Abstract
Purpose
To examine the prevalence of isolated IgA anti-β2Glycoprotein I (anti-β2GPI) positivity and the association of these antibodies, and a subgroup that bind specifically to domain IV/V of β2GPI, with clinical manifestations of the Antiphospholipid Syndrome (APS) in three patients groups. The pathogenicity of IgA anti-β2GPI was also evaluated in a mouse model of thrombosis.
Methods
Patients with systemic lupus erythematosus (SLE) from a multiethnic, multicenter cohort (LUpus in MInorities, NAture versus nurture [LUMINA]) (n=558), patients with SLE from the Hopkins Lupus Cohort (n=215), and serum samples referred to the Antiphospholipid Standardization Laboratory (APLS) (n=5,098) were evaluated. IgA anti-β2GPI titers and binding to domain IV/V of β2GPI were examined by enzyme-linked immunosorbent assay (ELISA). CD1 mice were inoculated with purified IgA anti- β2GPI antibodies, and surgical procedures and ELISAs were performed to evaluate thrombus development and tissue factor (TF) activity.
Results
A total of 198 patients were found to be positive for IgA anti-β2GPI isotype, and 57 patients were positive exclusively for IgA anti-β2GPI antibodies. Of these, 13 of 23 patients (56.5%) in the LUMINA cohort, 17 of 17 patients (100%) in the Hopkins cohort, and 10 of 17 patients (58.9%) referred to APLS had at least one APS-related clinical manifestation. Fifty-four percent of all the IgA anti-β2GPI positive serum samples reacted with domain IV/V of anti-β2GPI, and 77% of those had clinical features of APS. Isolated IgA anti-β2GPI positivity was associated with an increased risk for arterial thrombosis (p<0.001), venous thrombosis (p=0.015) and all thrombosis (p<0.001). The association between isolated IgA anti-β2GPI and arterial thrombosis (p=0.0003) and all thrombosis (p=0.0003) remained significant after adjusting for other risk factors for thrombosis. In vivo mouse studies demonstrated that IgA anti-β2GPI antibodies induced significantly larger thrombi and higher TF levels compared to controls.
Conclusion
Isolated IgA anti-β2GPI positive titers may identify additional patients with clinical features of APS. Testing for these antibodies when other antiphospholipid (aPL) tests are negative and APS is suspected is recommended. IgA anti-β2GPI antibodies directed to domain IV/V of β2GPI represent an important subgroup of clinically relevant antiphospholipids.
BACKGROUND
The current classification criteria for the antiphospholipid syndrome (APS) do not include determination of the presence of IgA anticardiolipin (aCL) or anti-β2glycoprotein I (β2GPI) antibodies [1]. IgA aCL antibodies are more frequently found in Afro-Caribbean populations, usually in association with other IgG and/or IgM aCL antibodies. IgA aCL antibodies have been shown to be pathogenic in animal models, but their clinical significance has remained elusive [2,3]. Previous studies have highlighted the association of IgA anti-β2GPI positivity with clinical manifestations of APS and have shown that systemic lupus erythematosus (SLE) patients with APS appear to be more prone to being positive for the IgA isotype [4-6]. Of particular interest is the study conducted by Fanopoulous et al, which demonstrated that IgA positivity occurred more frequently and at higher titers in SLE patients with APS manifestations [7]. Recently Mehrani et al reported that IgA anti-β2GPI antibodies were more strongly associated with deep venous thrombosis (DVT) and stroke than the IgM isotype [8]. In addition, it has been suggested that IgA anti-β2GPI antibodies may recognize epitopes in domains IV/V of β2GPI and these antibodies appear to be associated with certain manifestations of APS [9,10]. The majority of these studies however, describe patients that were also positive for other isotypes of antiphospholipid antibodies, limiting conclusions that can be drawn with respect to the clinical associations of IgA anti-β2GPI and aCL antibodies.
Recently, our group reported 5 isolated cases of individuals that were exclusively positive for IgA anti-β2GPI and had concomitant clinical manifestations of APS [11]. Subsequently, Sweiss et al found that the presence of isolated IgA anti-β2GPI positivity was associated with an increased occurrence of thromboembolic events in a small group of patients, especially among patients with SLE [12]. Isolated IgA anti-β2GPI isolated positivity has also been reported in scleroderma and in autoimmune hepatitis and has been shown to correlate with disease severity and endothelial damage [13,14]. However, the clinical importance of isolated IgA anti-β2GPI positivity is largely unknown.
Our aim was therefore to determine the prevalence of isolated IgA anti-β2GPI antibody and to correlate its presence with APS related clinical manifestations in 3 large groups of patients. In addition, we further examined the clinical relevance of IgA anti-β2GPI antibodies binding to domain IV/V of β2GPI and the pathogenicity of IgA anti-β2GPI antibodies in a mouse model of thrombosis.
METHODS
Patients and demographics
Patient serum samples were obtained from 3 independent sources: 588 from the Lupus in Minorities: Nature vs. Nurture (LUMINA) cohort, 215 from the Hopkins Lupus cohort (Johns Hopkins University, Baltimore, MD); and 5098 sent to the Antiphospholipid Standardization laboratory (APLS, University of Texas Medical Branch, TX) between January 2008 and March 2010 for antiphospholipid antibody evaluation. Of these 5098 APLS samples, 35 were found to be positive for IgA anti-β2GPI. We obtained APS-related clinical information about this subset of patients by medical chart review. LUMINA is a longitudinal study of outcome of multiethnic SLE patients [Hispanic (Mexican/ Central American and Puerto Rican), African American and Caucasian] enrolled within 5 years of fulfillment of the American College of Rheumatology criteria at participating institutions in Alabama, Houston, Galveston and Puerto Rico [15,16]. The Hopkins Lupus Cohort is a longitudinal study of lupus activity, organ damage, and quality of life in SLE patients. The demographic composition is balanced between Caucasian and African-American patients with SLE. The APLS Laboratory is a reference laboratory that routinely evaluates serum and plasma samples from patients referred for APS evaluation.
We evaluated all patients for classic manifestations of APS as per the revised Sapporo criteria [1]. We also explored nontraditional APS manifestations, such as seizures, valvular heart disease, pulmonary arterial hypertension, thrombocytopenia, livedo reticularis, skin ulcers, osteonecrosis, transverse myelitis and headache. Other information, such as age at time of enrollment, and history of smoking, and the presence of variables such as: obesity (body mass index [BMI] ≥ 30kg/m2), pregnancy, end-stage renal disease (serum creatinine level >3.0mg/dl), and hydroxychloroquine, non-steroidal anti-inflammatory drug (NSAID) and/or oral contraceptive use, at the time of the visit during which the blood sample used in this study was drawn, were also available for the 3 groups of patients.
The institutional review boards of the respective institutions approved the use of samples and clinical data from all patients. This study was conducted according to the guidelines of the Declaration of Helsinki for the inclusion of human subjects in research, and informed consent was obtained from all subjects.
Antiphospholipid antibody testing
Anticardiolipin (aCL) antibodies (IgG, IgM, IgA) were evaluated using an in-house enzyme-linked immunosorbent assay (ELISA) method as previously described [17]. The IgG, IgM and IgA anti-β2GPI antibodies were determined using at least 1 of 2 commercial ELISA kits (Kit A: TheraTest Laboratories, Lombard IL, USA, Kit B: INOVA Diagnostics, San Diego CA, USA). A number of samples were tested for IgA anti-β2GPI activity in both kits to assess the correlation of positivity between the two. To better characterize the antigenic target recognized by those IgA anti-β2 GPI antibodies, we performed an ELISA assay to detect IgA antibodies specific to domain IV and V of β2GPI (Research Use Only, INOVA) on a selected number of positive samples (n=126)
All assays were performed according to the manufacturer’s instructions and were considered positive when titers were above the manufacturer’s pre-established cut off points. (For aCL assays ≥10 IgG phospholipid units or IgM phospholipid units and ≥15 IgA phospholipid units, for IgA INOVA anti-β2GPI assays >20 standard anti-β2GPI antibody units (SAU), for Theratest IgA, IgG, and IgM anti-β2GPI assays, > 4 anti-β2GPI units, for Domain IV/V > 20 units).
IgA anti-β2GPI antibodies in a mouse model of thrombosis
IgA from the pooled sera of 4 patients with IgA anti-β2GPI titers ≥80 SAU (APS-IgA) (2 had strokes, 1 had a confirmed DVT and 2 had pregnancy losses) and IgA from the serum of a healthy subject with no evidence of inflammatory or thrombotic disease (control IgA) were purified by affinity chromatography using Immobilized Jacalin columns according to recommendations of the manufacturer (Pierce Biotechnology). Endotoxin was removed from solutions of purified IgA antibodies using Detoxi-Gel Endotoxin Removing Columns (Thermo Fisher Scientific) and confirmed to be endotoxin-free by the Limulus amebocyte cell lysate assay (Sigma-Aldrich). The concentration of purified IgA was determined by the Bradford method, and the total IgA anti-β2GPI activity and the specific IgA binding activity to domain I (DI) and domain IV/V (DIV/V) of β2GPI were determined by ELISA using commercial assays (INOVA Diagnostics). Lupus Anticoagulant (LAC) activity was measured using a modified silica clotting time (SCT) assay (HemosIL, Beckman Coulter).
CD1 mice (The Jackson Laboratory) were used for animal experiments. The mice (n=5 per group) were injected intraperitoneally with either APS-IgA (500μg) or control IgA (500μg) on 2 occasions at 48-hour intervals. Surgical procedures were performed to study thrombus dynamics at 72 hours after the first injection was administered, as described previously [3]. Tissue factor (TF) activity was subsequently determined in peritoneal macrophages and carotid homogenates using a chromogenic assay (AssayPro).
Statistical analysis
The strengths of the associations between IgA anti-β2GPI positivity and thromboses were measured by calculating the odds ratio (OR) with 95% confidence interval (95% CI) using univariate and multivariate logistic regression (SAS version 9.2 software, SAS Institute). The correlation of IgA anti-β2GPI titers between kits was evaluated by Spearman’s Rho (PASW version 18.0 software, SPSS). Student’s t-test was used to determine differences in mean thrombus size and TF activity between mice treated with APS-IgA and control IgA.
RESULTS
Prevalence of IgA anti-β2GPI antibodies
The demographic characteristics of the patients from the Hopkins and LUMINA cohorts are summarized in Table 1. Of the 35 patients in the APLS group found to be positive for IgA anti-β2GPI, 25 (71.4%) were women. Most were Caucasian (12 of 35 [34.3%]) or of African descent (10 of 35 [28.5%]); other ethnic groups accounted for the rest (13 of 35 [37.1%]). The mean age of these patients was 42.6 years (range 23-70 years) and 14 of 35 (40.0%) had SLE. In addition to the 35 patients from the APLS group, 129 of the 588 patients from the LUMINA cohort (21.9%) and 34 of the 215 patients from the Hopkins cohort (15.8%) were positive for IgA anti-β2GPI. A total of 57 of these patients were positive for IgA anti-β2GPI exclusively (negative for aCL and IgG and IgM anti-β2GPI; data not shown) including 23 from the LUMINA cohort, 17 from the Hopkins Lupus cohort and 17 from the APLS group.
Table 1.
Demographic characteristics of the 2 groups of patients with SLE*
| Patients Characteristics | LUMINA n = 588 |
Hopkins n = 215 |
p |
|---|---|---|---|
| Sex, % female/male | 86/14 | 90/10 | 0.117 |
| Ethnicity, % | < 0.001 | ||
| • African American | 43.0 | 39.1 | |
| • Caucasian | 22.0 | 52.1 | |
| • Other | 35.0 | 8.8 | |
| Age, mean (range) years | 36.8(19-66) | 45.9(19-81) | < 0.001 |
SLE= systemic lupus erythematosus;
LUMINA – Lupus in Minorities, Nature versus nurture
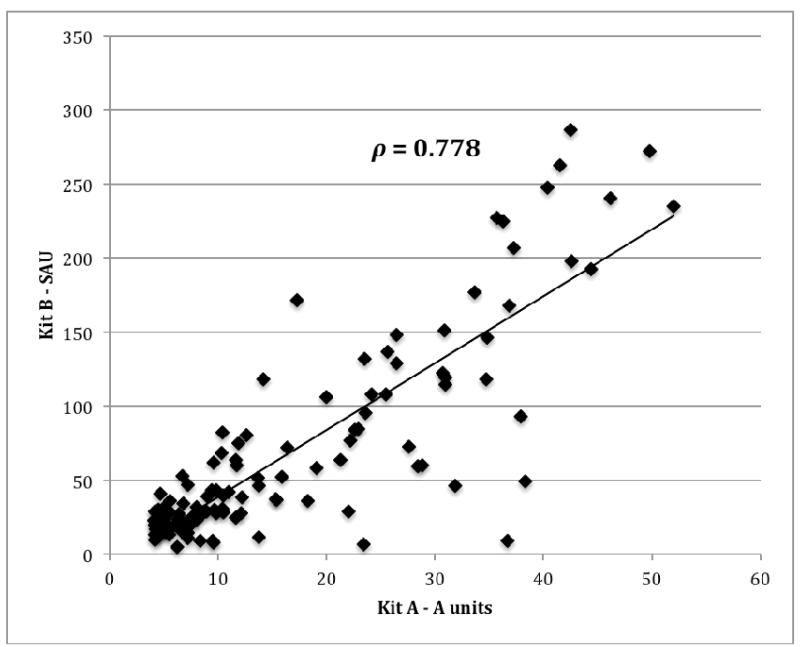
In order to confirm that these results were not kit specific, the IgA anti-β2GPI activity in 126 positive samples identified by kit A was re-evaluated in kit B and 97/126 (77.0%) were also positive by kit B. Despite both assays expressing results in different arbitrary units, there was good correlation between the kits (Spearman’s ρ=0.78, p<0.001) (Figure 1).
Figure 1.
Correlation of IgA anti-β2GPI titers in two assays. A scatterplot of IgA-anti-β2GPI titers of126 samples tested using kit A and in kit B, as described in Material and Methods, is shown. Results were expressed units/ml in kit A and in standard IgA anti-β2GPI units (SAU) in kit B.
Prevalence of IgA aCL
Of the 198 cases of individuals who were positive for IgA anti-β2GPI, 12 were also positive for IgA aCL. IgA aCL positivity occurred in complete isolation in only 2 cases.
Binding of IgA to anti-β2GPI domain IV/V
Out of the 115 IgA anti-β2GPI positive samples from the LUMINA and APLS cohorts (80 from LUMINA and 35 from APLS) that were evaluated for binding to domain IV/V of β2GPI, 62 (54%) were found to be positive in this assay. The correlation of IgA anti-β2GPI domain IV/V titers and IgA anti-β2GPI titers was 0.81 (p<0.0001).
Clinical manifestations in patients with isolated IgA anti-β2GPI positivity and IgA anti-β2GPI domain IV/V positivity
A considerable number of the subjects with isolated IgA anti-β2GPI positivity in the 3 groups had at least one APS-related clinical manifestation (Table 2). These included classic features such as DVT and arterial thrombosis (stroke, myocardial infarction and transient ischemic attacks) and miscarriages. Nontraditional APS manifestations included seizures, valvular heart disease, avascular necrosis, pulmonary hypertension, thrombocytopenia, superficial thrombophlebitis, livedo reticularis, skin ulcers, transverse myelitis and headache. Of the 62 patients whose samples were positive for binding of IgA to anti-β2GPI domain IV/V, 77% had clinical manifestations of APS that included DVT, strokes, myocardial infarction, pulmonary arterial hypertension, seizures, pregnancy losses, skin ulcers and livedo reticularis.
Table 2.
Clinical manifestations in patients with isolated IgA positivity*
| Clinical manifestations† | LUMINA n = 23 |
Hopkins n = 17 |
APLS n = 17 |
|---|---|---|---|
| All thrombosis (arterial and/or venous) | 6 (26.1) | 12 (70.6) | 6 (35.3) |
| Pregnancy loss/ complications | 3 (13.0) | 9 (52.9) | 1 (5.8) |
| Stroke | 1 (4.3) | 4 (23.5) | 2 (11.7) |
| Seizure | 1 (4.3) | 6 (35.3) | 0 (0) |
| Myocardial infarction | 2 (8.7) | 4 (23.5) | 0 (0) |
| Valvular heart disease | 1 (4.3) | 1 (5.8) | 0 (0) |
| Pulmonary Arterial hypertension | 0 (0) | 5 (29.4) | 0 (0) |
| Skin ulcers | 1 (4.3) | 0 (0) | 0 (0) |
| Livedo reticularis | N/A | 3 (17.6) | 0 (0) |
| Thrombocytopenia | 5 (21.7) | 7 (41.2) | 2 (11.7) |
| ESRD | 0 (0) | 3 (17.6) | 1 (5.8) |
| SLAM-R (mean±SD) | 7.30 ± 5.49 | N/A | N/A |
| SELENA-SLEDAI (mean±SD) | N/A | 4.04 ± 3.53 | N/A |
Except where indicated otherwise, values are the number (%) of patients.
LUMINA = Lupus in Minorities, Nature versus nurture; APLS = Antiphospholipid Standardization Laboratory; NA = not available; ESRD: end-stage renal disease; SLAM-R: Systemic Lupus Activity Measure, Revised; SELENA-SLEDAI: Safety of Estrogens in Lupus Erythematosus National Assessment version of the Systemic Lupus Erythematosus Disease Activity Index.
Includes traditional and non-traditional antiphospholipid syndrome-associated manifestations
Association of IgA anti-β2GPI with thrombosis in SLE patients
Of the 817 patients with SLE from the LUMINA and Hopkins cohorts, 202 (24.7%) had some form of thrombosis. Arterial thrombosis was seen in 95 patients (11.6%) and venous thrombosis in 132 (16.2%). Patients with overall IgA anti-β2GPI positivity (IgA positivity in the presence or absence of other isotypes) were significantly more likely to have arterial thrombosis than patients who were negative for this antibody [17.0% vs. 10.4%, OR 1.8 (1.1-2.8) p=0.018]. Overall IgA anti-β2 GPI positivity was also significantly associated with all forms of thrombosis [31.6% vs. 23.3% OR 1.5 (1.1-2.2) p = 0.027]. Although venous thrombosis occurred more frequently in patients that were positive for this antibody than in those who were not (20.5% vs. 15.3%), the difference was not statistically significant (p=0.103). After adjusting for age, obesity, pregnancy, use of oral contraceptives, end-stage renal disease, use of hydroxychloroquine or NSAID at the time of visit when the blood samples were obtained, and for history of smoking, IgA anti-β2GPI positivity remained significantly associated with both arterial (adjusted P=0.021) and all thrombosis (adjusted P=0.016) but still had no significant association with venous thrombosis (adjusted P= 0.12) (Table 3).
Table 3.
Association of anti-β2GPI IgA positivity with thrombosis*
| Antiphospholipid Antibody | Arterial Thrombosis | Venous Thrombosis | All Thrombosis | |
|---|---|---|---|---|
| IgA anti- β2GPI |
Positive, % | 17.0 | 20.5 | 31.6 |
| Negative, % | 10.4 | 15.3 | 23.3 | |
| OR (95%CI) | 1.8 (1.1 - 2.8) | 1.4 (0.9 - 2.2) | 1.5 (1.1 - 2.2) | |
| P | 0.018 | 0.103 | 0.027 | |
| Adjusted OR (95% CI) † | 2.2 (1.1 - 4.4) | 1.6 (0.9 - 2.8) | 1.9 (1.1 - 3.3) | |
| P | 0.021 | 0.120 | 0.016 | |
| Isolated IgA anti- β2GPI |
Positive, % | 31.8 | 29.5 | 50.0 |
| Negative, % | 10.6 | 15.6 | 23.6 | |
| OR (95%CI) | 3.9 (2.0 - 7.7) | 2.3 (1.2 - 4.5) | 3.2 (1.8 - 6.0) | |
| P | <0.001 | 0.015 | <0.001 | |
| Adjusted OR (95% CI) † | 5.8 (2.3 - 15.2) | 2.3 (1.0 - 5.4) | 5.1 (2.2 - 12.4) | |
| P | 0.0003 | 0.061 | 0.0003 | |
P values represent comparisons of autoantibody-positive versus autoantibody-negative patients anti-β2GPI = anti- β2-glycoprotein I; OR = odds ratio; 95% CI = 95% confidence interval
Adjusted for age at visit, smoking, obesity (BMI), pregnancy at visit, oral contraceptives, end-stage renal disease (measured by serum creatinine level), hydroxychloroquine treatment, and non-steroidal anti-inflammatory drug treatment.
Isolated IgA anti-β2GPI positivity was associated with all forms of thrombosis: 31.8% vs. 10.6% for arterial thrombosis (OR 3.9 [95% CI 2.0-7.7], p<0.001), 29.5% vs. 15.6% for venous thrombosis (OR 2.3 [95% CI 1.2-4.5], p = 0.015) and 50.0 % vs. 23.6% for all thrombosis (OR 3.2 [95% CI 1.8-6.0], p<0.001] in the 3 cohorts (Table 3). After adjusting for age, obesity, pregnancy, use of oral contraceptives, end-stage renal disease, use of hydroxychloroquine or NSAID at the time of visit when the blood samples were obtained, and for history of smoking, isolated IgA anti-β2GPI positivity remained significantly associated with arterial and all thrombosis and the strength of association increased (OR 5.8, adjusted p = 0.0003 and OR 5.1, adjusted p = 0.0003 respectively). However, the association with venous thrombosis did not reach statistical significance (adjusted p =0.061) (Table 3).
Pathogenicity of IgA anti-β2GPI antibodies in a thrombosis mouse model
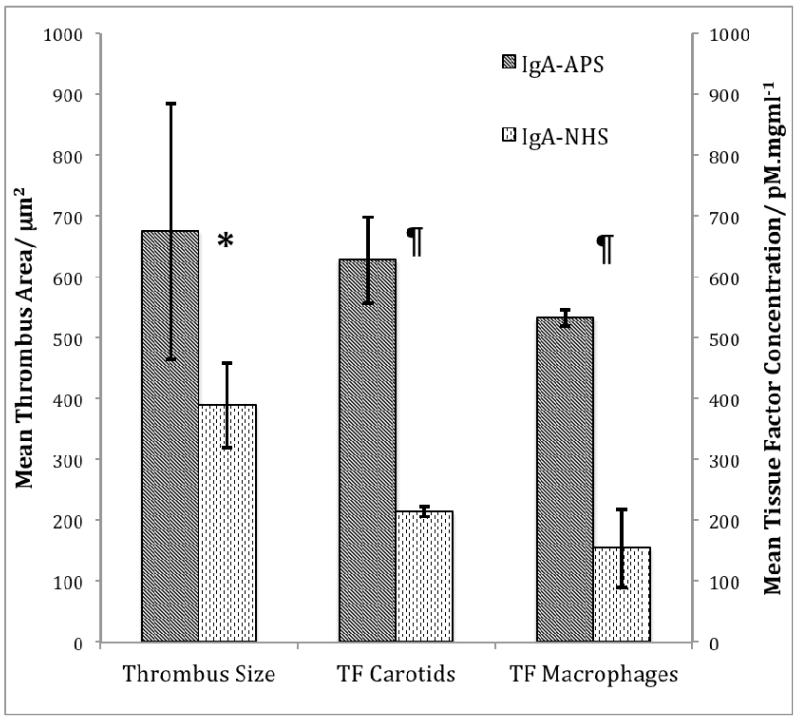
The final concentration of total IgA in the fraction obtained from APS patients (APS-IgA) was 40.4mg/dl, while the concentrations of IgG and IgM were below the lower limits of the assay. The APS-IgA preparation had high levels of total IgA anti-β2GPI (103.7 SAU) but was negative for IgG (8.7 SGU) and IgM (6.6 SMU) anti-β2GPI antibodies. The APS-IgA preparation also had high IgA binding activity for DI and DIV/V of β2GPI at several dilutions as shown in Table 4. The control IgA had a total IgA concentration of 67.6mg/dl but was negative for IgA, IgG and IgM anti-β2GPI antibodies. The LAC activity of the APS-IgA preparation was greater than that of the control IgA preparation (silica clotting time ratio APS-IgA to control IgA =2; normal <1.2). The mean area of the thrombi induced in mice inoculated with APS-IgA was significantly higher than that in mice inoculated with control IgA. Similarly, TF activity in the peritoneal macrophages and carotid homogenates of mice treated with APS-IgA was significantly greater than in mice treated with control IgA (Figure 2).
Table 4.
Specific IgA binding activity of purified whole IgA from APS patients to domains I and IV/V at several dilutions*
| Material | IgA DI | IgA DIV/V | ||
|---|---|---|---|---|
|
|
||||
| OD | Units | OD | Units | |
| Dilution of APS-IgA | ||||
| • Neat | 3.624 | 163.7 | 3.655 | 268.8 |
| • 1:20 | 0.658 | 29.7 | 0.802 | 59.0 |
| • 1:40 | 0.357 | 16.2 | 0.459 | 33.8 |
| • 1:80 | 0.196 | 8.9 | 0.279 | 20.5 |
| • 1:100 | 0.176 | 7.9 | 0.195 | 14.3 |
| Negative Control | 0.017 | 0.7 | 0.039 | 2.9 |
APS = antiphospholipid syndrome;
OD = optical density
Figure 2.
Thrombus formation and tissue factor activity in mice inoculated with IgA anti-β2GPI antibodies. Thrombus size (1.7 fold increase), TF activity in carotid homogenates (2.9 fold increase), and TF activity in peritoneal macrophages (3.5 fold increase) were significantly elevated in IgA anti-β2GPI-inoculated mice compared to controls inoculated with IgA antibodies from normal human serum (NHS). * = p = 0.02; ¶ = p <0.01
DISCUSSION
In this study, we demonstrated a fair prevalence of IgA anti-β2GPI positivity in the LUMINA and Hopkins cohorts of SLE patients. Our prevalence rates were similar to previous studies [5,8], including one of the Hopkins Lupus cohort that reported 20.2% positivity of the IgA isotype in 796 patients, but lower than reported rates in other studies [7,18]. Importantly, we demonstrated for the first time that IgA anti-β2GPI was the sole aPL antibody present in 18% and 50% of cases in the LUMINA cohort and in the Hopkins Lupus cohort, respectively. Additionally, we showed that a large number of the IgA anti-β2GPI antibodies present in our patients reacted preferentially against domain IV and V of β2GPI, confirming previous observations [9,10]. We have also shown quite clearly, and for the first time, that IgA anti-β2GPI antibodies, particularly in the absence of other aPL antibodies, are associated with clinically significant thrombosis, especially arterial thrombosis. Furthermore, we have demonstrated the pathogenicity of purified IgA anti-β2GPI antibodies, with binding activity for domains I and IV/V, in a mouse model of thrombosis.
Our study utilized two different commercial kits that express IgA anti-β2GPI titers in different units of measurements, have different cut-off points and reference intervals, and yet showed very good correlation, confirming that IgA anti-β2GPI positivity was not kit specific. Of note, kit A identified more positive samples when compared to kit B (127 vs. 96 positive samples). The differences observed cannot be attributed to cross-reactivity with similar antibodies of the IgG or IgM isotype, since both kits utilize α–chain specific affinity purified anti-human IgA antibodies as secondary antibody, but rather to increased sensitivity of one kit versus the other. Other kit related characteristics such as the type and/or amount of β2GPI to coat the ELISA plates or other features of the assays, not disclosed by the manufacturer, may account for these apparent discrepancies.
Prior studies have shown an association of IgA anti-β2GPI with clinical manifestations of APS, but these findings are still a subject of controversy. Several studies have demonstrated that SLE patients with APS are more prone to being positive for the IgA isotype [4,5]. Fanopoulous et al in their cohort of 48 patients with SLE found the most prevalent isotype was IgA anti-β2GPI antibody (58%). Importantly, significantly higher frequencies (p<0.001) and titers (p<0.05) of IgA anti-β2GPI were observed in patients with APS than in those without APS manifestations [7]. Lakos et al found that the prevalence of IgA anti-β2GPI antibody in their cohort of 70 patients was 59.3% and that this assay had the highest specificity (83%) for the diagnosis of APS [19]. They also reported an association of this isotype with venous thrombosis (p=0.007), thrombocytopenia (p=0.02), livedo reticularis (p=0.01), epilepsy (p=0.01), and valvular heart disease (p=0.02) [18,20,21].
Furthermore, it seems that IgA anti-β2GPI antibodies are independent risk factors for acute myocardial infarction and atherosclerosis in populations without APS [18,20,21] and the same positive association was found for acute cerebral ischemia [22]. Cucurul et al. studied both IgA aCL and anti-β2GPI antibodies in African-American patients with SLE and found an association between thrombotic events and elevated levels of both these autoantibodies [2]. Recently, Mehrani et al found that the IgA isotype was strongly associated with DVT and stroke as compared to the IgM isotype [8]. Other researchers have demonstrated the association of IgA anti-β2GPI antibodies with obstetric complications in APS patients [23,24].
It is clear that IgA anti-β2GPI antibodies have an association with many clinical manifestations in APS. What remains uncertain is the significance of these antibodies as sole entities in different patient populations and the pathogenic effects most attributed to their presence. We have recently published a case series, reporting 5 isolated cases of individuals who were exclusively positive for IgA anti-β2GPI and had concomitant clinical manifestations of APS [11]. Another study showed that IgA anti-β2GPI positivity was associated with an increase in thromboembolic events in a small subset of patients with SLE (n=31) [12]. In addition, Shen et al (25) retrospectively examined 472 patients for whom clinical information on thrombotic events and complete laboratory evaluation for antiphospholipid antibodies were available. Univariate analysis showed a statistically significant risk of thrombosis in patients with elevated titers of IgA of any ELISA-based antiphospholipid antibodies (aCL, anti-phosphatidylserine and anti-β2GPI; OR 1.77). Stepwise logistic regression (multivariate) analysis identified elevated titers of any ELISA-based IgA antibody as an independent risk factor for thrombosis (OR 1.6) in the entire cohort and in a subgroup of patients without concurrent LAC positivity (OR 1.8) [25]. Our analysis of the association of isolated IgA anti-β2GPI positivity with the increased risk of thromboembolic events included a total of 817 subjects from 3 independent groups of patients, using multivariate analysis and adjusting for possible confounding factors.
In the current study, we demonstrated an association of IgA anti-β2GPI antibodies with thrombosis, specifically arterial thrombosis and an association of isolated IgA anti-β2GPI positivity with both venous and arterial thrombosis. After adjusting for confounding factors, isolated IgA anti-β2GPI positivity remained significantly associated with arterial thrombosis, but not with venous thrombosis. Perhaps this indicates that IgA anti-β2GPI in certain subgroups of patients are more thrombogenic than other isotypes. Further subset analysis will be needed to identify possible markers that would identify these groups of patients and possibly identify predisposing clinical and ethnic components.
Thrombogenic effects of IgG and IgM antiphospholipid antibodies using in vitro and in vivo animal models have been shown [3,26,27]. However, similar evidence demonstrating the pathogenic effects of IgA antiphospholipid antibodies has been comparatively limited. In a mouse model designed to study thrombus formation, injected IgA immunoglobulins with aCL activity from 2 patients with APS were shown to cause thrombosis [28]. We have now also demonstrated, for the first time, that IgA anti-β2GPI antibodies induce increased thrombus formation and the up-regulation of TF activity using the same mouse model.
IgA anti-β2GPI antibody positivity was not included in the revised classification criteria for APS in 2006, due to lack of supporting evidence at that time [1]. The question of whether IgA anti-β2GPI may have diagnostic value for APS was subsequently addressed by the ‘non-criteria’ antiphospholipid task force during the 13th International Congress on Antiphospholipid Antibodies (APLA), held in April 2010 in Galveston Texas [29]. The task force concluded that the IgA anti-β2GPI antibodies should be tested in the presence of clinical signs and symptoms of SLE and/or APS, particularly when other antiphospholipid tests are negative. Importantly, the Systemic Lupus International Collaborating Clinics group has recently proposed that anti-β2GPI antibodies be included as serological markers of SLE as part of the revised classification criteria for SLE, including the IgA isotype [32]. Our study provides enough data to indicate that isolated IgA anti-β2GPI positivity should be considered for APS diagnosis and classification in the presence of clinical signs and symptoms. There is still a need for larger longitudinal studies in order to confirm the relative importance of this laboratory marker in the diagnosis of APS, either primary or secondary. Such studies would help to define its value as a marker for disease activity and risk for thrombosis.
Acknowledgements
The Hopkins Lupus cohort is supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS # RO1AR043727) and the Antiphospholipid Standardization Laboratory by a grant from the National Institutes of Health (NIH # 1R01AR056745). AMS received salary support from a NIH grant # T32 AR052283T32. Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Disease P01 AR49084, General Clinical Research Centers (NCRR/NIH) M01-RR02558 (UTH) and M01-RR00032 (UAB) and from the National Center for Research Resources (NCRR/HIH) RCMI Clinical Research Infrastructure Initiative (RCRII) 1P20RR11126. We thank INOVA Diagnostics for providing kits for these studies.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite Antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Cucurul E, Gharavi AE, Diri E, et al. IgA anticardiolipin and anti-β2glycoprotein I are the most prevalent isotypes in African American patients with systemic lupus erythematosus. Am J Med Sci. 1999;318:53–60. doi: 10.1097/00000441-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Pierangeli SS, Liu SW, Anderson G, Barker JH, Harris EN. Thrombogenic properties of murine anti-cardiolipin antibodies induced by beta 2 glycoprotein 1 and human immunoglobulin G antiphospholipid antibodies. Circulation. 1996;94:1746–51. doi: 10.1161/01.cir.94.7.1746. [DOI] [PubMed] [Google Scholar]
- 4.Diri E, Cucurull E, Gharavi AE, et al. Antiphospholipid (Hughes’) syndrome in African-Americans: IgA aCL and anti-β2 glycoprotein I is the most frequent isotype. Lupus. 1999;8:263–8. doi: 10.1191/096120399678847812. [DOI] [PubMed] [Google Scholar]
- 5.Bertolaccini ML, Atsumi T, Escudero Contreras A, et al. The value of IgA antiphospholipid testing for diagnosis of antiphospholipid (Hughes) syndrome in systemic lupus erythematosus. J Rheumatol. 2001;28:2637–43. [PubMed] [Google Scholar]
- 6.Danowski A, Kickler TS, Petri M. Anti-beta2 glycoprotein I: prevalence, clinical correlations, and importance of persistent positivity in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Rheumatol. 2006;33:1775–9. [PubMed] [Google Scholar]
- 7.Fanopoulos D, Teodorescu MR, Varga J, Teodorescu M. High frequency of abnormal levels of IgA anti-ß2-glycoprotein I antibodies in patients with systemic lupus erythematosus: relationship with antiphospholipid syndrome. J Rheumatol. 1998;25:675–80. [PubMed] [Google Scholar]
- 8.Mehrani T, Petri M. Association of IgA anti-β2 glycoprotein I with clinical and laboratory manifestations of systemic lupus erythematosus. J Rheumatol. 2011;38:64–8. doi: 10.3899/jrheum.100568. [DOI] [PubMed] [Google Scholar]
- 9.Iverson GM, von Muhlen CA, Staub HL, et al. Patients with atherosclerotic syndrome, negative in anti-cardiolipin assays, make IgA autoantibodies that preferentially target domain 4 of beta2-GPI. J Autoimmun. 2006;27:266–71. doi: 10.1016/j.jaut.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Martinez LA, Aguilar-Valenzuela R, Seif A, et al. Do clinically relevant IgA anti-β2 glycoprotein I (anti-β2 GPI) antibodies bind to DIV/V of β2 GPI? Lupus. 2010;19:C130. (abstract) [Google Scholar]
- 11.Kumar S, Papalardo E, Sunkureddi P, et al. Isolated elevation of IgA anti-β2 glycoprotein I antibodies with manifestations of antiphospholipid syndrome: a case series of five patients. Lupus. 2009;18:1011–4. doi: 10.1177/0961203309103048. [DOI] [PubMed] [Google Scholar]
- 12.Sweiss NJ, Bo R, Kapadia R, et al. IgA anti-beta2-glycoprotein I autoantibodies are associated with an increased risk of thromboembolic events in patients with systemic lupus erythematosus. PLoS One. 2010;5:e12280. doi: 10.1371/journal.pone.0012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabeta S, Norman GL, Gatselis N, et al. IgA anti-β2 glycoprotein I antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501–11. doi: 10.1007/s10875-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 14.Boin F, Franchini S, Colantuoni E, et al. Independent association of anti-β2 glycoprotein I antibodies with macrovascular disease and mortality in scleroderma patients. Arthritis Rheum. 2009;60:2480–9. doi: 10.1002/art.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reveille JD, Moulds JM, Ahn C, et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA study group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1161–72. doi: 10.1002/1529-0131(199807)41:7<1161::AID-ART4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Pierangeli SS, Harris EN. A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc. 2008;3:840–8. doi: 10.1038/nprot.2008.48. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Cho M, Joo Y, et al. Isotypes of anti-β2-glycoprotein I antibodies: association with thrombosis in patients with systemic lupus erythematosus. J Rheumatol. 2001;28:520–4. [PubMed] [Google Scholar]
- 19.Lakos G, Kiss E, Regëczy N, et al. Isotype distribution and clinical relevance of anti-beta2-glycoprotein I (beta2-GPI) antibodies: importance of IgA isotype. Clin Exp Immunol. 1999;117:574–9. doi: 10.1046/j.1365-2249.1999.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staub HL, Franck M, Ranzolin A, et al. IgA antibodies to β2 glycoprotein I and atherosclerosis. Autoimmun Rev. 2006;6(2):104. doi: 10.1016/j.autrev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Ranzolin A, Bohn JM, Norman GL, et al. Anti-β2 glycoprotein I antibodies as risk factors for acute myocardial infarction. Arquivos Brasileiros de Cardiologia. 2004;83:141–4. doi: 10.1590/s0066-782x2004001400005. [DOI] [PubMed] [Google Scholar]
- 22.Staub HL, Norman GL, Crowther T, et al. Antibodies to the atherosclerotic plaque components β2 glycoprotein I and heat-shock proteins as risk factors for acute cerebral ischemia. Arq Neuropsiquiatr. 2003;61:757–63. doi: 10.1590/s0004-282x2003000500010. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Tsutsumi A, Ichikawa K, et al. IgA-class anti-β2glycoprotein I in women with unexplained recurrent spontaneous abortion. Arthritis Rheum. 1999;42:2727–2730. doi: 10.1002/1529-0131(199912)42:12<2727::AID-ANR33>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Lee RM, Branch DW, Silver RM. Immunoglobulin A anti-β2 glycoprotein antibodies in women who experience unexplained recurrent spontaneous abortion and unexplained fetal death. Am J Obstet Gynecol. 2001;185:748–753. doi: 10.1067/mob.2001.117659. [DOI] [PubMed] [Google Scholar]
- 25.Shen YM, Lee R, Frenkel E, Sarode R. IgA Antiphospholipid antibodies are an independent risk factor for thromboses. Lupus. 2008;17:996–1003. doi: 10.1177/0961203308093460. [DOI] [PubMed] [Google Scholar]
- 26.Pierangeli SS, Liu X, Espinola R, et al. Functional analyses of patient-derived IgG monoclonal anticardiolipin antibodies using in vivo thrombosis and in vivo microcirculation models. Thromb Haemost. 2000;84:388–95. [PubMed] [Google Scholar]
- 27.Pierangeli SS, Barker JH, Stikovac D, et al. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb Haemost. 1994;71:670–4. [PubMed] [Google Scholar]
- 28.Pierangeli SS, Liu X, Barker JH, Anderson G, Harris EN. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 1995;74:1361–1367. [PubMed] [Google Scholar]
- 29.Bertolaccini ML, Amengual O, Atsumi T, et al. Pierangeli SS. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;2:191–205. doi: 10.1177/0961203310397082. [DOI] [PubMed] [Google Scholar]
- 30.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]