Abstract
Newborns and young infants are particularly susceptible to infections, including Mycobacterium tuberculosis. Further, immunogenicity of vaccines against tuberculosis and other infectious diseases appears suboptimal early in life, compared with later in life. We hypothesized that developmental changes in innate immunity would underlie these observations.
To determine evolution of innate responses to mycobacteria early in life, whole blood from newborns, 10-week old and 36-week old infants was incubated with viable Mycobacterium bovis Bacille Calmette Guerin (BCG) or TLR ligands. Innate cell expression of cytokines and maturation markers was assessed, as well as activation of the pro-inflammatory NF-κB and MAPK signaling pathways.
BCG-induced production of pro-inflammatory cytokines TNF-α, IL-6 and IL-12p40 increased from the newborn period to 9 months of age in monocytes, but not in myeloid dendritic cells (mDCs). No changes in production of anti-inflammatory IL-10 were observed. CD40 expression increased with age in both cell populations. Older infants displayed substantial activation of all three signal transduction molecules: degradation of NF-κB inhibitor IκBα and phosphorylation of MAPK Erk and p38 upon TLR1/2 triggering, compared with predominant activation of only one of any of these molecules in newborns.
Maturation of innate pro-inflammatory responses during the first 9 months of life may underlie more effective control of mycobacteria and other pathogens observed later in infancy, and age-related differential induction of Th1 responses by vaccination.
Keywords: infants, monocytes, mycobacteria, pro-inflammatory, signal transduction
Introduction
Innate cells such as granulocytes, macrophages/monocytes and dendritic cells (DCs) form a cornerstone of host defense against infection with bacteria such as Mycobacterium tuberculosis (M.tb). These cells recognize conserved pathogen-associated molecular patterns (PAMPs) through germ-line encoded pattern recognition receptors (PRRs) (1), such as toll-like receptors (TLRs) (2). PRR triggering leads to the activation of complex signaling networks, including MAPKs and NF-κB pathways (3, 4), and regulates phagocytosis (5), antimicrobial activities, cytokine production (6), and the induction and nature of adaptive immune responses (7).
The neonatal immune system is specifically adapted for transition from a sterile intra-uterine compartment to an environment ripe with microbial challenges. At birth, the innate response to pathogens is less vigorous, characterized by lower pro-inflammatory cytokine production and greater regulatory cytokine production, than that typically observed in adults (reviewed in (8) and (9)). Cord blood monocytes and DCs express low levels of co-stimulatory molecules and are relatively unresponsive to LPS or IFN-κ, compared to monocytes and DCs from adults (10). Neonatal monocytes also have reduced capacity to differentiate into DCs in vitro, and cord blood monocyte-derived DCs (MDDCs) produce lower levels of IL-12 in response to toll-like receptor (TLR) and CD40 signaling, than adult monocytes (9, 11). Most of these responses were observed upon stimulation with purified TLR agonists and it is not currently known how innate cells respond to mycobacteria, which express multiple ligands recognized by various PRRs. The focus of this study was to evaluate innate responses to the live mycobacterium, attenuated M. bovis Bacille Calmette-Guerin (BCG), which is the currently licensed vaccine against tuberculosis (TB), during the first 9 months of life.
TB is a major cause of morbidity and mortality in young children in developing countries (12, 13). At least 74,000 HIV-uninfected children died from TB in 2012, and children account for half a million new TB cases every year (14). Newborns and older infants up to 6 months of age are more likely to develop pulmonary and disseminated forms of TB disease than older children (12, 13,15, 16). Before TB treatment was available, mortality rates of infants less than 6 months of age were ~55%, compared with ~30% in children aged 1 to 2 years (17). The immature innate immune response in early life may contribute to the higher susceptibility of newborns and older infants to infections, including M.tb (18, 19).
Vaccination-induced immunity is less effective for many vaccines when administered during the first months of life than at older ages (8). The only vaccine currently available for the prevention of TB, BCG, is given at birth in most countries endemic for TB. BCG protects infants against disseminated forms of TB disease but confers limited protection against pulmonary disease, at all ages (20). We previously showed that frequencies of BCG-specific T cell responses measured in 1 year old infants were higher when BCG vaccination was delayed to 10 weeks of age, compared with administration at birth (21).
If our hypothesis of a causal relation between innate immune status and susceptibility to M.tb and other infections were correct, we would expect that as the risk for disease decreases over the first year of life, innate immune responses to mycobacteria should become more adult-like. To test this hypothesis, we investigated production of pro- and anti-inflammatory cytokines, expression of co-stimulatory molecules, phagocytosis of mycobacteria and TLR-induced signaling pathways in innate cells over the first 9 months of life.
Materials and methods
Participants and blood collection
This study was completed at the South African TB Vaccine Initiative (SATVI) site in the Cape Town region of South Africa. The aim was to enroll 25 pregnant mothers for collection of cord blood immediately after giving birth by Ceasarian section, a separate group of 35 infants aged 10 weeks, and a separate group of 35 infants aged 36 weeks. All infants aged 10 and 36 weeks received BCG vaccine at birth as is routine in South Africa. Pregnant mothers and infants with TB disease or possibly exposed to M. tb, who were HIV-infected or HIV-exposed (infants), who had any other acute or chronic disease and who were receiving medication other than sedation/anesthetics administered surrounding birth, were excluded. Cord blood was not collected from newborns with a birth weight <2.5kg. The project was approved by the Research Ethics Committee of the University of Cape Town; written informed consent was obtained from pregnant women and parents/legal guardians of infants.
TLR agonists, bacteria and antibodies
Ultrapure lipopolysacharide (LPS, TLR4 ligand, 100ng/mL used in whole blood assays) isolated from Salmonella minnesota, was obtained from InvivoGen. PAM3 (Pam3CSKKKK, N-palmitoyl-S-[2,3-bis- (palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine, TLR2/1 ligand, 300ng/mL used in whole blood assays, 10 µg/mL used in PBMC phosphoflow assays) was obtained from EMC Microcollections. Log-phase derived live BCG expressing green fluorescent protein (BCG-GFP, Pasteur strain; used at 3.5 × 105 CFU/mL in assays) was donated by Muazzam Jacobs, University of Cape Town.
The following antibodies were used for whole blood flow cytometry: CD14-QDot605 (clone Tuk4, Invitrogen), TNF-α-PECy7 (Mab11), CD11c-V450 (B-ly6), CD86 (B70/B7–2)-PE (both from BD Pharmingen), CD11c-PerCPCy5.5 (Bu15), HLA-DR-AlexaFluor700 (L243), IL-12/23p40-Pacific Blue (C11.5), IL-10-PE (JES3-19F1), IL-6-APC (MQ2-13A5), CD40-PerCP-Cy5.5 (5C3) and CD83-APC (HB15e) (all from Biolegend). CD66a/c/e (ASL-32, Biolegend) was conjugated in-house to QDot565 (Invitrogen) using the manufacturer’s protocol. The following antibodies were used for PBMC phosphoflow experiments: CD14-Pacific Blue (clone M5E2), p-p38 MAPK-PECy7 (36/p38: pT180/pY182), p-Erk1/2-AlexaFluor647 (20A: pT202/pY204), IκBα-PE (25/IκBα/MAD-3), all from BD Biosciences.
Whole blood incubation and cryopreservation
Peripheral blood from infants and cord blood from newborns were collected into sterile, heparinized blood collection tubes or bags, immediately transported to the laboratory and processed within 30 minutes of collection. One hundred and eighty L whole blood was added to wells of a 96-well round bottom plate, already containing 20µL RPMI (Lonza) with individual TLR agonists or BCG. Polymixin B (BioChemika, 10mg/mL) was added to the wells containing PAM3 to minimize possible effects of LPS contamination. RPMI only was used as medium control (no antigen). Incubation proceeded at 37°C, 5% CO2 in humidified conditions for 6 hours for an intracellular cytokine staining (ICS) assay (unstimulated, BCG and LPS) as previously optimized (22), and for 18 hours to measure maturation marker expression on innate cells and soluble cytokine production (unstimulated, BCG, PAM3 and LPS). For the 6-hour ICS assay, brefeldin A (BFA, 10µg/mL, Sigma-Aldrich) was added to each well after 3 hours of incubation; for the 18-hour assay, no BFA was added. Further, after a total incubation of 18 hours, 100µL assay supernatant was removed and stored at −80°C. Whole blood from 25 infants of each age group was stimulated in duplicate wells and cells and supernatants from each duplicate were pooled for analyses. After incubation, 2mM EDTA (final concentration, Sigma-Aldrich) was added for 10 minutes, followed by lysis of red cells and fixing of white cells with FACS Lysing Solution (BD Biosciences) for 10 minutes, repeated once after a wash. Fixed white cells were cryopreserved in 10% DMSO in heat inactivated fetal calf serum (FCS) in liquid nitrogen.
Staining of cells and flow cytometric acquisition
Cryopreserved, stimulated cells were thawed in batch, and washed twice with either phosphate buffered saline (PBS, without calcium and magnesium) or BD Perm/Wash buffer (BD Biosciences). Samples from the 6h incubation were stained with fluorescent antibody cocktails in BD Perm/Wash, at 4°C for 1 hour. Stained cells were washed and 1 million cells acquired on a BD LSR II flow cytometer. Only results from samples with an acquired total leukocyte number exceeding 500,000 were included in the analysis. None failed to meet this criterion.
The cells yielded by the longer incubation were washed twice in PBS. A two-step staining protocol was employed; cells were first stained with non-QDot-conjugated antibodies in PBS, for 1 hour followed by a second 1-hour staining step with QDot-conjugated antibodies in BD Perm/Wash buffer. We have previously observed that PBS adversely affects fluorescence of QDot-conjugated antibodies (22). Stained cells were washed and acquired on a BD LSR II flow cytometer. Only results from samples with an acquired total leukocyte number exceeding 500,000 were included in the analysis. None failed to meet this criterion.
Flow cytometric measurement of signaling pathways in PBMC
PBMC were isolated by gradient centrifugation of infant blood (cord blood n=10; 10-week-old n=7; 36-week-old n=7), and cryopreserved in FCS containing 10% DMSO within 4 hours of phlebotomy. Cryopreserved PBMC were thawed, washed and rested in RPMI containing 1% L-Glutamine (Lonza), 10% human AB serum (Sigma) and DNAse (30 Kunitz/mL, Sigma) for 6 hours at 37°C, 5% CO2 in humidified conditions. PBMC were then washed and rested in the absence of serum for an additional 30 to 60 minutes in RPMI containing 1% L-Glutamine. PBMC viability was assessed by Trypan Blue exclusion. Cells were left unstimulated or incubated with PAM3 for exactly 30 minutes at 37°C and then fixed in PBS containing 2% paraformaldehyde. Fixed PBMC were washed with ice-cold PBS and permeabilized using Perm Buffer IV 0.5× (BD Phosflow) for 20 minutes. Cells were washed twice in PBS containing 1% FCS and stained for 1 hour at room temperature with antibodies. Stained cells were washed and acquired on a BD LSR II flow cytometer.
Secreted cytokine measurement
Supernatants from whole blood stimulated for 18 hours were thawed. Concentrations of IL-12p40, IL-12p70, IL-10, IL-6 and TNF-α were measured for all samples by Milliplex MAP Multiplex Immunoassay (based on Luminex MAP technology; Millipore Corporation) on a Bio-Rad Luminex 100 Bio-Plex Liquid Array Multiplexing System Fluorescent Reader, according to the manufacturer’s instruction. Concentrations of the standards in 200µL assay buffer ranged from 10000, 2000, 400, 80, 16 to 3.2 pg/mL. A sample volume of 25µL either diluted 2- or 25-fold was used.
Data analysis
Flow cytometry data were analyzed using FlowJo v9.2. Results from single-stained and unstained mouse κ beads were used to calculate compensations for each run. Cell doublets were excluded using forward scatter-area versus forward scatter-height parameters. Cytokine co-expression by monocytes and DCs and signaling molecule co-activation by monocytes were assessed by Boolean gating. Subtraction of background cytokine expression (unstimulated samples) was done using Pestle V1.6.2, while data sorting and analysis were done with Spice V5.1 (Roederer et al., 2011; http://exon.niaid.nih.gov/spice, accessed February 25th, 2011). The median fluorescent intensity for each cytokine was determined by Flowjo, and samples were excluded if there were less than 25 cells in a given cytokine gate.
For analysis the Milliplex MAP Multiplex Immunoassay data, standard curve values were considered as outliers and excluded if the observed/expected × 100 ((obs/exp)*100) was outside the range of 10030%, according to the manufacturer’s instruction. Cytokine values below the lowest level of detection were assigned half the lower value of the lowest standard.
For intracellular signaling assays, only samples showing >50% viable cells after resting were further processed and only those for which more than 1,000 monocytes were acquired were included in the analysis.
GraphPad Prism v5 was used for data presentation and statistical analysis. Comparisons within the same age group were done using a Wilcoxon matched-pairs signed rank test. Comparisons between the different age groups were assessed with a Kruskal-Wallis test, followed by Mann-Whitney U test, and p values less than 0.05 were considered statistically significant.
Results
Participants
We enrolled three groups of participants: newborns, infants aged 10 weeks and infants aged 36 weeks (Table I). Gender distributions between the groups were similar. Newborns were from Black African, South African Mixed Ancestry and Caucasian ethnic groups, whereas 10 and 36-week old infants were all from the mixed race group.
Table I.
Demographic characteristics of enrolled study participants.
| Newborns | 10 week old infants | 36 week old infants | ||||
|---|---|---|---|---|---|---|
| Whole blood assays |
PBMC assay |
Whole blood assays |
PBMC assay |
Whole blood assays |
PBMC assay |
|
| n | 25 | 10 | 25 | 7 | 25 | 7 |
| Median age (weeks) (IQR) |
0 | 0 | 10.1 (9.8–10.2) |
10.0 (9.2–10.4) |
36.2 (35.7–36.8) |
36.1 (34.7–37.1) |
| Gender | ||||||
| Female | 12 | 4 | 14 | 4 | 12 | 5 |
| Ethnicity | ||||||
| Mixed race | 10 | 4 | 25 | 7 | 25 | 7 |
| Black African | 3 | 0 | 0 | 0 | 0 | 0 |
| Caucasian | 12 | 6 | 0 | 0 | 0 | 0 |
Pro-inflammatory cytokine production in response to mycobacteria over the first 9 months of life
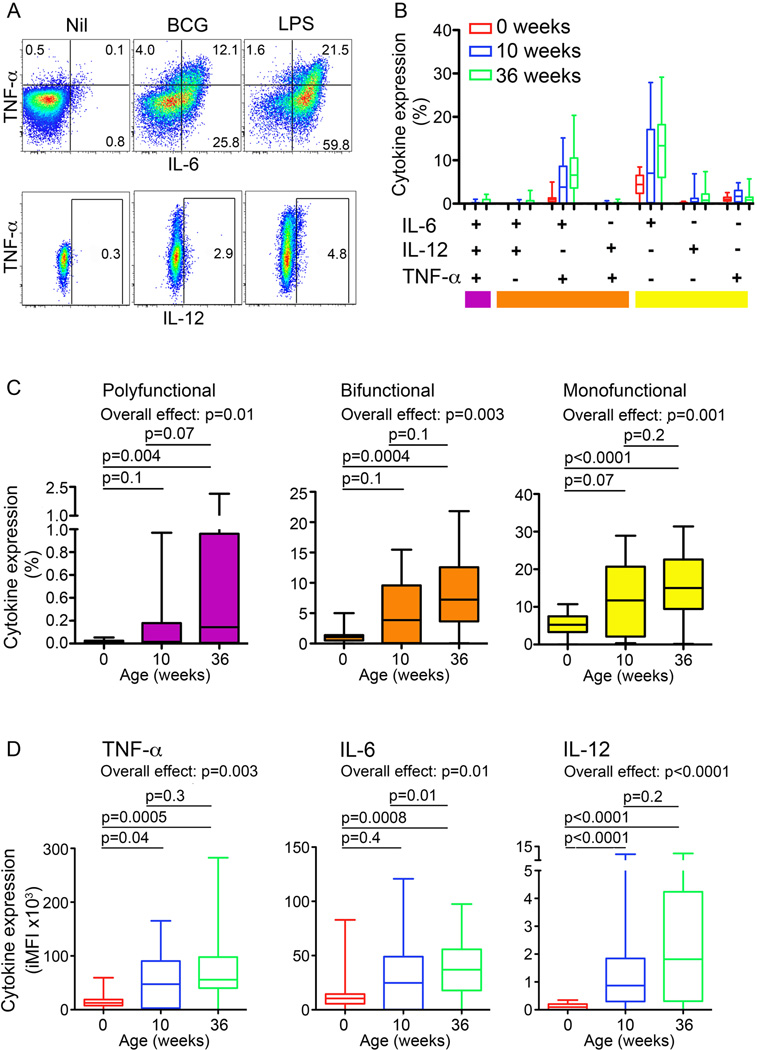
To investigate changes in innate immunity during the first 9 months of life, we evaluated intracellular expression of the pro-inflammatory cytokines TNF-α, IL-6 and the p40 subunit of IL-12/IL-23 (here referred to as IL-–12) by monocytes following incubation of whole blood with BCG or LPS (Fig. 1A and Supplementary Fig. 1).
Figure 1. Monocyte expression of pro-inflammatory cytokines upon BCG stimulation of whole blood from newborns and infants.
(A) Monocyte expression of TNF-α, IL-6 and/or IL-12 from a representative infant at 36 weeks of age. Frequencies (%) of cells falling into each gate are shown for each plot. (B) Frequencies of monocyte subsets co-expressing pro-inflammatory cytokines upon BCG stimulation of whole blood from newborns, 10 and 36-week old infants (n = 25 for each group). (C) Frequencies of BCG-stimulated monocytes expressing all 3 pro-inflammatory cytokines (polyfunctional), any 2 of the 3 cytokines (bifunctional) or any 1 of the 3 cytokines (monofunctional). (D) Integrated median fluorescence intensity (iMFI) of BCG-induced TNF-α, IL-6 and IL-12 expression by monocytes, representing the product of the frequency and the MFI of cytokine+ monocytes, from newborns, 10 and 36-week old infants. For box and whisker plots, horizontal lines represent the median, boxes represent the interquartile range (IQR) and whiskers represent the range. Group comparisons were done using the Kruskal-Wallis test (Overall effect), followed by the Mann-Whitney test.
Frequencies of monocytes expressing any combination of TNF-α, IL-6 and IL-12, or any single cytokine, in response to BCG increased over the study period (Fig. 1B and C). At all ages, frequencies of monocytes expressing IL-6 and/or TNF-α were higher than those expressing IL-12. Co-expression of IL-6 and TNF-α, and single expression of IL-6 dominated among possible cytokine combinations (Fig. 1B), regardless of age.
The median fluorescence intensity (MFI) of TNF-α and of IL-12 expression among monocytes positive for these cytokines also increased from the newborn period to 36 weeks of age (Supplementary Fig. 2, second panel and data not shown); IL-6 MFI did not change (Data not shown). To enable measurement of age-related changes in both frequencies and relative cytokine expression levels of innate cells, we calculated the product of cytokine+ monocyte frequencies and their median fluorescence intensities (MFI), the so-called integrated MFI (iMFI, Supplementary Fig. 2), described previously (23). iMFI levels of TNF-α, IL-6 and IL-12 expression in BCG-stimulated monocytes were lowest in newborns and increased with age (Fig. 1D).
Similar age-associated increases in monocyte expression of pro-inflammatory cytokines upon LPS stimulation were observed. These increases were not necessarily stepwise from birth to 10 and then 36 weeks of age (Supplementary Table I).
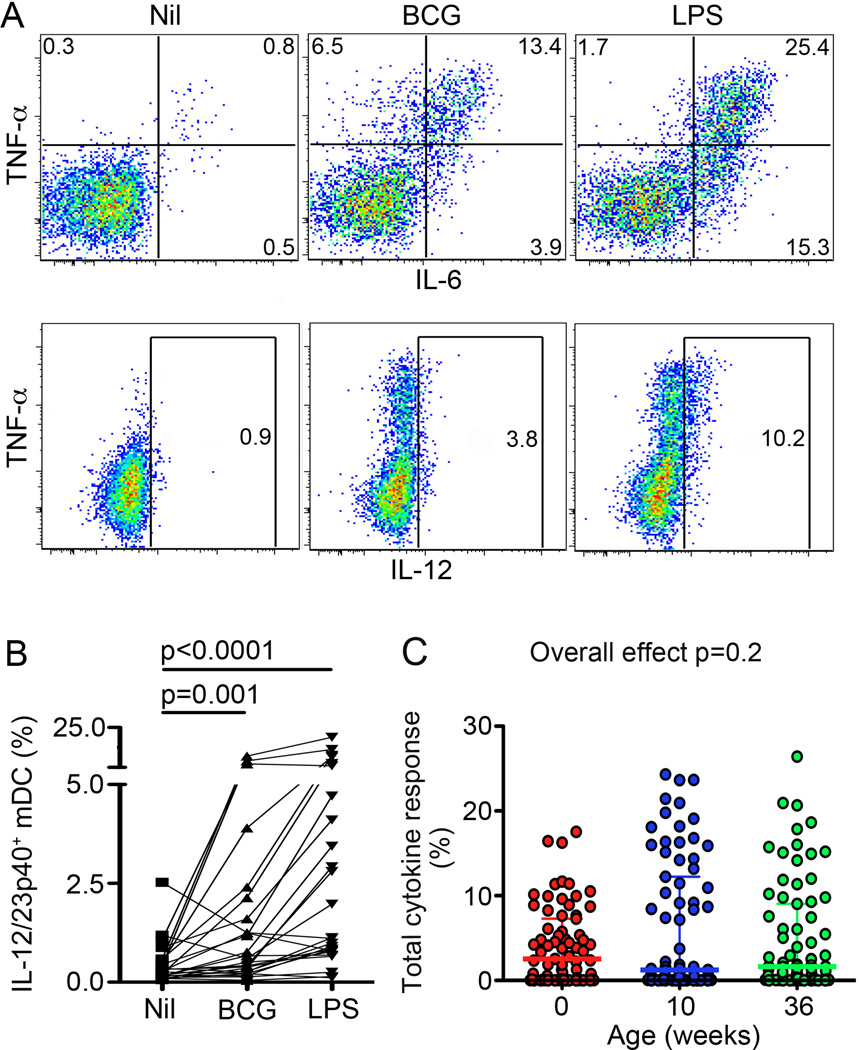
Similar to monocytes, the mDC response to BCG was characterized by a predominance of cells expressing IL-6 alone, or co-expressing IL-6 and TNF-α (data not shown). IL-12 expression was also relatively low, but detectable in mDCs in only a subset of participants (Fig. 2B).
Figure 2. Myeloid DC expression of pro-inflammatory cytokines upon BCG stimulation of whole blood from newborns and infants.
(A) mDC expression of TNF-α, IL-6 and/or IL-12 from a representative infant at 36 weeks of age. Frequencies of cells falling into each gate are shown for each plot. (B) Frequencies of mDCs expressing IL-12 in unstimulated (Nil), BCG- or LPS-stimulated whole blood from 36 week old infants (n = 25 for each group). (C) Frequencies of BCG-stimulated mDCs expressing any combination of TNF-α, IL-6 and/or IL-12. In scatter plots, horizontal lines represent the median and whiskers represent the interquartile range (IQR). Intra-group comparisons were done using the Wilcoxon test, while inter-group comparisons were done using the Kruskal-Wallis test (Overall effect).
In contrast to monocytes, frequencies and iMFIs of these cytokines in mDCs following incubation with BCG were not different in newborns, 10 and 36-week old infants (Fig. 2C and not shown).
By contrast, in response to LPS, higher expression of IL-12 and TNF-α was observed in older infants, as early as at 10 weeks of age, compared with newborns in both monocytes and mDCs (Supplementary Table I). Also, LPS-induced frequencies of mDCs co-expressing 2 or 3 cytokines were higher in older infants, compared with newborns, while frequencies of single cytokine-expressing mDCs were lower in older infants, compared with newborns (Supplementary Table I).
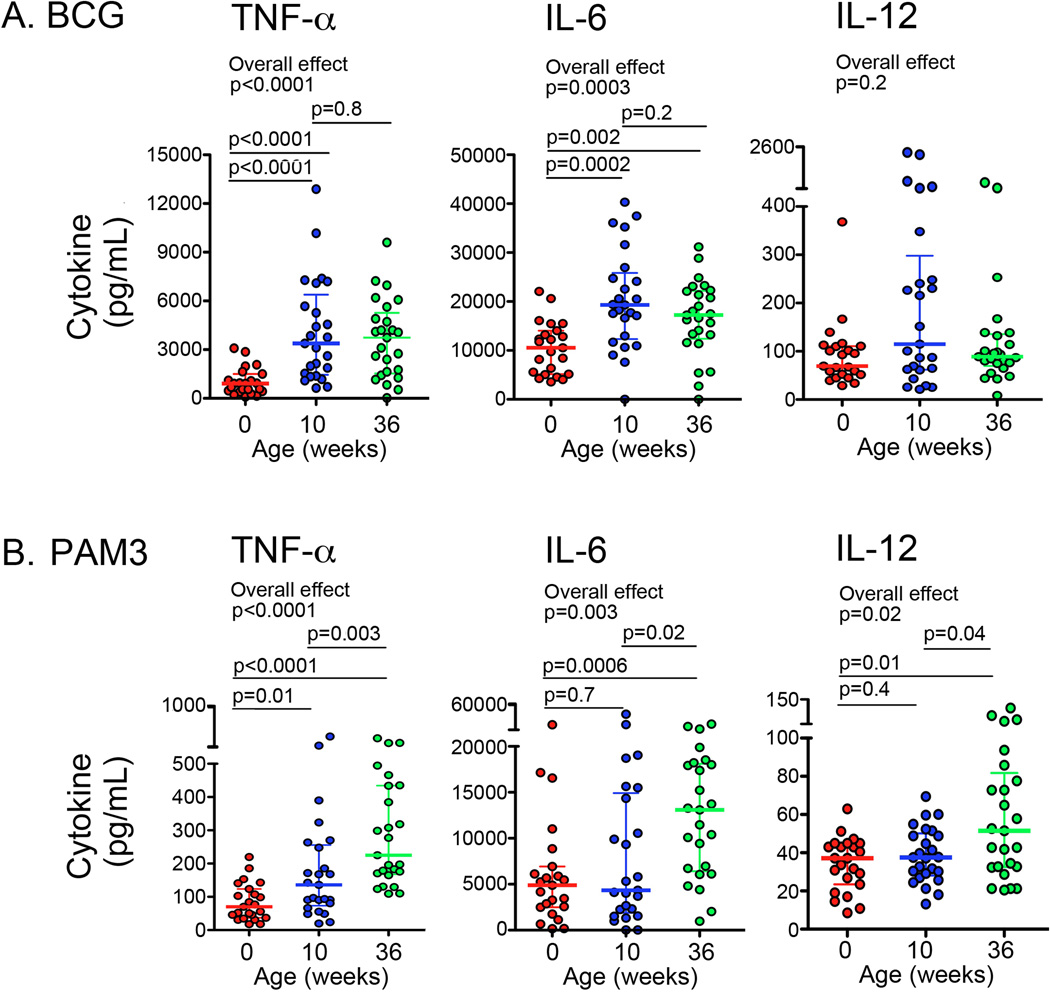
Next, we measured soluble levels of secreted pro-inflammatory cytokines after stimulation of whole blood for 18 hours with live BCG or with PAM3. Consistent with our results of monocyte responses, levels of TNF-α and IL-6 were lowest in newborns and increased with age in response to both BCG and PAM3 (Fig. 3A and 3B). Levels of IL-12 did not increase significantly with age upon BCG stimulation (Fig. 3A), but were higher in response to PAM3 in 36-week old infants, compared with younger infants (Fig. 3B). Soluble levels of TNF-α, IL-6 and IL-12 after LPS stimulation were again lower in newborns and increased with age (data not shown). Levels of these pro-inflammatory cytokines in response to BCG and LPS were not different in 10 and 36-week old infants, but in response to PAM3 these pro-inflammatory cytokines were higher at 36 weeks of age (p=0.02 for IL-6; p=0.04 for IL-12; and p=0.003 for TNF-α; data not shown).
Figure 3. Levels of soluble pro-inflammatory cytokines in plasma of whole blood from newborns and infants.
Levels of soluble TNF-α, IL-6, and IL-12/p40 after incubation of cord or peripheral blood from newborns, 10 or 36-week old infants (n = 25 for each group) with BCG (A) and PAM3 (B). In scatter plots, horizontal lines represent the median and whiskers represent the interquartile range (IQR). Group comparisons were done using the Kruskal-Wallis test (Overall effect), followed by the Mann-Whitney test.
Taken together, our results suggest that the anti-mycobacterial pro-inflammatory cytokine response by peripheral blood mononuclear innate cells is lowest at birth and increases during the first 9 months of life. The differences in responses observed between BCG and purified TLR ligands could be attributed to a complex array of ligands expressed by BCG that could be both activating and inhibitory to the immune system.
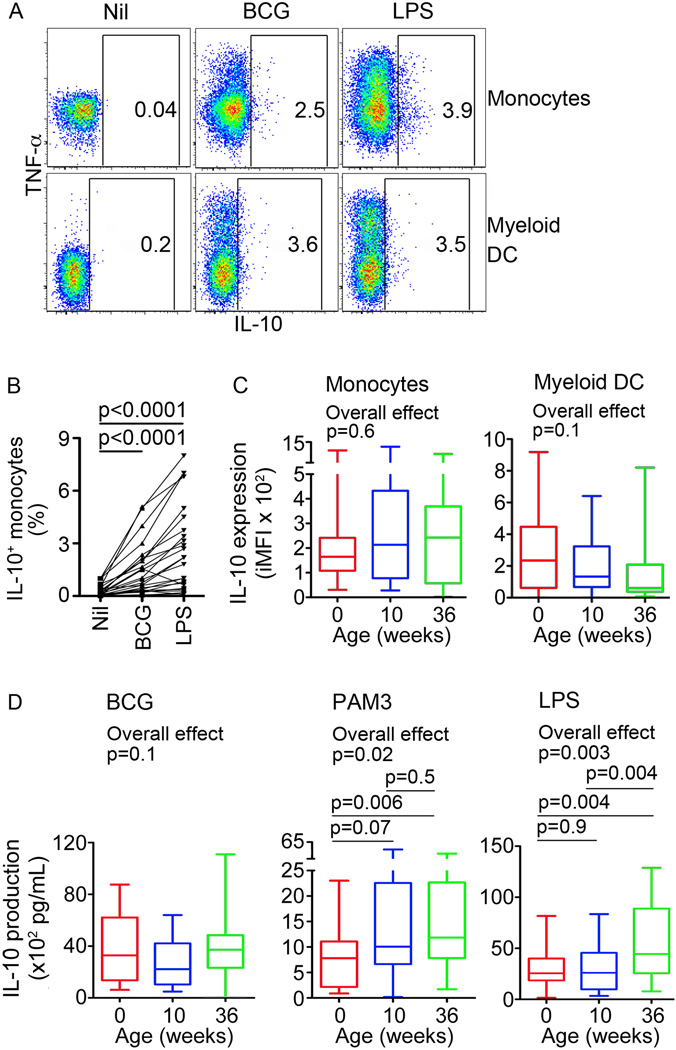
No change in anti-inflammatory IL-10 response to mycobacteria over the first 9 months of life
IL-10 is a key anti-inflammatory cytokine with a well-described role as suppressive regulator of pro-inflammatory immune responses during infection (24). To determine whether this anti-inflammatory response to mycobacteria changes during the first 9 months of life, we measured monocyte and mDC expression of IL-10 upon incubation of whole blood with BCG. Low levels of IL-10-expressing monocytes and mDCs were detected after BCG stimulation (Fig. 4A and B). In contrast to the pro-inflammatory monocyte response to BCG, frequencies and iMFI levels of IL-10-expressing monocytes and mDCs were not different in newborns and older infants (data not shown and Fig. 4C). Similarly, levels of secreted IL-10 after incubation with BCG for 18 hours were also not different in newborns and older infants (Fig. 4D). However, lower levels of secreted IL-10 were observed in newborns, compared with older infants after stimulation with PAM3 or LPS (Fig. 4D). LPS-induced intracellular IL-10 expression by monocytes or mDCs in newborns, 10 and 36-weeks old infants was also not different (Supplementary Table I).
Figure 4. Innate cell expression of IL-10 upon stimulation of whole blood from newborns and infants.
(A) Monocyte and mDC expression of IL-10 in whole blood from a 36-week old infant, stimulated with media (Nil), BCG or LPS. (B) Frequencies of cells falling into the IL-10 gate are shown for each participant. (C) Integrated median fluorescence intensity (iMFI) of BCG-induced IL-10 expression by monocytes or mDCs, representing the product of the frequency and the MFI of IL-10+ cells, from newborns, 10 and 36-week old infants (n = 25 for each group). (D) Levels of soluble IL-10 after incubation of cord or peripheral blood from newborns, 10 or 36-week old infants with BCG, PAM3 and LPS. For box and whisker plots, horizontal lines represent the median, boxes represent the interquartile range (IQR) and whiskers represent the range. Intra-group comparisons were done using the Wilcoxon test, while inter-group comparisons were done using the Kruskal-Wallis test (Overall effect), followed by the Mann-Whitney test.
Changes in expression of co-stimulatory molecules over the first 9 months of life
Upregulation of co-stimulatory molecules in innate cells upon PRR triggering is critical for optimal interaction between the innate and adaptive immune response. To determine whether expression and/or stimulation-induced upregulation of co-stimulatory molecules changed during the first 9 months of life, we evaluated expression of CD40, CD83 and CD86 on monocytes and mDCs after incubation of whole blood with BCG, LPS or media (Fig. 5).
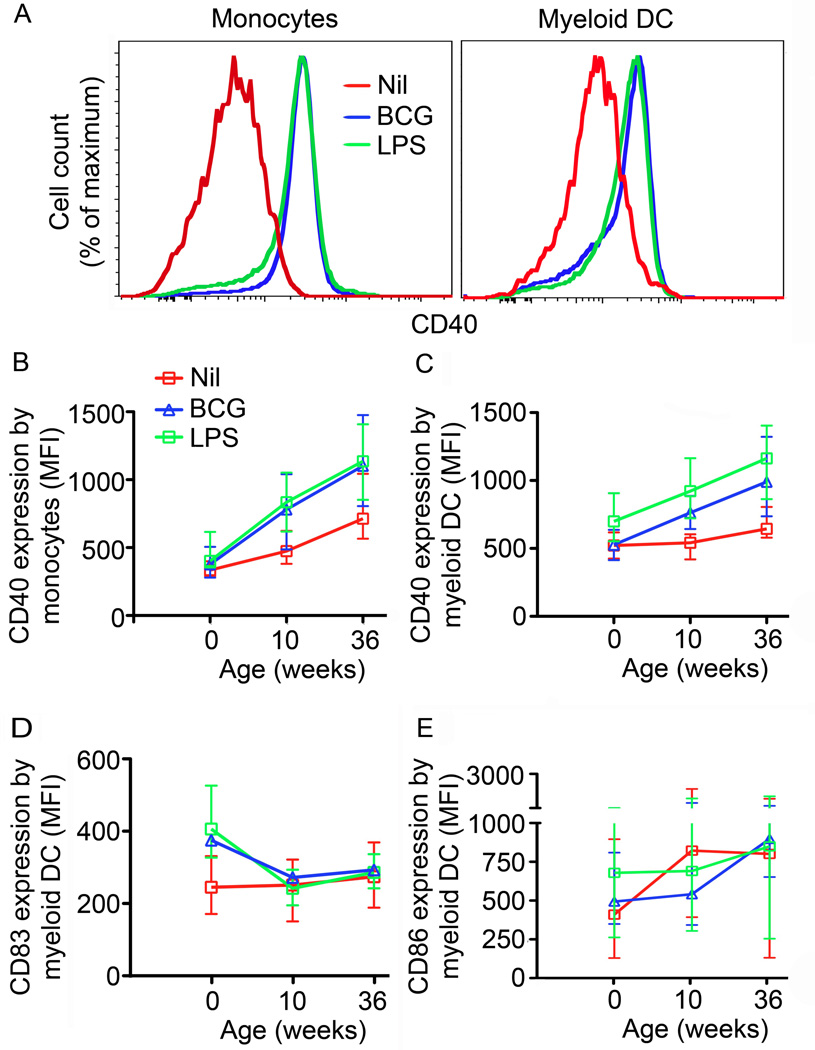
Figure 5. Expression of maturation markers by monocytes and mDCs upon BCG stimulation of whole blood from newborns and infants.
(A) CD40 expression on monocytes (left) or mDCs (right) after incubation of whole blood from a representative 36-week old infant for 18 hours with BCG (Blue line), LPS (Green line) or media (Red line). Median levels of CD40 expression by monocytes (B) or mDCs (C) in whole blood from newborns, 10 and 36-week old infants (n=24 for newborns; n=25 for 10- and 36-week old infants). Background expression of CD40 in mDC, p=0.7 for Newborn compared with infants at 10 weeks; p=0.0014 for newborns compared with infants at 36weeks; p= 0.0079 for infants at 10 weeks compared with 36 weeks. Background expression of CD40 in monocytes, p=0.0006 for Newborns compared with infants at 10 weeks; p<0.0001 for newborns compared with infants at 36weeks; p= 0.0016 for infants at 10 weeks compared with 36 weeks. Median levels of CD83 (D) or CD86 (E) expression by mDCs in blood from newborns, 10 and 36-week old infants stimulated for 18 hours with BCG (Blue triangles), LPS (Green squares) or media (Red squares). Error bars represent the IQR. The Mann-Whitney test was used for statistical comparisons.
Levels of basal CD40 expression increased consistently with age on both unstimulated monocytes and mDCs (p<0.0001 and p=0.003 respectively; Fig. 5B and C). Marginal or no upregulation of CD40 was observed after BCG stimulation on monocytes or mDCs in newborns (p=0.04 and p=0.727 respectively; Fig. 5B and C). However, monocytes and mDCs from 10 and 36 week old infants did upregulate CD40 expression in response to BCG (p<0.001 for all; Fig. 5B and C). This age-dependency appeared to be unique to mycobacterial stimulation, as LPS-induced CD40 upregulation was already observed in cord blood mDCs (p= 0.0006; Fig. 5C).
CD83 expression on unstimulated mDCs did not change with age. Interestingly, mDCs from newborns significantly upregulated CD83 expression upon BCG stimulation, while expression of this molecule was not upregulated upon stimulation in the older age groups (Fig. 5D). While CD86 expression on unstimulated mDCs did increase with age, at any age no significant upregulation of CD86 expression upon BCG stimulation was observed on mDCs (Fig. 5E) or monocytes (data not shown). CD86 upregulation was however observed upon LPS stimulation in cord blood mDCs (Fig. 5E).
Mechanisms for differential innate responses over the first 9 months of life
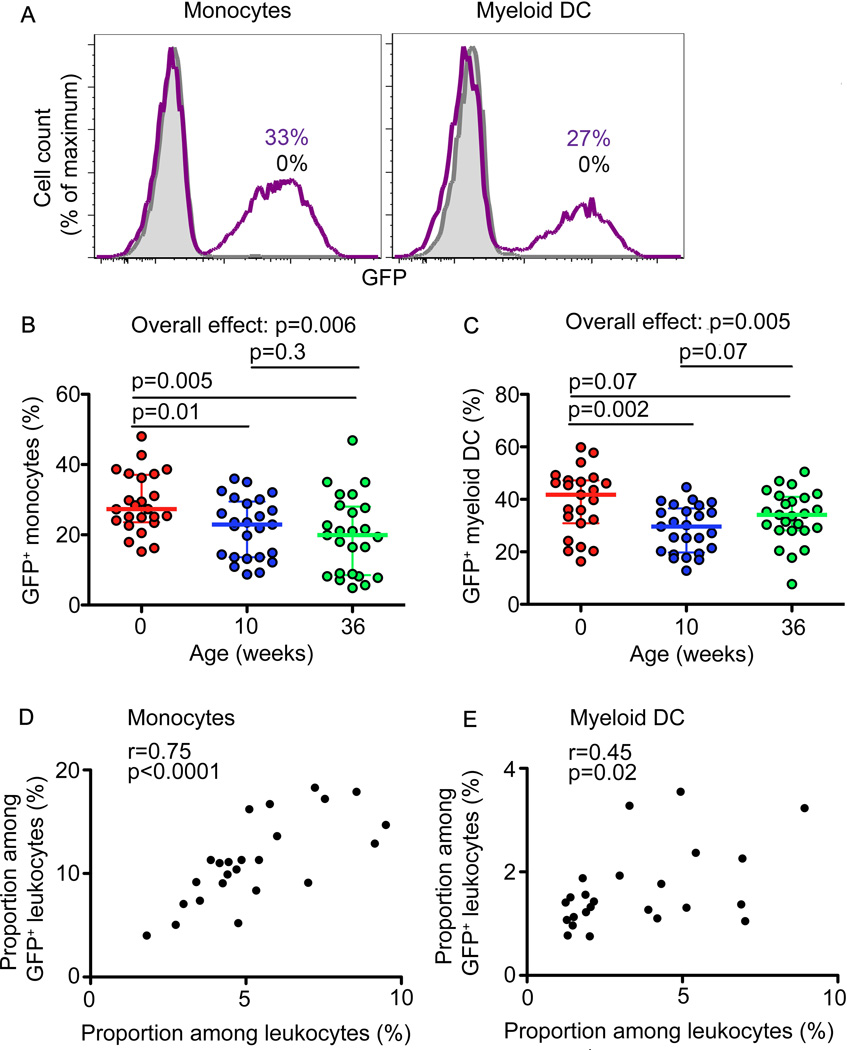
To determine if differential BCG uptake and binding by monocytes associated with observed age-dependent differential cytokine and CD40 expression, we quantified proportions of cell subsets that phagocytosed or bound GFP-expressing BCG upon whole blood incubation for 6 hours (Fig. 6A). Higher proportions of GFP+ monocytes and mDCs were observed in newborns, compared with older infants (Fig. 6B and C), suggesting that the greater pro-inflammatory monocyte response in older infants is not driven by greater binding or uptake of BCG by their innate cells.
Figure 6. Phagocytosis of BCG-GFP by mDCs and monocytes.
(A). GFP fluorescence of monocytes and mDCs after 6 hour incubation of whole blood from a representative 36-week old infant with 3.5 × 105 CFU/mL of GFP-expressing BCG. Proportions of BCG-GFP+ monocytes (B) and mDCs (C) in blood from newborns, 10 and 36-week old infants after 6 hours of incubation (n=24 for newborns; n=25 for 10- and 36-week old infants). In scatter plots, horizontal lines represent the median and whiskers represent the interquartile range (IQR). Group comparisons were done using the Kruskal-Wallis test (Overall effect), followed by the Mann-Whitney test.
We also compared cytokine-expression in cells that had bound/internalized BCG (BCG-GFP+) with BCG-GFP-negative cells. As previously reported (22), both the BCG-GFP+ and BCG-GFP-negative monocyte and mDC subsets expressed IL-6, TNF-α and IL-12, as well as IL-10. These data suggest that bystander activation of innate cells in the absence of direct pathogen recognition may lead to cytokine expression. However, significantly higher frequencies of BCG-GFP+ monocytes and mDCs expressed these cytokines, relative to BCG-GFP- cells at all ages (data not shown) (22).
We next investigated whether differential activation of TLR signaling through NF-κB and MAPK pathways may underlie the greater pro-inflammatory responses observed in older infants. Only cryopreserved PBMC were available for these signaling experiments and we evaluated BCG and PAM3 stimulation in pilot studies. Stimulation of thawed PBMC with BCG yielded variable responses, even in cells from the same donor, while PAM3-induced signaling was highly reproducible. Because a limited amount of PBMC were available from infants, we prioritized PAM3 stimulation. We measured activation of the NF-κB pathway by detecting degradation of IκBα, an NF-κB-inhibitor, in TLR1/2 ligand PAM3-stimulated PBMC in comparison to unstimulated controls (Figure 7A and Supplementary Figure 3A and 3B). Activation of the MAPK pathway was measured by detecting phosphorylation of p38 and Erk.
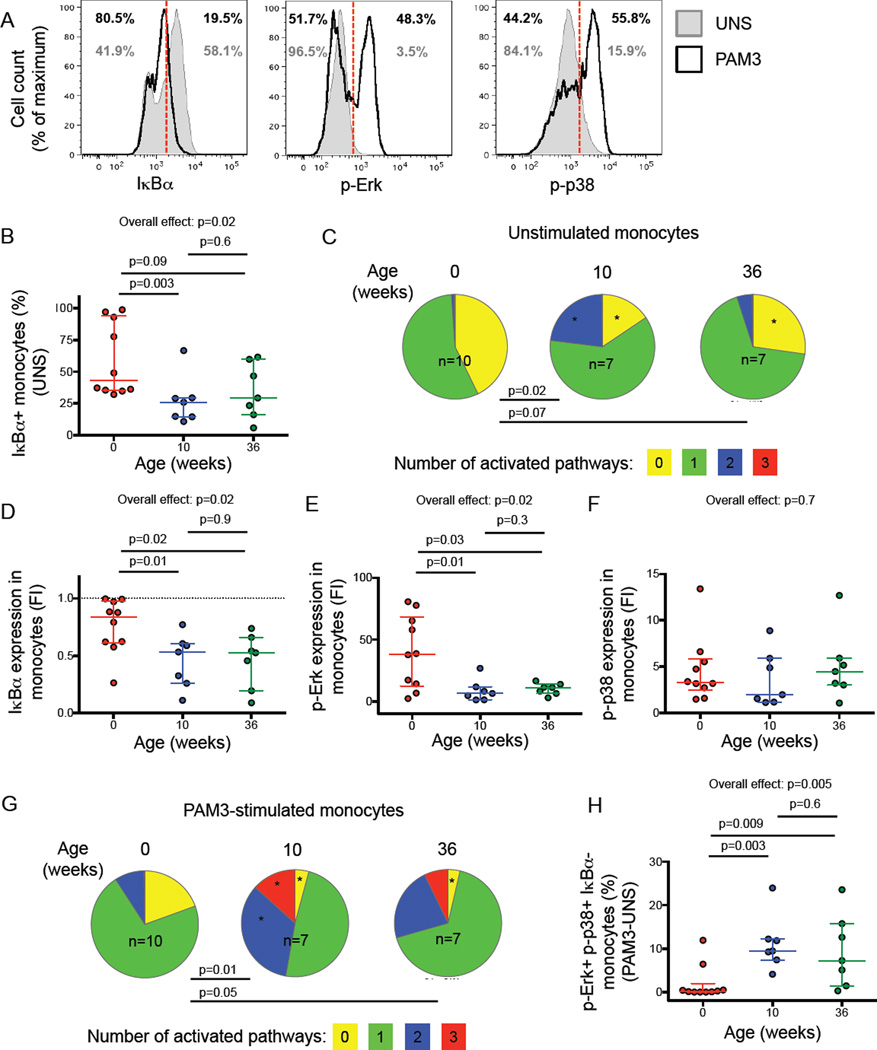
Figure 7. Differential pro-inflammatory cell signaling in monocytes from newborns and older infants.
Degradation of IκBα and phosphorylation of Erk (p-Erk) and p38 (p-p38) were measured by flow cytometry (gating strategy is shown in Supplementary figure 3A and 3B) in monocytes from newborns (n=10), 10-week-old (n=7) and 36-week-old (n=7) infants. (A) Representative IκBα, p-Erk and p-p38 staining in unstimulated (solid grey histograms) and PAM3 stimulated (open black histograms) monocytes from a single 36-week old infant. (B) Basal expression of IαBα in unstimulated monocytes. (C) Simultaneous activation of pro-inflammatory signaling pathways in unstimulated monocytes from infants at different ages. Pie charts represent the total monocyte population. Slices of each pie show the median proportion of monocytes exhibiting simultaneous activation of 3 (red), any 2 (blue), one (green) or no (yellow) signaling pathways (i.e. IκBα degradation, phosphorylation of Erk or p38). (D–F) Proportions of monocytes expressing IκBα (D), p-Erk (E) and p-p38 (F) after PAM3 stimulation, expressed as fold induction (FI) over marker+ monocyte proportions in unstimulated samples. (G) Simultaneous activation of pro-inflammatory signaling pathways in PAM3-stimulated monocytes from infants at different ages, represented as in (C). (H) Relative increase (stimulated minus unstimulated) in proportions of monocytes with simultaneous activation of the three signaling pathways in response to PAM3 at different ages. In scatter plots, horizontal lines represent the median and whiskers represent the interquartile range (IQR). Group comparisons were done using the Kruskal-Wallis test (overall effect), followed by the Mann-Whitney test for pairwise comparisons. Pies and slices were compared using permutation test (p values are shown) or T-test (stars indicate p<0.05 in comparison to newborns), respectively.
After resting, median PBMC viability was 90% (interquartile range 76.7% – 94%). Lower basal activation of TLR signaling pathways in unstimulated monocytes was observed in newborns, compared with older infants. Newborns had higher frequencies of IκBα+ monocytes (Figure 7B) and generally displayed lower proportions of activated monocytes, as reflected by p38 and Erk phosphorylation, compared with older infants (Figure 7C).
When cells were stimulated with PAM3, IαBα degradation and p38 and Erk phosphorylation were observed in monocytes at all ages (Supplementary Figure 3C). However, IκBα degradation was significantly lower in monocytes from newborns, compared with older infants (Figure 7D). By contrast, higher Erk phosphorylation was observed in monocytes from newborns than in those from older infants (Figure 7E). Phosphorylation of p38 did not differ according to age (Figure 7F). No activation or activation of only one signal transduction molecule was observed in PAM3-stimulated monocytes from newborns (Figure 7G). A markedly higher proportion of monocytes with simultaneous activation of two or three signal transduction molecules was observed in 10 and 36-week-old infants (Figure 7G, and 7H). No significant differences in activation of signal transduction molecules were observed between 10 and 36 week old infants. Our data indicate that upon TLR1/2 triggering monocytes from newborns activate pro-inflammatory signaling pathways to a lower degree than monocytes from older infants.
Discussion
Newborns are exquisitely susceptible to infection, including M.tb, and respond poorly to vaccination. To explore possible immunological mechanisms underlying these observations, we investigated changes in innate immune responses to BCG and mycobacterial ligands during the first 9 months of life. Three key observations suggest increasing capacity for pro-inflammatory monocyte responses to mycobacteria or mycobacterial ligands as infants mature: (1) BCG-induced proportions of monocytes expressing TNF-α, IL-6 and/or IL-12, and the relative expression levels of these pro-inflammatory cytokines, were lower in newborns than in older infants. (2) Upregulation of the innate cell maturation marker CD40 upon BCG stimulation was lower in monocytes and myeloid DCs from newborns than in those from older infants. (3) These functional and phenotypic differences were possibly due to differential activation of key pro-inflammatory signal transduction pathways downstream of mycobacterial recognition receptor triggering; specifically, lesser TLR1/2-mediated co-activation of monocyte NF-κB and MAPK pathways was observed in newborns than in older infants.
The magnitude and nature of innate immune responses is a critical component of host resistance to infection and the generation of adaptive immunity. Several characteristics of the monocyte pro-inflammatory responses to BCG were lowest in newborns and increased during the first 9 months of life. These included the proportions of TNF-α, IL-6 and/or IL-12-expressing monocytes, the capacity of these cytokine+ monocytes to simultaneously express multiple pro-inflammatory cytokines, the relative amounts of TNF-α, IL-6 and/or IL-12 on a per cell level and the levels of TNF-α and IL-6 released upon BCG stimulation. Taken together, our study is the first to show that innate responses to a live mycobacterium develop to a more pro-inflammatory capacity in early life. These findings are consistent with the previously described development of innate immune responses in response to other purified TLR agonists (25, 26).
We also observed an increase in CD40 expression by monocytes and mDCs during the first 9 months of life. CD40, which belongs to the TNF receptor superfamily, binds to its ligand CD154, expressed on T cells, providing co-stimulatory signals to CD40 expressing cells (27, 28). This signaling is required for long term DC activation, cytokine production and T cell polarization (27, 29, 30), implying that these processes may be relatively immature in newborns.
The increasing pro-inflammatory capacity and CD40 expression during the first 9 months of life suggest that BCG vaccination at birth may elicit poorer inflammatory responses than vaccination weeks or months after birth, when the infant immune system has matured to be more pro-inflammatory. This may underlie our previous observation that BCG vaccination at birth induced lower frequencies of specific Th1 responses, measured at 1 year of age, compared with BCG vaccination at 10 weeks of age (21). These results may also explain why infants who are exposed to M. tb in the first few months of life are highly likely to progress to TB disease.
Our findings of reduced pro-inflammatory cytokine production in response to whole mycobacteria in newborns compared with older infants build on the previous observations showing lower levels of purified TLR agonist-induced IL-12 and TNF-α in newborns, compared with older infants and/or adults (25, 31–35). In contrast to our results, Reikie et al. recently characterized innate responses during the first year of life and reported decreasing IL-6, and no changes in TNF-α expression in PAM3-stimulated whole blood over the first year of life (36). Reasons underlying the different outcomes between the latter study and ours are not clear. One possibility may involve the different ages at which blood was collected. For example, we studied cord blood from newborns, while the earliest blood collection by Reikie et al. was at 2 week of age (36). BCG and the poliomyelitis vaccine, given at birth, are likely to profoundly influence the activation and function of innate immune responses, effects that would have been detected by by Reikie et al. Different experimental protocols may also underlie the discordant results.
We observed that expression of IL-10 in response to live M. bovis BCG by monocytes and mDCs was not different between newborns and older infants. By contrast, lower production of IL-10 was observed in newborns compared with older infants when blood was stimulated with the TLR2/1 ligand, PAM3. The reasons underlying these differences are not clear, but likely include the well-recognized immune subversion strategies employed by mycobacteria, including BCG (37), and concomitant activation of multiple PAMPs and signaling pathways triggered by BCG. Our results of increasing PAM3 and LPS-induced soluble IL-10 with age are at odds with several studies reporting higher IL-10 expression in response to TLR agonists in newborns, compared with older infants or adults (25, 32,33, 36, 38). Belderbos et al. (39) observed greater IL-10 production in newborns, compared with older infants for some TLR agonists, and the opposite pattern for other TLR agonists. Genetic variations in populations, epidemiological factors, such as exposure to cigarette smoking and helminths (40–45), and differences in experimental protocols may also underlie the discrepancies in cytokine expression. Previous studies comparing innate responses in newborns and older infants or adults typically used purified TLR agonists or evaluated soluble cytokines rather than cell-associated cytokine expression (25, 32,33, 36, 38, 39).
Other factors may also lead to active suppression of immune responses in newborn infants. Cord blood typically contains high levels of adenosine, which has been shown to negatively influence production of TNF-α, but not IL-6 (46). A recent study also reported that pro-inflammatory innate and adaptive immune responses were suppressed by a novel subset of CD71+ erythroid cells, which are enriched in the neonatal period (47). This study suggested that active immunosuppression during the perinatal period may represent a fundamental adaptation to protect against excessive inflammation triggered by colonization of the neonate with commensal microbes. We did not study the role of these CD71+ erythroid cells in our study, but they should be investigated in future studies of mycobacterial immunity in newborns and infants.
Given the discordance between higher acquisition of BCG-GFP and lower cytokine expression by monocytes from newborns compared with older infants, and previous studies showing that levels of TLR expression may not change markedly during the first 9 months of life (48, 49), we investigated pro-inflammatory signaling pathways downstream of TLR1/2 triggering. We observed lower basal activation of pro-inflammatory signaling pathways in unstimulated monocytes from newborns compared with older infants. Activation of signaling pathways after birth may result from sensitization to foreign antigens and/or inflammation due to infections, vaccinations and ingested substances, or simply lower levels of inhibitory molecules such as adenosine. Interestingly, changes in epigenetic regulation of genes involved in the NF-κB and MAPK pathways have been observed during the perinatal period (50). Moreover, BCG-induced epigenetic changes in monocytes that may augment pro-inflammatory responses to other pathogens were recently described (51). Whether such BCG-induced epigenetic reprogramming is driving activation of pro-inflammatory responses in monocytes from infants is the focus of ongoing studies.
To our knowledge, we are the first to demonstrate lower TLR1/2-mediated co-activation of the NF-κB and MAPK pathways in monocytes from newborns, compared with older infants. Our findings suggest that co-activation of different pathways may underlie higher innate pro-inflammatory cytokine responses to mycobacteria observed in older infants, compared with newborns. Indeed, others have shown that co-activation of MAPKs and NF-κB pathways are important for production of pro-inflammatory cytokines in response to mycobacteria (52–54), as simultaneous inhibition of multiple molecules abrogated cytokine production to a larger extent than blockade of individual pathways. Furthermore, the combination of activated signaling pathways may determine which cytokines are produced: activation of both p38 and Erk pathways is essential for TNF-α production, whereas activation of p38 but not Erk induces IL-10 in response to M.tb (55). We could not determine if cytokine production and monocyte signaling were directly associated. These outcomes were measured in samples from different individuals because only limited volumes of blood could be safely collected from infants. Future studies will focus on cytokine production in whole blood stimulated with BCG and a panel of TLR ligands in presence or absence of specific cell signaling inhibitors.
Our study was subject to a number of limitations. A longitudinal study design in which individual newborns are followed up would have been ideal to evaluate changes in maturation of innate responses over the first 9 months of life. This was not possible within the context of available resources; a cross-sectional design was more practical and feasible. Despite the lower statistical power of our cross-sectional design, we observed striking age-associated differences. Another limitation was that ethnicity distributions of the newborn and older infant groups were not the same. However, innate responses to BCG did not differ between Caucasian and mixed ethnicity infants (not shown), suggesting that ethnicity is unlikely to be an important confounder in our study.
Another potential confounder in our study resulted from the fact that infants were vaccinated with BCG after birth. All analyses at 10 and 36 weeks of age were therefore potentially influenced by BCG-induced immune responses, whereas analyses of cord blood, taken before the newborn was vaccinated, were not subjected to the same external influences. In our functional assay whole blood was stimulated for a total of 6 hours, which, in our experience ((56) and upublished observations), is too short for optimal processing and presentation of BCG-derived antigens. Activation of BCG-specific memory T cells and downstream effects on innate cells would therefore have been minimal. Further, stimulation with BCG and purified TLR ligands showed similar results. Since PAM3 and LPS are known not to directly activate antigen-specific T cells, this further supports that antigen-specific responses did not markedly influence our outcomes. However, we cannot rule out that other effects of BCG vaccination, not detected with these approaches, may have confounded age-associated innate responses. In fact, natural microbiome colonisation as well as other vaccines administered during infancy are also likely to influence innate immune responses (57). Regardless, given the risk of infant morbidity and mortality due to infectious diseases in developing countries these routine health interventions should not be withheld. We propose that effects of routine vaccinations be considered part of the normal development of the infant immune system.
Finally, we acknowledge that in the context of TB it would have been more appropriate to measure immune responses to M.tb rather than BCG. We did not have access to a BSL-3 laboratory at our rural clinical site to inoculate blood specimens with virulent M.tb, and opted for M. bovis BCG instead.
Taken together, our study is the first to show that innate immune responses to BCG, a live mycobacterium routinely used as vaccine, mature to a more pro-inflammatory capacity from birth to 9 months of age. Limited innate pro-inflammatory capacity at birth may underlie suboptimal responses by newborns to vaccines and their particular susceptibility to infections, including M.tb. Different vaccine formulations or adjuvants may be required to achieve optimal activation of innate immunity in the perinatal period, compared to later in life.
Supplementary Material
Acknowledgements
We would like to thank the study participants.
Grant support: This work was supported by the TB Research Unit of the NIH (NO1 AI 70022). WAH was supported by the NIH (RO1AI087915) and by the Wellcome Trust-supported Clinical Infectious Disease Research Initiative of the University of Cape Town. MSS was supported by a South African Tuberculosis and AIDS Training scholarship (SATBAT: D0711100-22.CM), National Research Foundation (NRF) scholarship, Carnegie Corporation scholarship and the University of Cape Town. EN was supported by South African National Research Foundation (NRF) and Claude Leon Foundation fellowships. T.R.K. is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and a Michael Smith Foundation for Health Research Career Investigator Award.
Footnotes
Conflict of interest: The authors declare that they have no commercial or financial conflicts of interest.
References
- 1.Kleinnijenhuis J, Oosting MB, Joosten LAB, Netea MG, van Crevel R. Innate Immune Recognition of Mycobacterium tuberculosis. Clinical and Developmental Immunology. 2011;2011:1–12. doi: 10.1155/2011/405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Publishing Group. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003;5:133–142. doi: 10.1046/j.1462-5822.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal A, Ehlers S, Ernst M, Flad H-D, Reiling N. Control of mycobacterial replication in human macrophages: roles of extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinase pathways. Infection and Immunity. 2002;70:4961–4967. doi: 10.1128/IAI.70.9.4961-4967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu J, Shin D-M, Jo E-K. Mycobacterial signaling through toll-like receptors. Front Cell Infect Microbiol. 2012;2:145. doi: 10.3389/fcimb.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Seminars in Immunology. 2004;16:35–41. doi: 10.1016/j.smim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 9.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clinical Immunology. 2006;121:251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han P, Mcdonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113:26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 12.Moyo S, Verver S, Mahomed H, Hawkridge A, Kibel M, Hatherill M, Tameris M, Geldenhuys H, Hanekom W, Hussey G. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int. J. Tuberc. Lung Dis. 2010;14:149–154. [PubMed] [Google Scholar]
- 13.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int. J. Tuberc. Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 14.World Health Organization, UNICEF, Center for Disease Control U.S, I. U. A. L. Disease, S. T. Partnership, USAID. Roadmap for childhood tuberculosis: towards zero deaths. 2013:1–44. [Google Scholar]
- 15.Heyns L, Gie R, Goussard P, Beyers N, Warren R, Marais B. Nosocomial transmission of Mycobacterium tuberculosis in kangaroo mother care units: A risk in tuberculosis-endemic areas. Acta Paediatrica. 2006;95:535–539. doi: 10.1080/08035250600636560. [DOI] [PubMed] [Google Scholar]
- 16.Goussard P, Gie RP, Kling S, Schaaf HS, Kritzinger F, Andronikou S, Beyers N, Rossouw GJ. The outcome of infants younger than 6 months requiring ventilation for pneumonia caused byMycobacterium tuberculosis. Pediatr. Pulmonol. 2008;43:505–510. doi: 10.1002/ppul.20812. [DOI] [PubMed] [Google Scholar]
- 17.LINCOLN EM. Course and prognosis of tuberculosis in children. Am. J. Med. 1950;9:623–632. doi: 10.1016/0002-9343(50)90212-9. [DOI] [PubMed] [Google Scholar]
- 18.Lewinsohn DA, Lewinsohn DM. Immunologic susceptibility of young children to Mycobacterium tuberculosis. Pediatr Res. 2008;63:115. doi: 10.1203/PDR.0b013e3181652085. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Driessche K, Persson A, Marais BJ, Fink PJ, Urdahl KB. Immune vulnerability of infants to tuberculosis. Clinical and Developmental Immunology. 2013;2013:781320. doi: 10.1155/2013/781320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colditz GA, BTBCEA Efficacy of bcg vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA: The Journal of the American Medical Association. 1994;271:698–702. [PubMed] [Google Scholar]
- 21.Kagina BMN, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, Erasmus M, Nene N, Walzl G, Black G, Hussey GD, Hesseling AC, Hanekom WA. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine. 2009;27:5488–5495. doi: 10.1016/j.vaccine.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shey MS, Hughes EJ, de Kock M, Barnard C, Stone L, Kollmann TR, Hanekom WA, Scriba TJ. Optimization of a whole blood intracellular cytokine assay for measuring innate cell responses to mycobacteria. J. Immunol. Methods. 2012;376:79–88. doi: 10.1016/j.jim.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 24.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 25.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal Innate TLR-Mediated Responses Are Distinct from Those of Adults. The Journal of Immunology. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 27.Miga AJ, Masters SR, Durell BG, Gonzalez M, Jenkins MK, Maliszewski C, Kikutani H, Wade WF, Noelle RJ. Dendritic cell longevity and T cell persistence is controlled by CD154-CD40 interactions. Eur. J. Immunol. 2001;31:959–965. doi: 10.1002/1521-4141(200103)31:3<959::aid-immu959>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez MGH, Shen L, Rock KL. CD40-CD40 ligand interaction between dendritic cells and CD8+ T cells is needed to stimulate maximal T cell responses in the absence of CD4+ T cell help. J. Immunol. 2007;178:2844–2852. doi: 10.4049/jimmunol.178.5.2844. [DOI] [PubMed] [Google Scholar]
- 29.Ellis JH, Burden MN, Vinogradov DV, Linge C, Crowe JS. Interactions of CD80 and CD86 with CD28 and CTLA4. J. Immunol. 1996;156:2700–2709. [PubMed] [Google Scholar]
- 30.Yasumi T, Katamura K, Yoshioka T, Meguro T-A, Nishikomori R, Heike T, Inobe M, Kon S, Uede T, Nakahata T. Differential requirement for the CD40-CD154 costimulatory pathway during Th cell priming by CD8 alpha+ and CD8 alpha- murine dendritic cell subsets. J. Immunol. 2004;172:4826–4833. doi: 10.4049/jimmunol.172.8.4826. [DOI] [PubMed] [Google Scholar]
- 31.Levy O. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES, Kollmann TR. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of Human Blood Mononuclear Cells. PLoS ONE. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of Adult-Like TLR4 and TLR9 Responses during the First Year of Life. PLoS ONE. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, Jaye A, Flanagan KL, Levy O. Age-Dependent Maturation of Toll-Like Receptor-Mediated Cytokine Responses in Gambian Infants. PLoS ONE. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu E-M, Law HKW, Lau Y-L. Mycobacterium bovis bacillus Calmette-Guerin treated human cord blood monocyte-derived dendritic cells polarize naïve T cells into a tolerogenic phenotype in newborns. World J Pediatr. 2010;6:132–140. doi: 10.1007/s12519-010-0019-0. [DOI] [PubMed] [Google Scholar]
- 36.Reikie BA, Adams RCM, Ruck CE, Ho K, Leligdowicz A, Pillay S, Naidoo S, Fortuno ES, de Beer C, Preiser W, Cotton MF, Speert DP, Esser M, Kollmann TR. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of South African Infants throughout the First Year of Life. PLoS ONE. 2012;7:e44763. doi: 10.1371/journal.pone.0044763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y. Mycobacteria Target DC-SIGN to Suppress Dendritic Cell Function. Journal of Experimental Medicine. 2002;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vosters O, Lombard C, André F, Sana G, Sokal EM, Smets F. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clinical & Experimental Immunology. 2010;162:494–499. doi: 10.1111/j.1365-2249.2010.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JLL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: Low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clinical Immunology. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semnani RT, Venugopal PG, Leifer CA, Mostbock S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood. 2008;112:1290–1298. doi: 10.1182/blood-2008-04-149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pace E, Ferraro M, Siena L, Melis M, Montalbano AM, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology. 2008;124:401–411. doi: 10.1111/j.1365-2567.2007.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noakes PS. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. European Respiratory Journal. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 43.Adegnika AA, Köhler C, Agnandji ST, Chai SK, Labuda L, Breitling LP, Schonkeren D, Weerdenburg E, Issifou S, Luty AJF, Kremsner PG, Yazdanbakhsh M. Pregnancy-Associated Malaria Affects Toll-Like Receptor Ligand-Induced Cytokine Responses in Cord Blood. Journal of Infectious Diseases. 2008;198:928–936. doi: 10.1086/591057. [DOI] [PubMed] [Google Scholar]
- 44.van den Biggelaar AHJ, PhD, Prescott P Susan L, MD, MR, MAN-SB, CJDB, SP, WP, MKT, DL, PM, Peter C Richmond P, MD, Patrick G Holt F., DSc Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. Journal of Allergy and Clinical Immunology. 2009;124:544–550. e2. doi: 10.1016/j.jaci.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. BCG Vaccination Induces Different Cytokine Profiles Following Infant BCG Vaccination in the UK and Malawi. Journal of Infectious Diseases. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71. Nature. 2014;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 49.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martino DJ, Tulic MK, Gordon L, Hodder M, Richman TR, Metcalfe J, Prescott SL, Saffery R. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. epigenetics. 2011;6:1085–1094. doi: 10.4161/epi.6.9.16401. [DOI] [PubMed] [Google Scholar]
- 51.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JWM, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Natarajan P, Narayanan S. Mycobacterium tuberculosisH37Rv induces monocytic release of interleukin-6 via activation of mitogen-activated protein kinases: inhibition by N-acetyl-l-cysteine. FEMS Immunology & Medical Microbiology. 2007;50:309–318. doi: 10.1111/j.1574-695X.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 53.Lewthwaite JC, Clarkin CE, M. Coates AR, Poole S, Lawrence RA, Wheeler-Jones CPD, Pitsillides AA, Singh M, Henderson B. Highly homologous Mycobacterium tuberculosis chaperonin 60 proteins with differential CD14 dependencies stimulate cytokine production by human monocytes through cooperative activation of p38 and ERK1/2 mitogen-activated protein kinases. International Immunopharmacology. 2007;7:230–240. doi: 10.1016/j.intimp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Vallejo JG, Knuefermann P, Mann DL, Sivasubramanian N. Group B Streptococcus induces TNF-alpha gene expression and activation of the transcription factors NF-kappa B and activator protein-1 in human cord blood monocytes. J. Immunol. 2000;165:419–425. doi: 10.4049/jimmunol.165.1.419. [DOI] [PubMed] [Google Scholar]
- 55.Song C-H, Lee J-S, Lee S-H, Lim K, Kim H-J, Park J-K, Paik T-H, Jo E-K. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J. Clin. Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- 56.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PAJ, Ress S, Hussey GD, Kaplan G. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Netea MG. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest. 2013;43:881–884. doi: 10.1111/eci.12132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.