Abstract
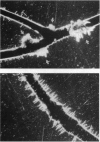
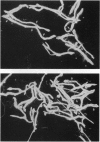
Arabidopsis thaliana mutants originally isolated as hypersensitive to irradiation were screened for the ability to be transformed by Agrobacterium transferred DNA (T-DNA). One of four UV-hypersensitive mutants and one of two gamma-hypersensitive mutants tested showed a significant reduction in the frequency of stable transformants compared with radioresistant controls. In a transient assay for T-DNA transfer independent of genomic integration, both mutant lines took up and expressed T-DNA as efficiently as parental lines. These lines are therefore deficient specifically in stable T-DNA integration and thus provide direct evidence for the role of a plant function in that process. As radiation hypersensitivity suggests a deficiency in repair of DNA damage, that plant function may be one that is also involved in DNA repair, possibly, from other evidence, in repair of double-strand DNA breaks.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaad F. F., Signer E. R. Somatic and germinal recombination of a direct repeat in Arabidopsis. Genetics. 1992 Oct;132(2):553–566. doi: 10.1093/genetics/132.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurazzi M., Signer E. R. Termini and telomeres in T-DNA transformation. Plant Mol Biol. 1994 Nov;26(3):923–934. doi: 10.1007/BF00028859. [DOI] [PubMed] [Google Scholar]
- Davies C., Howard D., Tam G., Wong N. Isolation of Arabidopsis thaliana mutants hypersensitive to gamma radiation. Mol Gen Genet. 1994 Jun 15;243(6):660–665. doi: 10.1007/BF00279575. [DOI] [PubMed] [Google Scholar]
- Gheysen G., Villarroel R., Van Montagu M. Illegitimate recombination in plants: a model for T-DNA integration. Genes Dev. 1991 Feb;5(2):287–297. doi: 10.1101/gad.5.2.287. [DOI] [PubMed] [Google Scholar]
- Harlow G. R., Jenkins M. E., Pittalwala T. S., Mount D. W. Isolation of uvh1, an Arabidopsis mutant hypersensitive to ultraviolet light and ionizing radiation. Plant Cell. 1994 Feb;6(2):227–235. doi: 10.1105/tpc.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B. J., Gardner R. C. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol. 1990 Jan;14(1):61–72. doi: 10.1007/BF00015655. [DOI] [PubMed] [Google Scholar]
- Jenkins M. E., Harlow G. R., Liu Z., Shotwell M. A., Ma J., Mount D. W. Radiation-sensitive mutants of Arabidopsis thaliana. Genetics. 1995 Jun;140(2):725–732. doi: 10.1093/genetics/140.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Barrena E. Prospects for reverse genetics in plants using recombination. Plant Mol Biol. 1993 Mar;21(6):v–xii. doi: 10.1007/BF00023619. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Ito Y., Hosoi T., Takahashi Y., Machida Y. Integration of Agrobacterium T-DNA into a tobacco chromosome: possible involvement of DNA homology between T-DNA and plant DNA. Mol Gen Genet. 1990 Dec;224(3):309–316. doi: 10.1007/BF00262423. [DOI] [PubMed] [Google Scholar]
- Mayerhofer R., Koncz-Kalman Z., Nawrath C., Bakkeren G., Crameri A., Angelis K., Redei G. P., Schell J., Hohn B., Koncz C. T-DNA integration: a mode of illegitimate recombination in plants. EMBO J. 1991 Mar;10(3):697–704. doi: 10.1002/j.1460-2075.1991.tb07999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R. J., Linard C. G., Signer E. R. Cytosine deaminase as a negative selective marker for Arabidopsis. Plant Mol Biol. 1993 Nov;23(4):793–799. doi: 10.1007/BF00021534. [DOI] [PubMed] [Google Scholar]
- Perez P., Tiraby G., Kallerhoff J., Perret J. Phleomycin resistance as a dominant selectable marker for plant cell transformation. Plant Mol Biol. 1989 Oct;13(4):365–373. doi: 10.1007/BF00015548. [DOI] [PubMed] [Google Scholar]
- Thaler D. S., Stahl F. W. DNA double-chain breaks in recombination of phage lambda and of yeast. Annu Rev Genet. 1988;22:169–197. doi: 10.1146/annurev.ge.22.120188.001125. [DOI] [PubMed] [Google Scholar]