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry patients have to be dialysis-dependent for 3 months, so scleroderma patients with early renal functional recovery are excluded [45▪▪]. Even considering these issues, the rates of renal functional recovery reported for the scleroderma population far exceed those reported in other diseases combined (1–2.4%) [46].
The use of renal biopsy to make prognostic recommendations in SRC has been investigated by several groups. A study of 58 biopsies evaluated at the Royal Free Hospital found no association between renal biopsy chronicity index [47] and renal survival [11]. However, a study [48] of 17 biopsies from Pittsburgh found that in addition to vascular thrombosis and glomerular ischemic collapse, elevated peritubular capillary C4d staining (a product of classical complement degradation associated with antibody-mediated injury and renal allograft failure) was associated with worse outcome.
Role of prophylactic angiotensin-converting enzyme inhibitors and other prophylactic interventions
There are no interventions that prevent SRC, but many clinicians advocate teaching high-risk patients how to self-monitor BP, educating them regarding the importance of seeking care, and discussing the risks of prednisone and other nephrotoxic drugs.
‘Prophylactic’ ACEi do not reduce the risk of SRC; they are associated with worse outcome and increased likelihood of requiring permanent dialysis [13, 29▪▪]. ARBs have not been studied in the prophylactic setting, but as there are case reports of SRC developing while on ARBs, these agents are likely to be ineffective [11,49].
Renal transplantation in scleroderma renal crisis
Renal transplantation is effective in patients with SRC. Survival for scleroderma patients with end-stage renal failure (ESRF) who undergo transplant is better than those with ESRF awaiting transplant [50]. However, due to the relatively high rates of renal functional recovery, it is usually advised to delay transplantation until patients have been dialysis dependent for at least 2 years. Renal transplant survival rates are similar to those seen in patients undergoing renal transplantation for lupus and other connective tissue diseases. In the ANZDATA registry, renal allograft survival rates in the scleroderma population were 53% for deceased donor allografts, and 100% for live donor allografts [45▪▪].
Scleroderma renal crisis recurrence rates
Recurrence of SRC has been reported but is extremely rare. In patients with SRC who undergo renal transplant, recurrence in the transplanted kidney occurs in less than 5% of cases [51]. Recurrence is more common in patients with early loss of native renal function during the original SRC episode [51].
ANTINEUTROPHIL CYTOPLASMIC ANTIBODIES-ASSOCIATED GLOMERULONEPHRITIS IN SCLERODERMA
Glomerulonephritis associated with myeloperoxidase-specific antineutrophil cytoplasmic antibodies (ANCA) has been reported in scleroderma [52–54].
Epidemiology
In contrast to SRC, this manifestation develops in patients with longstanding limited cutaneous scleroderma. The prevalence is unknown, but approximately 40 cases have been reported in the English language literature.
Clinical presentation
Typically patients present with cresentic glomerulo-nephritis, progressive renal failure, mild hypertension, and proteinuria. Some cases have reported associated pulmonary hemorrhage.
Pathologic features and pathogenesis
Renal biopsy is required to exclude SRC and demonstrates cresentic glomerulonephritis. Although the pathogenesis is unknown, it has been postulated that established scleroderma vasculopathy exacerbates the interaction of ANCA with the endothelium near the vascular pole resulting in neutrophil activation in the glomerulus.
Management
Similarly to ANCA-associated vasculitis in patients without scleroderma, this complication requires therapy with high doses of steroids and immunosuppression. Rituximab has recently been approved by the US Food and Drug Administration for induction therapy in ANCA-associated vasculitis, and there are case reports suggesting efficacy of this drug for ANCA-associated glomerulonephritis in scleroderma [55].
PENICILLAMINE-ASSOCIATED RENAL DISEASE
Although rarely used in scleroderma now, approximately 20% of patients treated with D-Penicillamine develop reversible membranous glomerulonephritis and proteinuria, a complication with approximately 40% mortality [56].
RENAL FUNCTIONAL IMPAIRMENT WITHOUT OTHER MANIFESTATIONS OF RENAL DISEASE IN SCLERODERMA
Histopathologic renal injury is common in scleroderma even without clinically evident SRC [14,15]. Asymptomatic renal functional impairment affects 10–55% of scleroderma patients [7,15,57–59] and comorbidities contributing to renal impairment include congestive heart failure, gastrointestinal involvement, medication exposures, and dehydration. Proteinuria or albuminuria is reported in up to 25% of scleroderma patients, with intermediate-weight proteinuria in 31.3% [60,61]. Intermediate-weight proteinuria is a predictor of glomerular permeability and correlates with gastrointestinal involvement. Although asymptomatic renal changes are typically nonprogressive in scleroderma [7,58], some investigators have suggested that they indicate reduced renal functional reserve [62,63]. Reduced baseline renal functional reserve, measured by intravenous amino acid load test, was associated with significant reduction in mean annual creatinine clearance over the subsequent 5 years [62,63].
Reduced glomerular filtration rate (GFR) correlates with vasculopathic manifestations and prognosis [64–66]. In scleroderma-associated pulmonary hypertension, estimated GFR (eGFR) is a strong predictor of survival, with eGFR less than 60 ml/min per 1.73 m [2] associated with a three-fold increased risk of mortality [65]. The Chronic Kidney Disease Epidemiology Collaboration Equation strongly correlates with measured GFR in scleroderma patients with normal creatinine, suggesting this may be a helpful method for estimating GFR in scleroderma [67].
Renal vascular resistive index is a measure of intrarenal vascular elasticity and complicance assessed using Doppler ultrasound. Resistive index is very sensitive to renal vascular disease and correlates with GFR and digital microvascular damage in scleroderma [68▪]. The antioxidant N-acetylcysteine reduces renal resistive index in scleroderma patients with early capillaroscopic changes, but has the opposite effect in patients with late capillaroscopic changes [69]. Identifying patients with early vasculopathy in scleroderma remains an area of active investigation. Soluble CD163, a biomarker for oxidative stress, is elevated in scleroderma and may have promise as a biomarker of renal vascular disease. Although there is no correlation between CD163 levels and skin score, levels correlate with renal artery Doppler pulsatility index (r=0.534, P=0.0009) [70▪▪]. These observations require further validation in a larger population of scleroderma patients.
CONCLUSION
Renal disease remains an important cause of morbidity and mortality in scleroderma. Although SRC survival has significantly improved, mortality remains high outside of specialized centers. Anti-RNA polymerase III antibodies are a strong a predictor for the development of SRC and should be incorporated into clinical care to identify high-risk patients. Recent recommendations from EULAR, EUSTAR, and the Scleroderma Clinical Trials Consortium confirm ACEi remain first-line therapy for SRC.
Subclinical renal impairment affects approximately 50% of scleroderma patients and may be associated with other vascular manifestations. Subclinical renal involvement rarely progresses to ESRF; however, it may predict mortality in patients with other vasculopathic manifestations.
KEY POINTS.
Anti-RNA polymerase III antibodies are strongly associated with SRC and may be used to identify patients at high risk for SRC.
ACEi inhibitors are the first-line therapy for SRC, but outside of specialized centers, the mortality of SRC remains high.
‘Prophylactic’ ACEi do not reduce the risk of SRC; they are associated with worse outcome and increased likelihood of requiring permanent dialysis.
Renal transplantation is effective in patients with SRC, but due to the possibility of late renal functional recovery in SRC, it is recommended that transplantation be delayed for 2 years after becoming dialysis dependent.
Asymptomatic nonprogressive renal impairment is common in scleroderma and correlates with vasculopathic manifestations and prognosis.
Footnotes
Conflicts of interest
Dr V.K.S. has no relevant scientific disclosures related to this article. She is currently supported by award numbers KL2RR031974 and UL1RR031975 from the National Center for Research Resources.
Dr V.D.S. has no relevant scientific disclosures related to this article. She has received research funding in the past 2 years from the NIH (R01HL089758-01A1 and N01-AR-0-2251) and from Acterlion, United Therapeutics, Gilead, Roche and Celgene. She has received consulting income from United Therapeutics, Gilead, Roche and Sanofi, and is on the Speakers Bureau for Actelion and Gilead.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
All authors were involved in drafting the article and revising it critically for important intellectual content. All authors approved the final version to be published. The authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 699)
- 1.LeRoy EC, Black CM, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 2.LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–1576. [PubMed] [Google Scholar]
- 3.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34:1–15. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Hissaria P, Lester S, Hakendorf P, et al. Survival in scleroderma: results from the population-based South Australian register. Intern Med J. 2011;41:381–390. doi: 10.1111/j.1445-5994.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 6.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steen V. Scleroderma renal crisis. Rheum Dis Clin North Am. 2003;29:315–333. doi: 10.1016/s0889-857x(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 8.Steen VD, Medsger TA, Jr, Osial TA, Jr, et al. Factors predicting development of renal involvement in progressive systemic sclerosis. Am J Med. 1984;76:779–786. doi: 10.1016/0002-9343(84)90986-0. [DOI] [PubMed] [Google Scholar]
- 9.Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66:754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto A, Endo H, Kondo H, Hirohata S. Clinical features of 405 Japanese patients with systemic sclerosis. Mod Rheumatol. 2012;22:272–279. doi: 10.1007/s10165-011-0515-7. [DOI] [PubMed] [Google Scholar]
- 11.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM. 2007;100:485–494. doi: 10.1093/qjmed/hcm052. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira L, Mouthon L, Mahr A, et al. Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis. 2008;67:110–116. doi: 10.1136/ard.2006.066985. [DOI] [PubMed] [Google Scholar]
- 13.Helfrich D, Banner B, Steen V, Medsger TJ. Normotensive renal failure in systemic sclerosis. Arthritis Rheum. 1989;32:1128–1134. doi: 10.1002/anr.1780320911. [DOI] [PubMed] [Google Scholar]
- 14.Trostle D, Bedetti C, Steen V, et al. Renal vascular histology and morphometry in systemic sclerosis: a case-control autopsy study. Arthritis Rheum. 1988;31:393–400. doi: 10.1002/art.1780310311. [DOI] [PubMed] [Google Scholar]
- 15.Kovalchik M, Guggenheim S, Silverman M, et al. The kidney in progressive systemic sclerosis: a prospective study. Ann Intern Med. 1978;89:881–887. doi: 10.7326/0003-4819-89-6-881. [DOI] [PubMed] [Google Scholar]
- 16.Meyer O, De Chaisemartin LUC, Nicaise-Roland P, et al. Anti-RNA polymerase iii antibody prevalence and associated clinical manifestations in a large series of French patients with systemic sclerosis: a cross-sectional study. J Rheumatol. 2010;37:125–30. doi: 10.3899/jrheum.090677. [DOI] [PubMed] [Google Scholar]
- 17. Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a crosssectional analysis of data from an Australian cohort. Arthritis Res Ther. 2011;13:R211. doi: 10.1186/ar3544. This cohort study assessed prevalence and clinical correlations of anti-RNA polymerase III antibodies in an Australian cohort. The prevalence of anti-RNA polymerase III antibodies was 15.3% and as with other populations these antibodies were strongly correlated with diffuse disease, skin and joint involvement, and SRC.
- 18.Graf SW, Hakendorf P, Lester S, et al. South Australian Scleroderma Register: autoantibodies as predictive biomarkers of phenotype and outcome. Int J Rheum Dis. 2012;15:102–109. doi: 10.1111/j.1756-185X.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- 19. Emilie S, Goulvestre C, Bérezné A, et al. Anti-RNA polymerase III antibodies are associated with scleroderma renal crisis in a French cohort. Scand J Rheumatol. 2011;40:404–406. doi: 10.3109/03009742.2011.569753. This study from France found that 38% of SRC patients had anti-RNA polymerase III antibodies.
- 20. Hesselstrand R, Scheja A, Wuttge D. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. 2012;41:39–43. doi: 10.3109/03009742.2011.610032. In a Swedish cohort of SRC patients, anti-RNA polymerase III antibodies were a strong risk factor for SRC.
- 21.Satoh T, Ishikawa O, Ihn H, et al. Clinical usefulness of anti-RNA polymerase III antibody measurement by enzyme-linked immunosorbent assay. Rheumatology. 2009;48:1570–1574. doi: 10.1093/rheumatology/kep290. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen B, Assassi S, Arnett F, Mayes M. Association of RNA polymerase III antibodies with scleroderma renal crisis. J Rheumatol. 2010;37:1068. doi: 10.3899/jrheum.091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen B, Mayes MD, Arnett FC, et al. HLA-DRB1*0407 and *1304 are risk factors for scleroderma renal crisis. Arthritis Rheum. 2011;63:530–534. doi: 10.1002/art.30111. Using a well phenotyped population of scleroderma patients from the Scleroderma Family Registry, the University of Texas Health Science Center and the Genes versus Environment in Scleroderma Outcomes Study repository HLA typing was performed and correlated with autoantibody profiles and clinical features using multivariate analysis. his study found that HLA DRB1*0407 and *1304 are independent risk factors for SRC.
- 24.Aggarwal R, Lucas M, Fertig N, et al. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009;60:1112–1118. doi: 10.1002/art.24409. [DOI] [PubMed] [Google Scholar]
- 25.Steen VD, Medsger TA. Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000;133:600–603. doi: 10.7326/0003-4819-133-8-200010170-00010. [DOI] [PubMed] [Google Scholar]
- 26.Avouac J, Walker U, Tyndall A, et al. Characteristics of Joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR Scleroderma Trial and Research Group (EUSTAR) Database. J Rheumatol. 2010;37:1488–1501. doi: 10.3899/jrheum.091165. [DOI] [PubMed] [Google Scholar]
- 27.Steen V, Medsger T., Jr The palpable tendon friction rub: an important physical examination finding in patients with systemic sclerosis. Arthritis Rheum. 1997;40:1146–1151. doi: 10.1002/1529-0131(199706)40:6<1146::AID-ART19>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Steen V. Medsger TA Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41:1613–1619. doi: 10.1002/1529-0131(199809)41:9<1613::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29. Guillevin L, Bérezné A, Seror R, et al. Scleroderma renal crisis: a retrospective multicentre study on 91 patients and 427 controls. Rheumatology. 2012;51:460–467. doi: 10.1093/rheumatology/ker271. This retrospective multicenter study from France assessed 91 SRC cases seen in various centers throughout France. They found a mortality rate of 40.7% overall, with 1-year survival of 70.9% and 5-year survival of 60%. This suggests that outside of specialized centers, the mortality of SRC remains high.
- 30. Trang G, Steele R, Baron M, Hudson M. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatol Int. 2012;32:645–653. doi: 10.1007/s00296-010-1697-6. This meta-analysis confirmed other large cohort studies demonstrating the association between corticosteroid exposure and SRC.
- 31.Fonseca C, Renzoni E, Sestini P, et al. Endothelin axis polymorphisms in patients with scleroderma. Arthritis Rheum. 2006;54:3034–3042. doi: 10.1002/art.22036. [DOI] [PubMed] [Google Scholar]
- 32.Penn H, Quillinan N, Khan K, et al. Targeting the endothelin axis in scleroderma renal crisis: rationale and feasibility. doi: 10.1093/qjmed/hct111. [DOI] [PubMed] [Google Scholar]
- 33. Mouthon L, Mehrenberger M, Teixeira L, et al. Endothelin-1 expression in scleroderma renal crisis. Hum Pathol. 2011;42:95–102. doi: 10.1016/j.humpath.2010.05.018. This renal biopsy study demonstrated that endothelin-1 is overexpressed in glomeruli and arterioles of SRC patients.
- 34.Vancheeswaran R, Magoulas T, Efrat G, Wheeler-Jones C, et al. Circulating endothelin-1 levels in systemic sclerosis subsets: a marker of fibrosis or vascular dysfunction? J Rheumatol. 1994;21:1838–1844. [PubMed] [Google Scholar]
- 35. Yanaba K, Asano Y, Tada Y, et al. Increased serum soluble CD147 levels in patients with systemic sclerosis: association with scleroderma renal crisis. Clin Rheumatol. 2012;31:835–839. doi: 10.1007/s10067-012-1949-9. This prospective study investigated the extracellular matrix metalloproteinase-inducer CD147 in 61 scleroderma patients and 24 healthy controls. CD147 was elevated in patients with scleroderma compared with controls. Within the population with scleroderma, CD147 levels at first visit correlated with development of SRC. However, it should be noted that the antibody profile of this Japanese population was not typical for that seen in Europe and North America as anti-RNA-polymerase III antibodies were underrepresented in this cohort.
- 36.Fleischmajer R. Gould AB Serum renin and renin substrate levels in scleroderma. Proc Soc Exp Biol Med. 1975;50:374–379. doi: 10.3181/00379727-150-39039. [DOI] [PubMed] [Google Scholar]
- 37. Walker KM, Pope J. Treatment of systemic sclerosis complications: what to use when first-line treatment fails: a consensus of systemic sclerosis experts. Seminars in Arthritis and Rheumatism. Semin Arthritis Rheum. 2012;42:42–55. doi: 10.1016/j.semarthrit.2012.01.003. This study was designed to obtain a consensus of opinion from members of the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group on the management of common scleroderma manifestations including SRC.
- 38. Walker K, Pope J. Expert Agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheum. 2011;38:1326–1328. doi: 10.3899/jrheum.101262. This study surveyed members of the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group for their level of aggreement with the EULAR/EUSTAR recommendations.
- 39.Steen V, Costantino J, Shapiro A, Medsger TA., Jr Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin converting enzyme (ACE) inhibitors. Ann Intern Med. 1990;113:352–357. doi: 10.7326/0003-4819-113-5-352. [DOI] [PubMed] [Google Scholar]
- 40.Whitman Hr, Case D, Laragh J, et al. Variable response to oral angiotensin-converting-enzyme blockade in hypertensive scleroderma patients. Arthritis Rheum. 1982;25:241–248. doi: 10.1002/art.1780250301. [DOI] [PubMed] [Google Scholar]
- 41.Caskey F, Thacker E, Johnston P, Barnes J. Failure of losartan to control blood pressure in scleroderma renal crisis. Lancet. 1997;349:620. doi: 10.1016/s0140-6736(05)61568-1. [DOI] [PubMed] [Google Scholar]
- 42.Cheung WY, Gibson IW, Rush D, et al. Late recurrence of scleroderma renal crisis in a renal transplant recipient despite angiotensin II blockade. Am J Kidney Dis. 2005;45:930–934. doi: 10.1053/j.ajkd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Dhaun N, MacIntyre IM, Bellamy COC, Kluth DC. Endothelin receptor antagonism and renin inhibition as treatment options for scleroderma kidney. Am J Kidney Dis. 2009;54:726–731. doi: 10.1053/j.ajkd.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 45. Siva B, McDonald SP, Hawley CM, et al. End-stage kidney disease due to scleroderma outcomes in 127 consecutive ANZDATA registry cases. Nephrol Dial Transplant. 2011;26:3165–3171. doi: 10.1093/ndt/gfq861. This important study utilized data from more than 40 000 dialysis-dependent patients in the ANZDATA registry. The investigators found that scleroderma was not only an independent predictor of mortality in dialysis patients but also an independent predictor of renal functional recovery.
- 46.Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23:606–613. doi: 10.1111/j.1525-139X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 47.Howie AJ, Ferreira MAS, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16:1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 48.Batal I, Domsic RT, Shafer A, et al. Renal biopsy findings predicting outcome in scleroderma renal crisis. Hum Pathol. 2009;40:332–340. doi: 10.1016/j.humpath.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Penn H, Denton CP. Diagnosis, management and prevention of scleroderma renal disease. Curr Opin Rheumatol. 2008;20:692–696. doi: 10.1097/BOR.0b013e3283108df7. [DOI] [PubMed] [Google Scholar]
- 50.Gibney EM, Parikh CR, Jani A, et al. Kidney transplantation for systemic sclerosis improves survival and may modulate disease activity. Am J Transplant. 2004;4:2027–2031. doi: 10.1111/j.1600-6143.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 51.Pham P-TT, Pham P-CT, Danovitch GM, et al. Predictors and risk factors for recurrent scleroderma renal crisis in the kidney allograft: case report and review of the literature. Am J Transplant. 2005;5:2565–2569. doi: 10.1111/j.1600-6143.2005.01035.x. [DOI] [PubMed] [Google Scholar]
- 52.Anders H, Wiebecke B, Haedecke C, et al. MPO-ANCA-positive crescentic glomerulonephritis: a distinct entity of scleroderma renal disease? Am J Kidney Dis. 1999;33:e3. doi: 10.1016/s0272-6386(99)70244-1. [DOI] [PubMed] [Google Scholar]
- 53.Mimura I, Hori Y, Matsukawa T, et al. Noncrescentic ANCA-associated renal crisis in systemic sclerosis. Clin Nephrol. 2008;70:183–185. doi: 10.5414/cnp70183. [DOI] [PubMed] [Google Scholar]
- 54.Endo H, Hosono T, Kondo H. Antineutrophil cytoplasmic autoantibodies in 6 patients with renal failure and systemic sclerosis. J Rheumatol. 1994;21:864–870. [PubMed] [Google Scholar]
- 55.Arad U, Balbir-Gurman A, Doenyas-Barak K, et al. Anti-neutrophil antibody associated vasculitis in systemic sclerosis. Semin Arthritis Rheum. 2011;41:223–229. doi: 10.1016/j.semarthrit.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Derk C, Jimenez S. Goodpasture-like syndrome induced by D-penicillamine in a patient with systemic sclerosis: report and review of the literature. J Rheumatol. 2003;30:1616–1620. [PubMed] [Google Scholar]
- 57.Cannon P, Hassar M, Case D, et al. The relationship of hypertension and renal failure in scleroderma (progressive systemic sclerosis) to structural and functional abnormalities of the renal cortical circulation. Medicine (Baltimore) 1974;53:1–46. doi: 10.1097/00005792-197401000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Steen VD, Syzd A, Johnson JP, et al. Kidney disease other than renal crisis in patients with diffuse scleroderma. J Rheumatol. 2005;32:649–655. [PubMed] [Google Scholar]
- 59.Scheja A, Bartosik I, Wuttge DM, Hesselstrand R. Renal function is mostly preserved in patients with systemic sclerosis. Scand J Rheumatol. 2009;38:295–298. doi: 10.1080/03009740802629424. [DOI] [PubMed] [Google Scholar]
- 60.Seiberlich B, Hunzelmann N, Krieg T, et al. Intermediate molecular weight proteinuria and albuminuria identify scleroderma patients with increased morbidity. Clin Nephrol. 2008;70:110–117. doi: 10.5414/cnp70110. [DOI] [PubMed] [Google Scholar]
- 61.François B, Moulin G, Assenat H, et al. The kidney in systemic scleroderma: a report of 38 consecutive cases. Nephrologie. 1982;3:85–91. [PubMed] [Google Scholar]
- 62.Livi R, Guiducci S, Perfetto F, et al. Lack of activation of renal functional reserve predicts the risk of significant renal involvement in systemic sclerosis. Ann Rheum Dis. 2011;70:1963–1967. doi: 10.1136/ard.2011.152892. [DOI] [PubMed] [Google Scholar]
- 63.Livi R, Teghini L, Pignone A, et al. Renal functional reserve is impaired in patients with systemic sclerosis without clinical signs of kidney involvement. Ann Rheum Dis. 2002;61:682–686. doi: 10.1136/ard.61.8.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kingdon E, Knight C, Dustan K, et al. Calculated glomerular filtration rate is a useful screening tool to identify scleroderma patients with renal impairment. Rheumatology. 2003;42:26–33. doi: 10.1093/rheumatology/keg023. [DOI] [PubMed] [Google Scholar]
- 65.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohamed R, Zayed H, Amin A. Renal disease in systemic sclerosis with normal serum creatinine. Clin Rheumatol. 2010;29:729–737. doi: 10.1007/s10067-010-1389-3. [DOI] [PubMed] [Google Scholar]
- 67.Gigante A, Rosato E, Massa R, et al. Evaluation of Chronic Kidney Disease Epidemiology Collaboration equation to estimate glomerular filtration rate in scleroderma patients. Rheumatology. 2012;51:1426–1431. doi: 10.1093/rheumatology/kes049. [DOI] [PubMed] [Google Scholar]
- 68. Rosato E, Gigante A, Barbano B, et al. Intrarenal hemodynamic parameters correlate with glomerular filtration rate and digital microvascular damage in patients with systemic sclerosis. Semin Arthritis Rheum. 2012;41:815–821. doi: 10.1016/j.semarthrit.2011.11.005. This prospective study enrolled 30 patients with scleroderma and 30 healthy controls to assess Doppler ultrasound renal resistive index, measured GFR, and nailfold videocapillaroscopy findings. The authors found correlation between resistive index, measured GFR, and capillaroscopic findings.
- 69.Rosato E, Cianci R, Barbano B, et al. N-acetylcysteine infusion reduces the resistance index of renal artery in the early stage of systemic sclerosis. Acta Pharmacol Sin. 2009;30:1283–1288. doi: 10.1038/aps.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shimizu K, Ogawa F, Yoshizaki A, et al. Increased serum levels of soluble CD163 in patients with scleroderma. Clin Rheumatol. 2012;31:1059–1064. doi: 10.1007/s10067-012-1972-x. This study investigated CD163 levels in a cohort of Japanese scleroderma patients. Serum CD163 levels were elevated in scleroderma compared with control patients. Serum CD163 levels correlated with serum immunoglobulin G and with renal vascular resistance suggesting that CD163 has promise as a biomarker of oxidative stress particularly to identify scleroderma-associated vascular injury.