Abstract
Inhibition of return (IOR) refers to the slowing of reaction times to a target when a preceding stimulus has occupied the same location in space. Recently, we observed a robust inhibitory effect for color and shape in moderately complex displays: It is more difficult to detect a target with a particular nonspatial attribute if a stimulus with the same attribute was recently the focus of attention. Such nonspatial inhibitory effects have not generally been found in simpler displays. In the present study, we test how location-based and nonspatial inhibitory effects vary as a function of display complexity (8, 6, 4 and 2 locations). The results demonstrated that: (1) location based inhibition effects were much stronger in more complex displays, whereas the nonspatial inhibition was only slightly stronger in more complex displays; (2) Nonspatial inhibitory effects emerged at longer SOAs than location-based effects; and (3) Nonspatial inhibition only appeared when cues and targets occurred in the same locations, confirming that pure feature repetition does not produce a cost. Taken together, the results are consistent with perceptual processes based on object files that are organized by spatial location. Using somewhat more complex displays than are most commonly employed provides a more sensitive method to observe the role of inhibitory processes in facilitating visual search. In addition, using a relatively wide set of cue-target timing relationships is necessary in order to clearly see how inhibitory effects operate.
Keywords: Inhibition of Return, Location-based inhibition, Feature-based inhibition, Display complexity
As we navigate the world each day, we generate thousands of actions in response to countless objects and features of those objects in our environment. Among the many objects present at any moment, only a few are task relevant. The others are task irrelevant, and thus potentially distracting. The ability to efficiently direct visual attention to target objects or features, and ignore distracters, is thus critical for efficient functioning. And, once we have extracted information about a relevant object or feature, to ensure accurate and effective perception, we need an attentional control mechanism to discourage attention from re-orienting back to previously attended locations and features. The term Inhibition of Return (IOR) has been used to describe such a mechanism: a bias against attending to visual stimuli at recently attended locations (Posner & Cohen, 1984; Posner, Rafal, Choate, & Vaughan, 1985).
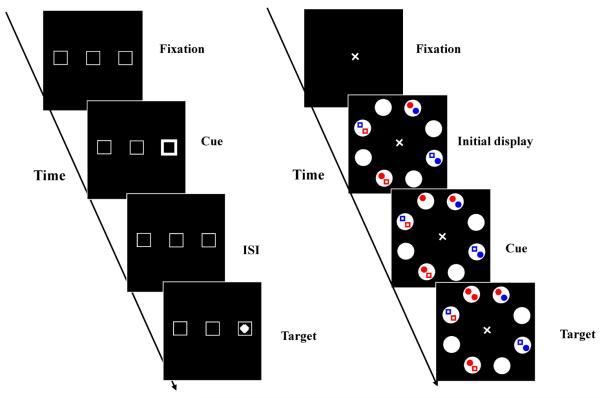
In Posner and Cohen's (1984) seminal study of IOR, subjects were presented with three boxes: One to the left, one to the right and one at the center of a screen (Figure 1, left panel). A trial began with the brightening of one of the peripheral boxes for 150 msec, which served as a visual cue. Later a small dot appeared in one of the three boxes 0–500 msec following the onset of the cue. This target appeared in the central box with probability 0.6 and in one of the peripheral boxes with probability 0.1 (catch trials occurred with a probability of 0.2). Subjects were required to hit a response button upon detection of the target. When the cue-target interval (stimulus onset asynchrony, SOA) was less than 250 msec, responses were faster to the visual targets that appeared at the cued location. However, this facilitation effect disappeared and reversed when the SOA exceeded 300 ms: Responses were actually slower at the cued location, as compared to the uncued location. Posner and Cohen proposed that IOR reflected a build-up of inhibition after attention was disengaged from the cued location, and the slowed responses were the result of a delay in attention returning to a previously examined location (for alternative theoretical perspectives, see Dukewich, 2009; Klein & Taylor, 1994; Lupiáñez, 2010).
Figure 1.
Left panel: the sequence of events in a typical trial of Posner and Cohen (1984); Right panel: Schematic procedure used in the spatial cueing task (Samuel & Weiner 2001). Each trial begins with the Fixation, then initial display, Cue, Inter stimulus interval, target, and ends with the response.
Research has begun to clarify the mechanisms underlying IOR, and more generally, the important principles that guide the visual system's selection and orienting processes (for reviews, see Klein, 2000; Lupiáñez, 2010; Lupiáñez, Klein & Bartolomeo, 2006). For example, the inhibitory effect is strongest at the location of the cue and falls off with distance from that location (Bennett & Pratt, 2001; Maylor & Hockey, 1985; Samuel & Weiner, 2001). The inhibition generally begins to appear 200–300 ms after the cue, and tends to dissipate about 3000 ms later (for a review, see Samuel & Kat, 2003). From a functional perspective, Klein (1988) has proposed that IOR facilitates foraging in tasks that require attention-demanding serial examination by discouraging orienting toward previously inspected regions and objects. Ivanoff and Taylor (2006) have suggested that IOR facilitates an observer's ability to operate in dynamic environments because reducing attention to one location or object may allow more efficient accrual of information about others (that have not yet been processed).
Most studies of IOR have focused on where and when: Studies have delineated the spatial distribution of IOR relative to where the cue was presented, and when the inhibition begins and ends. Less research has been done on the what question: Exactly what is being inhibited? There has been substantial work to test whether the inhibition applies to the location per se, or to an object that occurred at the critical location (e.g. Tipper, Driver & Weaver, 1991; Tipper et al., 1994). However, there has been relatively little research looking for evidence that processing of particular properties might be inhibited. For example, if a red triangle was the cueing event, is there reduced processing of red things, or of triangular things? One reason that there has not been a great deal of work on this question is that most early studies of it yielded negative findings (Kwak & Egeth, 1992; Tanaka & Shimojo, 1996), or very weak effects (Law, Pratt & Abrams, 1995; Taylor & Klein, 1998; Fox & de Fockert, 2001; Riggo, Patteri & Umilta, 2004).
However, there were hints in the studies finding weak effects that inhibition of particular stimulus properties might only be detectable when the test displays required a certain level of processing complexity. The highly-simplified types of displays that were used by Posner and Cohen (1984), and in much of the following work, may not provide conditions in which evidence can be found for feature-based inhibitory effects. With that in mind, we conducted a series of experiments using somewhat more complex displays, and found robust effects for inhibition based on both color, and on shape (Hu, Samuel & Chan, 2011). Figure 1 illustrates the quite different displays used in the classic Posner and Cohen types of experiments (left side) and the experiments that we reported (right side). Our displays had 8 regions that could each contain 0, 1, or 2 small objects, and each object could be one of two colors; the classic displays have two relevant locations, and a simple detection target. In the first use of these more complex displays, Samuel and Weiner (2001) reported location-based (standard) IOR effects that were about twice as large as those found with the more typical two-location displays. Thus, displays that reflect a bit more of the complexity of the visual world may allow us to see more of the operations that the attentional system performs to deal with the visual world. A central goal of the current study is to examine the pattern of location-based IOR and feature-based inhibition as a function of display complexity, combining this what question with the more typical when and where questions. To be clear about which effect we are discussing, we refer to the classic Location-based Inhibition of Return as LIOR, and to the Feature Repetition Inhibitory Effect as FRIE.
In the current study, we compare the LIOR and FRIE patterns as we decrease the display complexity from the 8-location displays used in our previous studies down to the simple 2-location displays used in many prior studies. We will examine the two different types of inhibitory effect (location vs feature) as a function of the complexity of the displays and the delay between the initial cue and target appearance. The results clarify the extent to which the classic IOR (LIOR) and newly-reported feature-based effects (FRIE) show common properties, and the extent to which they diverge.
We have noted that several theoretical accounts posit that inhibitory effects can improve foraging and visual search by preventing inefficient attentional allocation. These accounts predict that IOR should be larger when search is difficult, and there is evidence that this is the case (e.g., Klein & MacInnes, 1999; Snyder & Kingston, 2007). Consistent with this, Muller and von Muhlenen (2000) reported IOR effects for displays with 6 or 10 locations, but not for displays with only 2. However, there are also several studies that found no effect of display complexity on IOR (e.g., Klein, 1988; Takeda & Yagi, 2000; Thomas & Lleras, 2009; Wang & Klein, 2010). Pratt, Adam and McAuliffe (1998) actually found that when the number of target locations increased, the overall IOR effect seemed to decline, though work by Birmingham et al. (2007) indicates that this decline is probably a consequence of greater distances between the cue and target in the displays with more locations.
Collectively, the results from these studies are rather murky, and are limited to location-based IOR. Presumably the inconsistent pattern across studies can be attributed to variation in the methods used in different studies, both in terms of the placement of the target location, and critically, in terms of the particular cue-target onset times. In the present study we used a consistent procedure across multiple experiments, varying the display complexity across experiments while using a rich and consistent sampling of cue-target onset times. As the results will make clear, the inhibitory effects vary strongly across both complexity and cue-target delays, and the effects can only be understood with an appropriately dense sampling.
Our approach here is to start with displays of the same complexity that we used in our prior work (8 locations) together with still-complex but slightly simpler ones (6 locations). After establishing the inhibitory patterns with these complex displays in Section 1, we will then compare these to the results for displays like those used in most IOR studies (2 locations), and slightly more complex ones (4 locations) in Section 2.
Section 1: Inhibition Effects with Complex Displays
Method
Participants
55 participants were recruited for Experiment 1 (8 locations) and a different group of 56 participants were recruited for Experiment 2 (6 locations). All participants were from Peking University, were right-handed, and were naïve to the purpose of the experiment. All reported normal or corrected-to-normal (color) vision. Each was tested individually and was paid 15 RMB for participating.
Apparatus and procedure
All experiments were conducted on a Pentium IV computer running E-Prime software (Schneider, Eschman, & Zuccolotto, 2002), with subjects viewing the screen from a distance of approximately 63 cm. A computer keyboard was directly in front of the subject and its space bar was used as the response device.
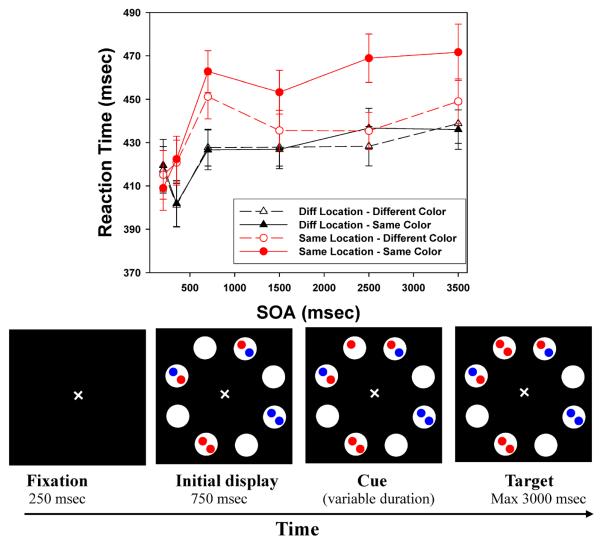
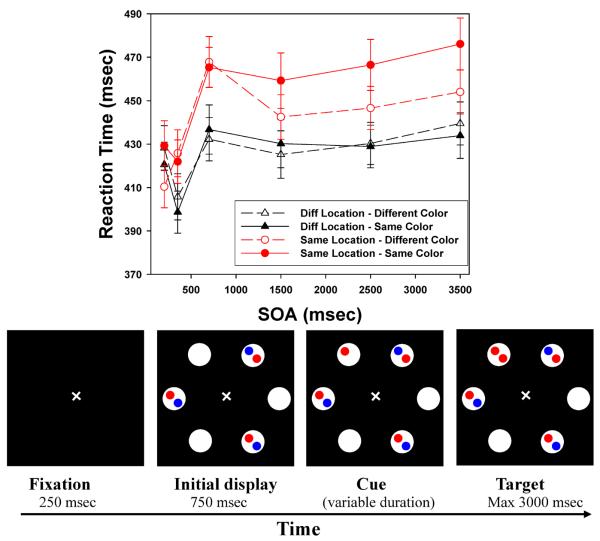
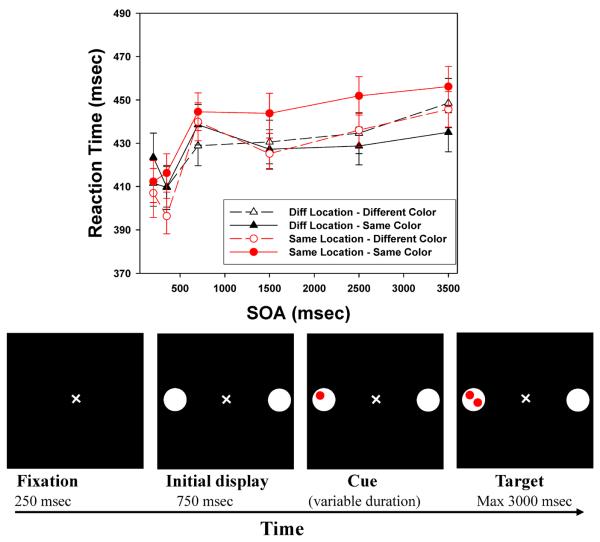
The stimuli and the general procedures were very similar to those of Hu et al. (2011). Figures 2 and 3 show the sequence of events on a trial in Experiment 1 (8 locations) and Experiment 2 (6 locations), respectively. Each trial began with a white fixation cross (10) on a dark background displayed for 250 ms. This was followed by a display which consisted of eight/six white circles (diameter: 3.70) presented for 750 ms. The white circles were arranged in a circular fashion around the fixation cross (radius: 6.80). Empty circles alternated with filled circles, each of which contained two small (10) disks. Each disk could be red or blue. The cue (a red or blue disk) then appeared in one of the empty circles. Finally, the target object was presented in the fourth display, with subjects instructed to respond when they detected target onset. The cue-target SOAs were chosen to provide both a dense sampling and to include longer intervals than many studies have used: 200, 350, 700, 1500, 2500, and 3500ms. Our previous work suggests that feature-based effects, in particular, may require relatively long SOAs. To look for feature repetition inhibition, half of the target disks matched the cue in color (feature repetition: red-red or blue-blue), and half mismatched (red-blue or blue-red). In all experiments, trials were terminated by the subject's response, or after a timeout period of 3000 msec.
Figure 2.
Top: Target detection times of Experiment 1, broken down by Color (repeated, nonrepeated), Location (Same, Diff), and stimulus onset asynchrony (SOA); Bottom, example of the sequence of events for a sample trial in Experiment 1 (size 8, cue stays on) (not drawn exactly to scale). Error bars indicate standard errors.
Figure 3.
Top: Target detection times of Experiment 2, broken down by Color (repeated, nonrepeated), Location (Same, Diff), and stimulus onset asynchrony (SOA); Bottom, example of the sequence of events for a sample trial in Experiment 2 (size 6, cue stays on) (not drawn exactly to scale). Error bars indicate standard errors.
Targets were equally likely to be presented at each possible location. In this respect, Experiment 1 was slightly different than the corresponding experiment in Hu et al. (2011). In that study, 33.3% of the targets were presented in the SAME location as the cue, 33.3% 900 away (split equally across the clockwise and counterclockwise locations), and 33.3% 1800 away.
Experiment 1 consisted of three blocks, with each block including 192 target-present trials and 16 target-absent trials. The 192 target-present trials represented the crossing of: 4 locations of the cue × 4 possible target locations relative to the cue × 6 SOAs × 2 color repetition cases (match or mismatch of cue-target color). Subjects were told that two small figures would be added to the initial display on each trial, and were required to push the space bar when the second figure appeared. The 16 target-absent trials in each block were catch trials in which a cue but no target appeared. Subjects were instructed to withhold responses on trials without targets. We divided blocks into two passes, offering a rest period after each pass. The subject was instructed to fixate on the central cross throughout the experiment. Before this experiment, and before each of the following experiments, each subject was given a practice block of 30 trials.
In Experiment 2, the 108 target-present cases represented the crossing of: 3 locations of the cue × 3 locations of the target × 6 SOAs × 2 color repetition cases [match or mismatch of cue-target color]). The 324 (3 × 108) target-present trials were broken into four passes. Each pass consisted of 81target-present trials plus 8 catch trials.
In all of the experiments, approximately half of the subjects were run with displays in which the cue remained present through the appearance of the target (cue on version); for the other subjects, the cue was removed from the display 100 msec after it appeared (cue off version). We included this manipulation based on Tipper et al.'s (1994) suggestion that because IOR can be associated with objects, the removal of the cueing object could remove object-based inhibition. While this prediction has been supported under some conditions (e.g. Klein & MacInnes, 1999; MacInnes & Klein, 2003; Muller & von Muhlenen, 2000; Takeda & Yagi, 2000), it has not been supported under others (e.g. Lleras, Rensink, & Enns, 2005, 2007; Thomas & Lleras, 2009). Because we found no effect of this manipulation in our experiments, in all of the analyses we report here we have collapsed across the on and off versions.
Results and discussion
In Experiment 1, the data from three subjects were removed because they did not correctly follow the instructions (these subjects reported during debriefing that they were mentally computing the rate of catch trials; their response times were extremely variable). One other subject's data were excluded due to very long reaction times (most RTs were greater than 600 msec). The grand mean reaction time was 433 msec. In Experiment 2, all 56 subjects performed well, with an overall mean reaction time of 436 msec. In both experiments, missed targets occurred on fewer than 1% of the trials; these trials were not included in the analyses. The false alarm rate on catch trials was under 2% in both experiments. Response times less than 100 ms or greater than 1500 ms were removed as outliers prior to analysis (less than 1%).
Preliminary analyses for each experiment indicated that there were no systematic differences between targets in the three (Experiment 1) or two (Experiment 2) Different location conditions. Therefore, in each experiment we created a single Different location condition by averaging across the Different locations. The top panels of Figures 2 and 3 present the mean target detection times (from correct trials) broken down by the location of the target, the SOA, and whether the target matched the cue in color.
For each experiment, the mean RTs were submitted to a 2 (Color Repetition: repeated vs non-repeated) × 6 (SOA: 200, 350, 700, 1500, 2500 and 3500 ms) × 2 (Location: Same vs. Different) analysis of variance (ANOVA). In both cases, all three main effects were significant. For the most complex (8-location) displays: Color Repetition, F(1,50) = 18.693, p<.001; Location, F(1,50) = 40.987, p <.001; SOA, F(5,250) = 24.841, p<.001. For the 6-location displays: Color Repetition, F(1,55) = 7.341, p=.009; Location, F(1,55) = 72.443, p <.001; SOA, F(5,275) = 22.416, p<.001. In Experiment 1, all three two-way interaction effects were also reliable: Location × Color Repetition, F(1,50) = 15.749, p <.001; SOA × Color Repetition, F(5,250) = 3.731, p = .003; and Location × SOA, F(5,250)=10.016, p <.001. In Experiment 2, the Location × Color Repetition interaction (F(1,55) =16.254, p <.001) and the Location × SOA interaction (F(5,275) = 9.049, p <.001) were significant, and the interaction of Color × SOA was marginally significant, F(5,275) = 1.997, p =.079. Finally, the three-way interaction effect was significant in both experiments, F(5,250) = 2.849, p =.016 (8-location), and F(5,275) = 2.527, p =.029 (6-location). The overall RT patterns are extremely similar to the results Hu et al. (2011) reported under similarly complex conditions.
Location repetition: LIOR
The classic location-based IOR effect is characterized by slower detection of targets that occur at a cued location than targets appearing at uncued locations, with the difference beginning to emerge after about 300 msec. The significant Location × SOA interaction in both experiments confirms the presence of this LIOR: Reaction times are much slower in the Same Location than in the Different Locations, but not at the shortest SOA. At SOA = 200 ms, there was a weak facilitation effect (Same faster than Different) in both experiments, t(50)= −1.449, p=.077 for the 8-location displays, and t(55) = −.875, p =.193 for the 6-location displays. For SOAs of 350 ms or greater, a robust LIOR effect was present, smallest t(50)=3.857, p <.001 for the most complex displays and smallest t(55)=4.840, p <.001for the 6-location displays. Statistical tests for all SOAs, for all experiments, are presented in the Appendix.
Feature (color) repetition: FRIE
Hu et al. (2011) reported that color repetition produced an inhibitory effect on target detection, but only when the target occurred at the Same location as the cue. As Figures 2 and 3 show, we observe the same pattern here, with no effect of Color Repetition when the target was presented at a Different location (the two curves plotted with triangles), but a large effect of Color Repetition for Same Location targets (the two curves plotted with circles). As in the previous study, the FRIE develops rather slowly. In both experiments, for the three longest SOAs (1500 ms, 2500 ms, and 3500 ms), color-based inhibition in the Same location was quite robust, smallest t(50) =2.606, p =.006 for the most complex displays, and smallest t(55) =2.556, p = 0.007 for the 6-location version. The effect may emerge a bit earlier in the most complex displays, as the effect at SOA = 700 ms was small but significant (a 12 msec difference, t(50) =1.899, p =.032) in Experiment 1, while it was not present in the 6-location case (t(55) = −.278, p =.391). In Experiment 1 there was no hint of FRIE based on Color Repetition at the 200 ms or 350 ms SOAs. This was also true at 350 msec SOA in Experiment 2. There was actually a significant effect at 200 msec for the 6-location displays, t(55)=2.637, p =.005, but that is almost certainly spurious, given the rest of the data.
Section 1 Conclusion
The goal of Section 1 was to replicate the LIOR and FRIE patterns observed by Hu et al. (2011), using equally complex displays (Experiment 1) and slightly simpler ones (Experiment 2). The observed inhibitory effects here nicely match those observed in the previous study, providing a basis for comparison when we reduce the complexity of the displays towards conditions more like the simpler displays used in most IOR studies. In both experiments, location repetition produced the classic pattern for location-based IOR: early facilitation followed by later inhibition. At the shortest SOA (200 ms), both experiments showed the usual facilitation, and both yielded reliable inhibition beginning at the 350 msec SOA. Both of the experiments also produced the feature (color) repetition inhibitory effect, and in each case the FRIE became apparent no sooner than 700 ms. After about 1 second, inhibition only remained for targets sharing features with the cue. Consistent with previous reports (e.g., Hu et al., 2011; Kwak & Egeth, 1992), the feature-based effect only appeared when the cue and the target occurred in the same location. The late-developing FRIE reflects the fact that inhibition is long-lasting when the target fully matches the cue but fades when there is featural mismatch.
Section 2: Inhibition Effects with Simpler Displays
In Section 2, we present two experiments to further investigate the effect of display complexity on LIOR and FRIE. As we noted previously, it seems likely that the simple displays used in many previous studies could potentially underestimate the degree to which the perceptual system suppresses distracters precisely because such displays do not have a great deal of distracting detail. In fact, Kat and Samuel (2003) reported that the location-based IOR effects obtained with 8-location displays similar to those in Experiment 1 were about twice as large as the typical effect found in most 2-location studies. Thus, we predict that LIOR effects in Section 2 will be significantly smaller than those in Section 1.
With respect to feature-based inhibition, recall that the existing literature is rather small, presumably because most early studies found little or no evidence for these effects. In our previous work (Hu et al., 2011) and in Section 1, in contrast, robust FRIE was observed. We hypothesize that the difference between the early failures and our more recent successes can be traced to a combination of the more complex displays we have used, and the wider range of cue-target SOAs. As we saw in Section 1, FRIE is only seen at quite long SOAs. Moreover, there is a hint across those two experiments that there may be an interaction between display complexity and the time needed for FRIE to emerge – greater complexity may allow the effect to be seen somewhat earlier. Section 2 provides information about the strength and time-course of both location-based and feature-based inhibitory effects in less complex displays, displays like those used in most prior IOR studies.
Method
Participants
120 paid subjects from Peking University, with the same qualifications as those in the prior experiments, participated. Half took part in Experiment 3, and half were in Experiment 4.
Apparatus and procedure
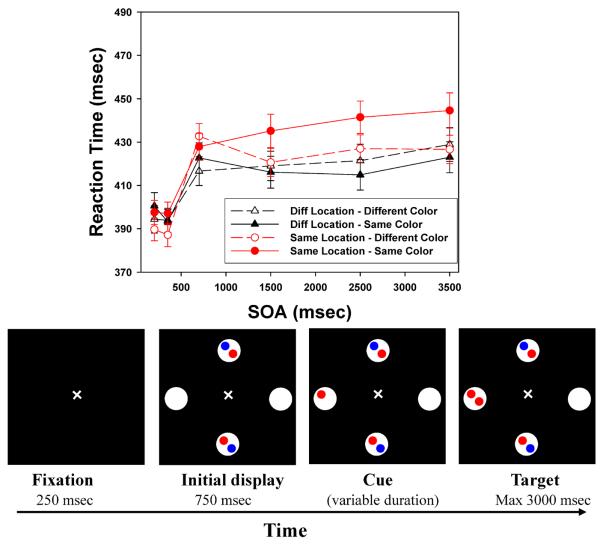
The apparatus and procedure matched those of Section 1, except that the displays now only included four or two large circles (see Figures 4 and 5). In Experiment 3, filled circles and empty circles alternated, with two (red or blue) disks present in the filled circles; in this respect, the method was identical to that in Experiments 1 and 2. The filled circles were eliminated in the displays for Experiment 4, producing 2-location conditions similar to those used in many IOR experiments. In both experiments, the cue appeared in one of the two empty circles and the target then appeared either within the Same circle or in the other originally empty circle (a Different Location trial). The target was presented equally often in two locations (50% Same, 50% Different). Again, these conditions are quite similar to those in classic IOR studies. There were 48 target-present cases in both Experiment 3 and Experiment 4: 2 locations of the cue × 2 locations of the target × 6 SOAs × 2 color repetition cases (match or mismatch of cue-target color); the 48 types were each presented eight times. These 384 trials were presented in 4 passes, with each pass consisting of 96 target-present trials plus 10 catch trials.
Figure 4.
Top: Target detection times of Experiment 3, broken down by Color (repeated, nonrepeated), Location (Same, Diff), and stimulus onset asynchrony (SOA); Bottom, example of the sequence of events for a sample trial in Experiment 3 (size 4, cue stays on) (not drawn exactly to scale). Error bars indicate standard errors.
Figure 5.
Top: Target detection times of Experiment 4, broken down by Color (repeated, nonrepeated), Location (Same, Diff), and stimulus onset asynchrony (SOA); Bottom, example of the sequence of events for a sample trial in Experiment 4 (size 8, cue goes off) (not drawn exactly to scale). Error bars indicate standard errors.
Results and discussion
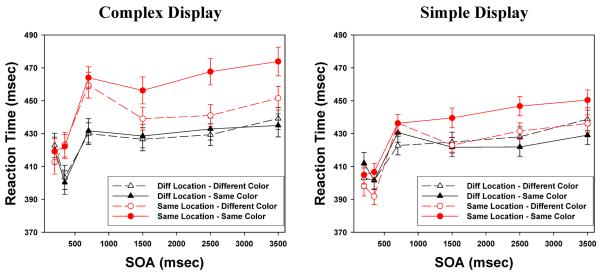
In both experiments, all subjects performed well, with miss rates under 1% and false alarm rates under 2%. The average response time was 416 msec for the 4-location displays, and 429 msec for the 2-location version. Figures 4 and 5 present the mean target detection times (from correct trials) broken down by target location, SOA, and the color repetition factor. Comparison of the results here to the results in Section 1 suggests that both the LIOR (circles versus triangles) and the FRIE (open versus filled circles) were reduced in these less complex displays.
Response times less than 100 ms or greater than 1500 ms were removed prior to analysis (less than 1%). In each experiment, the mean RTs were submitted to a 2 (Color Repetition: repeated vs non-repeated) × 6 (SOA: 200, 350, 700, 1500, 2500 and 3500 ms) × 2 (Location: Same vs. Different) ANOVA. In Experiment 3, all three main effects were significant: Color Repetition, F(1,59) = 11.325, p=.001; Location, F(1,59) = 15.219, p <.001; SOA, F(5,295) = 57.640, p<.001. In Experiment 4, two of the main effects were robust (Color Repetition, F(1,59) = 16.276, p<.001; SOA, F(5,295) = 27.651, p<.001), and the third was marginally significant (Location: F(1,59) = 3.431, p = .069). In both experiments, the two-way interaction of Location × SOA was significant (4-location: F(5,295) = 7.550, p <.001; 2-location: F(5,295) = 5.091, p <.001), as was the interaction of Location × Color Repetition (4-location: F(1,59) = 20.397, p <.001; 2-location: F(1,59) =14.961, p <.001) . The SOA × Color Repetition interaction was not significant in either experiment (4-location: F(5,295) = .741, p =.593; 2-location: F(5,295) = 1.276, p =.274). In both experiments, the three-way interaction reached significance (4-location: F(5,295) = 5.691, p <.001; 2-location: F(5,295) = 3.787, p =.002).
Location repetition: LIOR
The facilitation trend at the shortest SOA did not reach significance in the 4-location version (t(59)= −1.144, p=.129), but was significant for the 2-location displays (t(59)= −1.931, p=.029). Unlike the complex displays of Section 1, the displays in Section 2 did not produce a reliable inhibitory effect at the 350 msec SOA (4-location: t(59)= −.511, p =.306; 2-location: t(59)= −.939, p =.176). Beginning with the 700 msec SOA, LIOR was clearly present (4-location: t(59)=3.377, p =.001; 2-location: t(59)=2.671, p =.005). For the 4-location displays, LIOR was robust at the three longest SOAs (smallest t(59)= 2.819, p=.003). For the 2-location displays, LIOR was significant at the 2500 and 3500 msec SOAs (smaller t(59)=1.842, p=.035), and was marginally significant at an SOA of 1500, t(59)=1.515, p =.068. Thus, simpler displays support IOR, but the effect is smaller and does not reach significance as quickly.
Feature (color) repetition: FRIE
As in Experiment 2, a color-based inhibitory effect was not consistently observed for SOAs under one second. At the shortest SOA, the effect was significant for the 4-location displays (t(59)=2.505, p=.008) but not for the 2-location displays (t(59) = .983, p =.165). The effect was reliable at the 350 msec SOA in both experiments (4-location: t(59)=2.779, p=.004; 2-location: t(59) = 3.694, p <.001), but failed to reach significance at the 700 msec SOA in both cases (4-location: t(59)= −1.281, p =.103; 2-location: t(59)=.915, p =.182). For the three longest SOAs, color-based inhibition in the Same location was robust in both experiments (4-location: smallest t(59) =3.317, p = .001; 2-location: smallest t(59) =1.905, p = .031).
Section 2 Conclusion
Comparing Figures 4 and 5 to Figures 2 and 3, both LIOR and FRIE magnitudes in the simpler displays were reduced relative to the effects in the more complex displays. In Section 3, we conduct a more formal examination of the effect of display complexity.
Section 3: A Comparison of Inhibition Effects Across Display Complexity
Section 1included a pair of experiments that used displays similar to those we have used previously (Hu et al., 2011; Samuel & Kat, 2003; Samuel & Weiner, 2001). These displays are more complex than those used in most IOR research, and have revealed inhibitory effects of feature repetition that had been rather elusive in most prior IOR studies. Section 2 presented two experiments that used less complex displays, ones that were designed to be similar to those used in many prior IOR studies. To examine the effect of display complexity, we will test whether LIOR and FRIE were in fact more robust in Section 1 than in Section 2. In preliminary analyses of LIOR and FRIE, the 2- and 4-location cases were statistically indistinguishable; the same was true for the 6- and 8-location cases. In contrast, we found that LIOR was significantly stronger in the 6-location displays than in the 4-location displays, F(1,114) = 29.742, p<.001. We are not suggesting that there is a qualitative change between locations with four active locations versus those with six. However, the analyses do indicate that it would be reasonable to test the role of complexity by comparing the results for the two most complex cases to the two simplest ones.
Figure 6 provides a comparison of the overall patterns for the Complex displays (left panel: data collapsed across Display Sizes 6 and 8) and for the Simple ones (right panel: data collapsed across Display Sizes 2 and 4). As would be expected, reaction times are generally faster in the simpler displays than in the more complex ones. However, this difference is clearly limited to targets that were in the Same location as their cues. For Different-location trials, the curves are extremely similar across the two panels.
Figure 6.
Left panel: Mean Location IOR effect for complex display size experiments; Right panel: Mean feature repetition effect for simpler display size experiments. Error bars indicate standard errors.
To directly test how display complexity affected location-based IOR and feature-based repetition effects, we conducted two ANOVAs comparing the results from Section 1 (Complex) and Section 2 (Simple). One ANOVA tested for the effect of display complexity on LIOR, and the other tested whether FRIE depended on display complexity (in each, degrees of freedom were corrected for violations of the sphericity assumption using the Huynh-Feldt procedure). Both ANOVAs included the factors of Display Complexity (Complex vs. Simple) and SOA (700, 1500, 2500 and 3500 ms). The third factor in the LIOR analysis was Location (cue-target Same versus Different). For FRIE, the third factor was Color Repetition (Same versus Different), and as usual, in the FRIE analysis we only included data for targets in the Same location as the cues.
Figure 7 shows the effects being tested in these ANOVAS, with the LIOR effect (left panel) computed as the difference between Same and Different cue-target Locations, and the FRIE (right panel) computed as the difference between Same Color and Different Color. As Figure 7 shows, LIOR was much larger in the more complex displays, producing a significant interaction of Display Complexity with Location, F(1,225) = 29.769, p<.001. This difference was essentially constant between the 700 and 3500 ms SOAs, leading to a non-significant three-way interaction, F(3,645) = 1.60, p=.190. Feature-based effects also were more robust with the Complex displays than with the Simple ones, F(1,225) = 4.620, p=.033. The FRIE was significantly larger at longer SOAs, F(3,669) = 6.443, p<.001, reflecting its late development and thus small effect at the 700 ms SOA. Although there is a trend toward larger Complexity effects at longer SOAs, the three-way interaction was not significant, F(3,669) = 0.598, p=.612.
Figure 7.
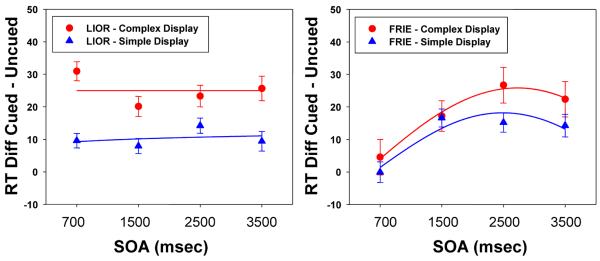
Left panel: Mean Location IOR effect for complex display size for SOAs greater than or equal to 700 msec (fitted with the equation: f= 28.16 − 1.27*X [complex display]; f= 8.89 + .56*X [simpler display]); Right panel: Mean feature repetition effect for complex and simpler display size, at the same SOAs, for the Same location (fitted with the equation: f= 25.81*sin(2*pi*x/9.88 + 5.80) [complex display]; f= 18.18*sin(2*pi*x/8.36 + 5.61) [simple display]). Error bars indicate standard errors.
General Discussion
Our goal here has been to clarify the properties of location-based and feature-based inhibitory effects, with a particular focus on whether these effects depend on the complexity of the stimulus displays. Our design has allowed us to simultaneously assess location-based and featural inhibition, and has provided evidence for different time-course patterns for the two.
Location-based IOR and display complexity
Our primary focus is the role that display complexity has on the location and feature repetition inhibitory effects. There is essentially no existing literature on this topic with respect to feature repetition, but there are a number of relevant prior findings regarding standard location-based IOR. For example, there is evidence that LIOR can co-occur at multiple locations (e.g. Danziger, Kingstone & Snyder, 1998) and that LIOR is larger when search is difficult (Klein & MacInnes, 1999; Snyder & Kingston, 2007). However, there is also a report that the strength of IOR declines with an increasing number of active locations (Pratt, Adam & McAuliffe, 1998). Birmingham et al. (2007) pointed out that displays with more locations also generally have greater distances between the locations of cues and targets than displays with fewer locations, and that we know IOR strength falls off with cue-target distance (Bennett & Pratt, 2001; Maylor & Hockey, 1985; Samuel & Weiner, 2001). In a clever set of experiments, Birmingham et al. isolated the distance factor, and demonstrated that it was in fact responsible for the drop in IOR size with an increased number of locations.
In the present study, we manipulated number of display locations, and found larger LIOR effects in the displays with more locations, despite the larger cue-target distance in more complex displays than in simpler displays. Critically, our displays did not just increase the number of locations, but also increased the complexity of the displays. That is, by interleaving “busy” locations between the actively tested locations, and by having multicolored displays, we increased the visual complexity of the scenes as display size increased. The observed increase in LIOR strength with display complexity complements prior results showing a similar effect of search difficulty. Both of these findings are predicted by the view that the function of IOR is to facilitate foraging (Klein, 1988; Posner & Cohen, 1984; Tipper et al., 1994).
We should add one caveat here. As Figure 7 showed, the LIOR effects for the more complex displays were in the range of 20–25 msec, while those for the simpler displays were about half as large. In absolute terms, the effects for the complex displays here are about the same size as those in typical IOR studies (see Samuel & Kat, 2003, for a graphical summary), rather than being larger. In contrast, in previous studies using similarly complex displays (Hu et al., 2011; Samuel & Kat, 2003; Samuel & Weiner, 2001), the effects were in the 40 msec range, larger than in typical studies. We suspect that the larger effects in those studies stem from the combination of the complex displays with more variability in the smaller embedded stimuli (multiple colors, multiple shapes) than in the current study, but this remains to be tested.
Feature repetition inhibitory effects and display complexity
Experiments 1 and 2 of the current study were similar to Hu et al.'s (2011) study of the inhibitory effect of feature repetition in relatively complex displays. Hu et al. found that there were indeed such costs, and the current study has clearly confirmed this. As we noted in the Introduction, the absence of much prior work on this topic probably stems from the mostly negative results that had been obtained in early studies. The current study provides substantial new information about the operation of feature-based inhibition. The right side of Figure 7 illustrates the major new findings, and shows two reasons why these effects were not usually found in early work. First, substantial effects are not consistently seen for SOAs under 1500 ms, and such long SOAs were rarely tested in prior IOR studies, let alone in studies looking for feature-based repetition costs. In fact, in Samuel and Kat's (2003) examination of 166 IOR tests in the literature, only 6 had SOAs this long (< 4%). Second, at the shortest SOA (700 ms) showing the feature repetition cost, the effect only was found with the complex displays (Sizes 6 and 8); such complex displays were not used in early feature-based tests.
Implications of the time-course of the two inhibitory effects
We have noted that the time-course of the LIOR and FRIE differ. The purely location-based inhibition arises more quickly than the feature-based effect, especially in more complex displays. This can be seen easily in the left panel of Figure 6: The two Same-Location curves (plotted with circles) clearly separate from the two Different-Location curves (plotted with triangles) by the 350 ms SOA, whereas the separation of the Same-Color (filled circles) and Different-Color (open circles) cases for Same-Location stimuli is only clear beginning at 1500 ms. In essence, targets that are extremely similar to cues (close location, same features) remain difficult to detect through the course of the trial, whereas targets with a featural difference from the cue become easier to detect. We should emphasize that accuracy was virtually perfect in both cases, so what we are seeing in the reaction times is a difference in processing difficulty, not a failure.
We suggested in our previous paper (Hu et al., 2011) that Lupiañez `s (2010) model of IOR could nicely explain the feature-based effects we observed, and this model is similarly well-suited to account for the results of the current study. The model assumes that in a given experimental situation, performance is determined by the effect of three factors on the particular task (e.g., detection of a target). The appearance of a cue can potentially facilitate performance due to two of the factors (spatial selection and spatial orienting), particularly at short cue-target SOAs: the cue can aid the observer by providing information about the target location on Same trials. However, the cue can potentially impair target detection due to the third factor (detection cost). In the model, both cues and targets are assumed to be represented in object files (Kahneman, Treisman & Gibbs, 1992). The detection cost is driven by the possibility that the target may be absorbed into the object file of the cue, impairing the ability to detect the target.
The model naturally accounts for the FRIE because sharing attributes should make cues and targets more likely to be mapped onto a single object file (Hommel, 2004). In fact, the pattern shown in Figure 6 is predicted if we assume that the likelihood of absorbing the target into the object file of the cue depends on an inclusive definition of similarity. That is, if similarity is a function of not only feature overlap but also of matching in time and in space, then targets should get absorbed most often in the Same Location / Same Color condition at short SOAs. The facilitative effect on spatial localization of the target initially offsets this cost, but by 350 ms LIOR appears as the detection cost outweighs the localization advantage. For SOAs of less than about one second the spatiotemporal overlap appears to be the dominant factor in impairing detection; we see LIOR but not FRIE. However, as the temporal separation of cue and target increases further, the featural overlap becomes critical, with fully-matching targets continuing to suffer a large detection cost, presumably because they continue to match the cue's object file parameters on all dimensions except time. This leads to the separation of the curves for fully-matching targets from those that differ in a feature, producing the FRIE.
For the current findings regarding display complexity, it seems reasonable to assume that such erroneous convergent mapping would happen more often in more complex displays, producing stronger LIOR, and potentially stronger FRIE. In fact, as shown in the right panel of Figure 6, the LIOR and FRIE patterns in the simpler displays are essentially dampened versions of what is seen in the complex ones. The initial rise in reaction times for both feature-matching and feature-mismatching targets in the Same location is smaller than the corresponding rise in the complex displays, i.e., LIOR is reduced. And, because this initial elevation is smaller, the size of the drop in reaction times for the feature-mismatching case (down to the level of the Different location results) is correspondingly reduced: the FRIE is smaller.
From a theoretical perspective, an attractive feature of the Lupiañez (2010) model is that it explicitly specifies multiple processes that reflect the complexity of attentional allocation in the complex visual environment in which we operate. Thus, certain aspects of a visual display can simultaneously produce positive effects (e.g., in helping to localize where an object is) and negative ones (e.g., by potentially matching an existing object file enough to be captured by it). Navigating the world is an extremely complex task, and presumably the neurocomputational processes that have evolved to accomplish this task will reflect this complexity. We believe that to fully engage these processes it is necessary to present the visual system with inputs that have enough in common with the real world. Clearly, there is a tension between this pressure and the need for strong experimental control. In the current study we have addressed this tension by systematically varying display complexity, and the results suggest that doing so can indeed provide further insights into how the perceptual system deals with the tremendously difficult problem that it successfully solves every day.
Author's notes and Acknowledgements
This research was financially supported by a research grant to Shuchang He (Natural Science Foundation of China, Grant no. 30870768) and a National Institutes of Health grant to Arthur G. Samuel (R01-059787). We thank all the participants for their contribution. We also thank Mike Dodd for his very helpful comments on an earlier version of this paper.
Appendix: T-test values for each experiment
Section I (Exp1. Size 8)
| LIOR tests [RT: Same Location– Different Location] | ||||
| Mean | t value | df | p | |
| SOA200 | −6.423 | −1.449 | 50 | 0.077 |
| SOA350 | 20.077 | 4.802 | 50 | 0.000 |
| SOA700 | 29.804 | 9.011 | 50 | 0.000 |
| SOA1500 | 17.061 | 4.468 | 50 | 0.000 |
| SOA2500 | 19.664 | 3.857 | 50 | 0.000 |
| SOA3500 | 22.958 | 4.127 | 50 | 0.000 |
| FRIE tests (from the “Same” Location condition) [RT: Same Color – Different Color] | ||||
| Mean | t value | df | p | |
| SOA200 | −6.153 | −0.967 | 50 | 0.169 |
| SOA350 | 1.632 | 0.264 | 50 | 0.397 |
| SOA700 | 11.591 | 1.899 | 50 | 0.032 |
| SOA1500 | 17.667 | 2.606 | 50 | 0.006 |
| SOA2500 | 33.523 | 4.250 | 50 | 0.000 |
| SOA3500 | 22.661 | 2.776 | 50 | 0.004 |
| Exp2. Size 6 | ||||
| LIOR tests [RT: Same Location– Different Location] | ||||
| Mean | t value | df | p | |
| SOA200 | −4.617 | −0.875 | 55 | 0.193 |
| SOA350 | 21.67 | 5.519 | 55 | 0.000 |
| SOA700 | 32.038 | 6.690 | 55 | 0.000 |
| SOA1500 | 23.163 | 4.840 | 55 | 0.000 |
| SOA2500 | 26.907 | 6.249 | 55 | 0.000 |
| SOA3500 | 28.309 | 5.548 | 55 | 0.000 |
| FRIE tests (from the “Same” Location condition) [RT: Same Color – Different Color] | ||||
| Mean | t value | df | p | |
| SOA200 | 18.921 | 2.637 | 55 | 0.005 |
| SOA350 | −3.877 | −0.551 | 55 | 0.292 |
| SOA700 | −2.449 | −0.278 | 55 | 0.391 |
| SOA1500 | 16.709 | 2.556 | 55 | 0.007 |
| SOA2500 | 19.834 | 2.627 | 55 | 0.006 |
| SOA3500 | 22.04 | 2.995 | 55 | 0.002 |
Section II (Exp3. Size 4)
| LIOR tests [RT: Same Location– Different Location] | ||||
| Mean | t value | df | p | |
| SOA200 | −3.865 | −1.144 | 59 | 0.129 |
| SOA350 | −1.416 | −0.511 | 59 | 0.306 |
| SOA700 | 10.646 | 3.377 | 59 | 0.001 |
| SOA1500 | 10.31 | 3.614 | 59 | 0.000 |
| SOA2500 | 16.08 | 5.171 | 59 | 0.000 |
| SOA3500 | 9.69 | 2.819 | 59 | 0.003 |
| FRIE tests (from the “Same” Location condition) [RT: Same Color – Different Color] | ||||
| Mean | t value | df | p | |
| SOA200 | 7.893 | 2.505 | 59 | 0.008 |
| SOA350 | 9.937 | 2.779 | 59 | 0.004 |
| SOA700 | −4.785 | −1.281 | 59 | 0.103 |
| SOA1500 | 14.519 | 3.536 | 59 | 0.000 |
| SOA2500 | 14.509 | 3.317 | 59 | 0.001 |
| SOA3500 | 17.966 | 4.292 | 59 | 0.000 |
| Exp4. Size 2 | ||||
| LIOR tests [RT: Same Location– Different Location] | ||||
| Mean | t value | df | p | |
| SOA200 | −7.884 | −1.931 | 59 | 0.029 |
| SOA350 | −3.404 | −0.939 | 59 | 0.176 |
| SOA700 | 8.537 | 2.671 | 59 | 0.005 |
| SOA1500 | 5.585 | 1.515 | 59 | 0.068 |
| SOA2500 | 12.304 | 3.524 | 59 | 0.000 |
| SOA3500 | 9.116 | 1.842 | 59 | 0.035 |
| FRIE tests (from the “Same” Location condition) [RT: Same Color – Different Color] | ||||
| Mean | t value | df | p | |
| SOA200 | 5.297 | 0.983 | 59 | 0.165 |
| SOA350 | 19.920 | 3.694 | 59 | 0.000 |
| SOA700 | 4.656 | 0.915 | 59 | 0.182 |
| SOA1500 | 18.648 | 5.042 | 59 | 0.000 |
| SOA2500 | 15.818 | 4.073 | 59 | 0.000 |
| SOA3500 | 10.506 | 1.905 | 59 | 0.031 |
References
- Bennett PJ, Pratt J. The spatial distribution of inhibition of return. Psychological Science. 2001;12(1):76–80. doi: 10.1111/1467-9280.00313. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Visser TAW, Snyder JJ, Kingstone A. Inhibition of return: Unraveling a paradox. Psychonomic Bulletin & Review. 2007;14(5):957–963. doi: 10.3758/bf03194128. [DOI] [PubMed] [Google Scholar]
- Danziger S, Kingstone A, Snyder JJ. Inhibition of return to successively stimulated locations in a sequential visual search paradigm. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(5):1467–1475. doi: 10.1037//0096-1523.24.5.1467. [DOI] [PubMed] [Google Scholar]
- Dukewich KR. Reconceptualizing inhibition of return as habituation of the orienting response. Psychonomic Bulletin & Review. 2009;16(2):238–251. doi: 10.3758/PBR.16.2.238. [DOI] [PubMed] [Google Scholar]
- Fox E, de Fockert JW. Inhibitory effects of repeating color and shape: Inhibition of return or repetition blindness? Journal of Experimental Psychology: Human Perception and Performance. 2001;27(4):798–812. doi: 10.1037//0096-1523.27.4.798. [DOI] [PubMed] [Google Scholar]
- Hommel B. Event files: feature binding in and across perception and action. Trends in Cognitive Sciences. 2004;8(11):494–500. doi: 10.1016/j.tics.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hu FK, Samuel AG, Chan AS. Eliminating inhibition of return by changing salient nonspatial attributes in a complex environment. Journal of Experimental Psychology: General. 2011;140(1):35–50. doi: 10.1037/a0021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff J, Taylor TL. Inhibition of return promotes stop-signal inhibition by delaying responses. Visual Cognition. 2006;13(4):503–512. [Google Scholar]
- Kahneman D, Treisman A, Gibbs BJ. The reviewing of object files: object-specific integration of information. Cognitive Psychology. 1992;24(2):175–219. doi: 10.1016/0010-0285(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibitory tagging system facilitates visual search. Nature. 1988;334:430–431. doi: 10.1038/334430a0. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, MacInnes WJ. Inhibition of return is a foraging facilitator in visual search. Psychological Science. 1999;10(4):346–352. [Google Scholar]
- Klein RM, Taylor TL. Categories of cognitive inhibition, with reference to attention. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 113–150. [Google Scholar]
- Kwak HW, Egeth H. Consequences of allocating attention to locations and to other attributes. Perception & Psychophysics. 1992;51(5):455–464. doi: 10.3758/bf03211641. [DOI] [PubMed] [Google Scholar]
- Law MB, Pratt J, Abrams RA. Color-based inhibition of return. Perception & Psychophysics. 1995;57(3):402–408. doi: 10.3758/bf03213064. [DOI] [PubMed] [Google Scholar]
- Lleras A, Rensink RA, Enns JT. Rapid resumption of interrupted visual search. Psychological Science. 2005;16(9):684–688. doi: 10.1111/j.1467-9280.2005.01596.x. [DOI] [PubMed] [Google Scholar]
- Lleras A, Rensink RA, Enns JT. Consequences of display changes during interrupted visual search: Rapid resumption is target specific. Perception & Psychophysics. 2007;69(6):980–993. doi: 10.3758/bf03193936. [DOI] [PubMed] [Google Scholar]
- Lupiáñez J. Inhibition of Return. In: Nobre AC, Coull JT, editors. Attention and time. Oxford University Press; Oxford: 2010. pp. 17–34. [Google Scholar]
- Lupiáñez J, Klein RM, Bartolomeo P. Inhibition of return: Twenty years after. Cognitive Neuropsychology. 2006;23(7):1003–1014. doi: 10.1080/02643290600588095. [DOI] [PubMed] [Google Scholar]
- MacInnes WJ, Klein RM. Inhibition of return biases orienting during the search of complex scenes. The Scientific World Journal. 2003;3:75–86. doi: 10.1100/tsw.2003.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylor EA, Hockey R. Inhibitory component of externally controlled covert orienting in visual space. Journal of Experimental Psychology: Human Perception and Performance. 1985;11(6):777–787. doi: 10.1037//0096-1523.11.6.777. [DOI] [PubMed] [Google Scholar]
- Muller HJ, von Muhlenen A. Probing distracter inhibition in visual search: Inhibition of return. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(5):1591–1605. doi: 10.1037//0096-1523.26.5.1591. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. Vol X. Erlbaum; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Pratt J, Adam J, McAuliffe J. The spatial relationship between cues and targets mediates inhibition of return. Canadian Journal of Experimental Psychology. 1998;52:213–216. [Google Scholar]
- Riggio L, Patteri I, Umilta C. Location and shape in inhibition of return. Psychological Research. 2004;68:41–54. doi: 10.1007/s00426-003-0136-7. [DOI] [PubMed] [Google Scholar]
- Samuel AG, Kat D. Inhibition of return: A graphical meta-analysis of its time course and an empirical test of its temporal and spatial properties. Psychonomic Bulletin & Review. 2003;10(4):897–906. doi: 10.3758/bf03196550. [DOI] [PubMed] [Google Scholar]
- Samuel AG, Weiner SK. Attentional consequences of object appearance and disappearance. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(6):1433–1451. [PubMed] [Google Scholar]
- Schneider W, Eschmann A, Zuccolotto A. E-Prime user's guide. Psychology Software Tools; Pittsburgh, PA: 2002. [Google Scholar]
- Snyder JJ, Kingstone A. Inhibition of return at multiple locations and its impact on visual search. Visual Cognition. 2007;15(2):238–256. [Google Scholar]
- Takeda Y, Yagi A. Inhibitory tagging in visual search can be found if search stimuli remain visible. Perception & Psychophysics. 2000;62(5):927–934. doi: 10.3758/bf03212078. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Shimojo S. Location vs feature: Reaction time reveals dissociation between two visual functions. Vision Research. 1996;36(14):2125–2140. doi: 10.1016/0042-6989(95)00272-3. [DOI] [PubMed] [Google Scholar]
- Taylor TL, Klein RM. Inhibition of return to color: A replication and nonextension of Law, Pratt, and Abrams (1995) Perception & Psychophysics. 1998;60(8):1452–1456. doi: 10.3758/bf03208005. [DOI] [PubMed] [Google Scholar]
- Thomas LE, Lleras A. Inhibitory tagging in an interrupted visual search. Attention Perception & Psychophysics. 2009;71(6):1241–1250. doi: 10.3758/APP.71.6.1241. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Driver J, Weaver B. Object-centered inhibition of return of visual attention. The Quarterly Journal of Experimental Psychology. 1991;43A:289–298. doi: 10.1080/14640749108400971. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Weaver B, Jerreat LM, Burak AL. Object-based and environment-based inhibition of return of visual attention. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(3):478–499. [PubMed] [Google Scholar]
- Wang Z, Klein RM. Searching for inhibition of return in visual search: A review. Vision Research. 2010;50:220–228. doi: 10.1016/j.visres.2009.11.013. [DOI] [PubMed] [Google Scholar]