Abstract
Neutral cues that predict emotional events (emotional harbingers) acquire emotional properties and attract attention. Given the importance of emotional harbingers for future survival, it is desirable to flexibly learn new facts about emotional harbingers when needed. However, recent research revealed that it is harder to learn new associations for emotional harbingers than cues that predict non-emotional events (neutral harbingers). In the current study, we addressed whether this impaired association learning for emotional harbingers is altered by one’s awareness of the contingencies between cues and emotional outcomes. Across three studies, we found that one’s awareness of the contingencies determines subsequent association learning of emotional harbingers. Emotional harbingers produced worse association learning than neutral harbingers when people were not aware of the contingencies between cues and emotional outcomes, but produced better association learning when people were aware of the contingencies. These results suggest that emotional harbingers do not always suffer from impaired association learning and can show facilitated learning depending on one’s contingency awareness.
Keywords: association learning, conditioning, contingency awareness, emotion and memory
Emotional reactions to events or materials related to physical or social survival influence how such stimuli are processed and remembered (Dolan, 2002; Pourtois, Schettino, & Vuilleumier, 2012; Sakaki, Niki, & Mather, 2012). For instance, emotional images often get more initial attention than neutral images (M. Knight et al., 2007; Mather & Knight, 2006; Öhman, Flykt, & Esteves, 2001; Öhman, Lundqvist, & Esteves, 2001; Schimmack, 2005). Emotional information also tends to be remembered better than neutral information (Dolcos & Denkova, 2008; Hamann, 2001; Kensinger & Corkin, 2004; LaBar & Cabeza, 2006; Mather & Knight, 2005). In particular, intrinsic features of emotional events are remembered better than those of neutral events (D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003; Kensinger, Garoff-Eaton, & Schacter, 2006; Mather, 2007; Mather & Nesmith, 2008).
Despite the large number of studies on the interaction between emotion and cognition, researchers have not paid much attention to the effects of previous emotional associations on subsequent association learning about cues. For example, imagine that you saw a large dog that ran over to a fence and barked at you every time you walked by a neighbors’ place. Even when this neighbor’s place originally had no emotional meaning, repeated exposures to the barking dog might establish associations between this place and negative emotions. How does this emotional association influence learning new features (e.g., new tenant; new car) about this place? Is it easier or harder to learn new associations for cues previously predictive of emotional events (emotional harbingers; for instance, the place where you repeatedly saw the barking dog) than for cues predictive of neutral events (neutral harbinger; for instance, another neighbor’s place)? The current study addressed this issue.
One plausible prediction that can be made based on past research is that previous emotional associations to something make it easier to learn new associations to that cue. Ample research on classical fear conditioning revealed that cues predictive of aversive events acquire emotional value and increase subjective emotions, physiological reactions, and brain activity in emotion-related areas (Bermpohl et al., 2006; Delgado, Olsson, & Phelps, 2006; Kelly & Forsyth, 2007; Mackiewicz, Sarinopoulos, Cleven, & Nitschke, 2006; Maren, 2001; Morris, Ohman, & Dolan, 1999; Phelps et al., 2001; Sehlmeyer et al., 2009). Neutral cues predictive of aversive events also attract attention (Beaver, Mogg, & Bradley, 2005; Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2005; Notebaert, Crombez, Van Damme, De Houwer, & Theeuwes, 2011; Smith, Most, Newsome, & Zald, 2006; Van Damme, Crombez, Hermans, Koster, & Eccleston, 2006; Van Damme, Crombez, & Notebaert, 2008) in a similar manner as inherently emotionally arousing stimuli (e.g., Anderson, 2005; Mather & Knight, 2006; Most, Chun, Widders, & Zald, 2005). Such enhanced emotional reactions and attention might enhance learning new associations to emotional harbingers. Indeed, people tend to show enhanced memory binding for features of emotionally arousing stimuli compared with features of neutral stimuli (D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003; Kensinger et al., 2006; Kensinger & Schacter, 2007; Mather, 2007; Mather & Nesmith, 2008; Nashiro & Mather, 2011). Intra-item associations are also sometimes learned better for emotionally arousing items than for neutral items (Maddox, Naveh-Benjamin, Old, & Kilb, 2012; Pierce & Kensinger, 2011).
In contrast, recent research revealed the opposite result: people have more difficulty learning new associations for emotional harbingers than neutral harbingers (Mather & Knight, 2008). For example, in their third experiment, the authors first asked participants to view neutral faces, followed by negative or neutral pictures (initial cue-learning phase). Unbeknownst to participants, some faces always preceded negative pictures (emotional harbingers) and the other faces always preceded neutral pictures (neutral harbingers). Participants’ task was to indicate whether the picture was negative or neutral. After this initial learning phase, participants were asked to learn a face-hat association for all faces they saw during initial learning. The results indicated that participants were worse at remembering face-hat pairings for emotional harbinger faces than for neutral harbinger faces. Similar results were reported irrespective of harbinger type (e.g., face, tone), association types (e.g., face-location; tone-digit), valence of emotional outcomes (positive or negative), and the duration of delay between encoding and memory tests across five experiments, suggesting that the effects are robust.
Effects of the contingency awareness
However, the previous study (Mather & Knight, 2008) did not consider one important factor: the contingency awareness between cue and emotional outcomes. In their study, participants were merely told to passively observe cues followed by emotional/neutral pictures during the initial learning. In addition, participants’ main task during the initial learning phase was to indicate the valence of each outcome picture (negative or neutral; positive or neutral). Thus, it seems possible that participants focused on outcomes more than cues. This selective attention to outcomes should lead to the lack of awareness about the contingencies between cues and emotional outcomes (Blask, Walther, Halbeisen, & Weil, 2012). Furthermore, the authors did not test participants’ awareness of cue-valence contingencies. Therefore, it is not clear whether and how people’s awareness of cue-valence contingencies influences subsequent association learning for harbinger cues.
In contrast, research on fear/evaluative conditioning suggests that the awareness of the contingency increases emotional reactions to cues, which might alter subsequent association learning for cues. In fact, although conditioning can sometimes occur in the absence of contingency awareness (e.g., Walther & Nagengast, 2006), many studies indicate that contingency awareness increases emotional reactions (due to conditioning) to cues predictive of emotional outcomes (Bar-Anan, De Houwer, & Nosek, 2010; Carter, Hofstötter, Tsuchiya, & Koch, 2003; Dawson, 1973; Dawson, Rissling, Schell, & Wilcox, 2007; Hofmann, De Houwer, Perugini, Baeyens, & Crombez, 2010; Kattner, 2011; Klucken et al., 2009; Pleyers, Corneille, Luminet, & Yzerbyt, 2007; Pleyers, Corneille, Yzerbyt, & Luminet, 2009; Tabbert et al., 2011).
Past studies on association learning also suggest the importance of the contingency awareness in subsequent learning for cues. Decades of research indicate that animals and humans are better at learning new associations to cues previously established as reliable predictors than for cues established as unreliable predictors (Beesley & Le Pelley, 2010; Kruschke & Blair, 2000; Le Pelley, 2004; Le Pelley & McLaren, 2003; Prados, Redhead, & Pearce, 1999; Reid, 1953; Trobalon, Miguelez, McLaren, & Mackintosh, 2003). These effects of cues’ historical predictiveness have been interpreted as reflecting attention (de Pasquale et al., 2010; Kruschke, 2003; Le Pelley, 2004; Mackintosh, 1975). That is, once animals or humans realize cues’ predictiveness, they should devote more attention to cues yielding accurate predictions and devote fewer resources to other cues. Since emotional outcomes have significance for individuals relative to neutral outcomes (Lang & Bradley, 2010), it seems possible that one’s contingency awareness increases attentional priority especially for emotional harbingers. This increased attention should result in stronger memory representations, leading to better association learning for emotional harbingers than neutral harbingers.
The Current Study
In the current study, we investigated the effects of contingency awareness on association learning for emotional and neutral harbingers. In Studies 1 and 2, we examined whether emotional harbingers produce worse association learning than neutral harbingers even when people are aware of the contingency between harbinger cues and emotional outcomes. Thus, we explicitly told and encouraged participants to learn associations between harbinger cues and emotional/neutral outcomes during the initial learning phase. Participants were then told to learn new associations to emotional and neutral harbinger cues. At the end of the study, we also included a contingency awareness memory test. Given that contingency awareness can increase emotional reactions and attentional priority for emotional harbinger cues, we expected that people would be better at learning new associations for emotional harbingers than for neutral harbingers under these conditions, in contrast with previous findings of impaired association learning for emotional harbingers (Mather & Knight, 2008). In Study 3, we manipulated people’s contingency awareness to address whether contingency awareness serves as a boundary condition between impaired and facilitated association learning for emotional harbingers relative to neutral harbingers.
Study 1
Study 1 tested whether association learning is easier for emotional harbingers than for neutral harbingers when people are aware of cue-outcome contingencies. As discussed above, in Mather and Knight (2008), participants were simply told to passively observe cues followed by emotional/neutral pictures without any explicit instruction about cue-valence contingencies during the initial learning phase. To increase participants’ contingency awareness, in Study 1, participants were explicitly asked to predict for each cue whether the cue would be predictive of negative or neutral outcomes. They were also encouraged to make as accurate predictions as possible. Furthermore, whereas the previous study used emotional/neutral pictures, we used 6-sec emotional/neutral sound clips as emotional or neutral outcomes during the initial learning phase. Since the contingency awareness can be enhanced when outcomes and cues are given in different modalities (Blask et al., 2012), this should also help participants learn the contingency.
Methods
Participants
Forty undergraduates (Mage = 20.00, SD = 1.65; 10 males) took part in the experiment for course credit.
Materials
We used 32 negative and 32 neutral sound clips of 6-s duration obtained from the International Affective Digital Sounds (IADS: Bradley & Lang, 1999; Verona, Patrick, Curtin, Bradley, & Lang, 2004) and other resources (e.g., the Internet). Because sound clips obtained from other resources did not have normative ratings, we quantified valence and arousal for all of the 64 sounds. Ten participants who did not take part in any studies reported in this paper rated each sound in terms of valence on a scale ranging from 1 (most unpleasant) to 9 (most pleasant) and the arousal on a scale ranging from 1 (least arousing) to 9 (most arousing). The average valence rating was 1.62 for negative (SD = 0.33) and 5.09 (SD = 0.14) for neutral sounds. The average arousal level was 7.83 (SD = 0.44) for negative and 1.38 (SD = 0.20) for neutral sounds. All of the sounds involved human voices regardless of the valence condition.
In addition, eight female faces with neutral expressions were obtained from a previous stimuli set (Mather & Knight, 2008). Half of the faces were paired with negative sounds, and the other faces were paired with neutral sounds. Whether the face was paired with negative or neutral sounds was counterbalanced across participants. Each face was paired with eight different sounds from the same valence category.
Procedure
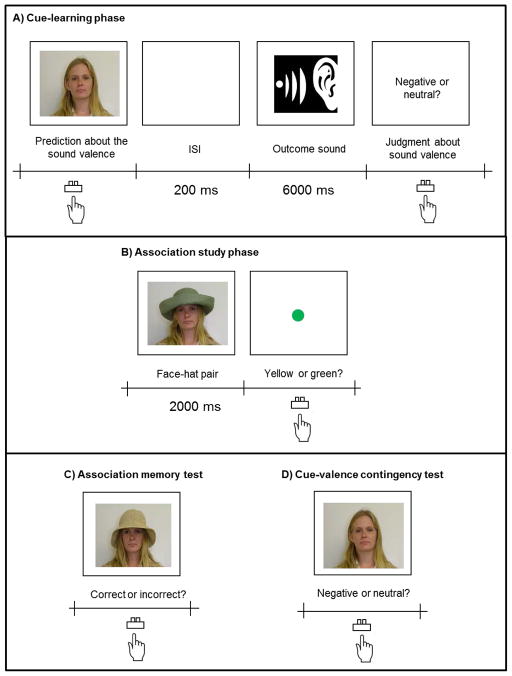
The procedure involved four phases: cue-learning phase, association study phase, association memory test, and valence contingency test.
Each trial of the cue-learning phase started with a presentation of a face (Figure 1A). Participants were asked to predict whether the face was followed by negative or neutral sounds. Following a 200-ms interval after their responses, they were presented with either a negative or neutral sound. Immediately after the sound, participants indicated whether the sound was negative or neutral. There was a 1000-ms intertrial interval (starting at the offset of participants’ response) before the next trial began. Each face was presented 8 times with different sound clips from the same valence category. Presentation order was random. Although the cue-learning phase involved the same number of trials for all participants, participants were told that they could move on to the next phase as soon as they had reached a certain criterion in their prediction accuracy. Thus, they were encouraged to learn cue-valence contingencies as accurately as possible.
Figure 1.
Schematic representations of procedures in Study 1.
Next, in the association study phase, participants learned face-hat associations for the faces they viewed in the cue-learning phase (Figure 1B). On each trial, participants saw a face wearing a hat for 2 sec and were instructed to learn the face-hat pairing for a later memory test. The face-hat pair was followed by either a yellow or green dot. Participants were told to indicate whether the dot was green or yellow by pressing a key. The dot task was included to make sure that participants maintained their attention on the screen. The dot remained on the screen until the participant pressed the key. After their response, there was a 1000-ms intertrial interval before the next trial began. Each of the eight face-hat pairs was shown once.
Following the association study phase, participants completed the association memory test (Figure 1C). As in the association study phase, they viewed a face wearing a hat. Half of the faces were paired with the same hat learned during the association study phase. In contrast, the other half of the faces were paired with hats previously paired with another face in the same valence condition. Participants were asked to indicate whether the faces and hats were correctly paired or incorrectly paired.
Finally, participants’ memory about cues-valence contingencies was tested by a valence contingency awareness test (Figure 1D). Participants viewed each of the eight faces without a hat and indicated whether the face had predicted negative or neutral sounds in the initial cue-learning phase.
Results and Discussion
Cue-hat associative memory
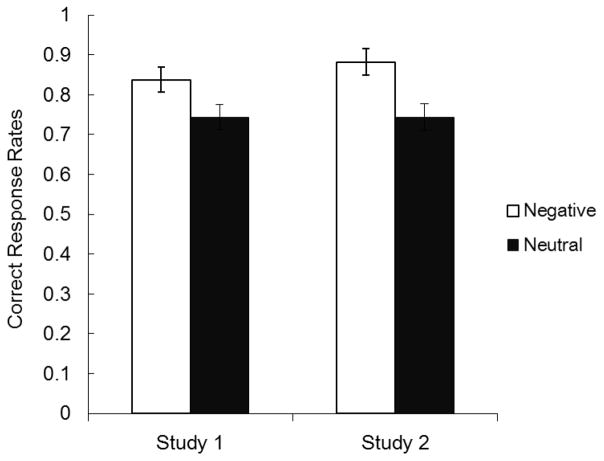
We analyzed the proportion of correct responses in the association memory test. Participants showed better face-hat memory for emotional harbinger faces (M = .84) than for neutral harbinger faces (M = .74; Figure 2), F (1, 39) = 4.63, ηp2 = .11, p < .05. Thus, contrary to previous findings (Mather & Knight, 2008), cues previously predictive of emotional outcomes produced better associative memory than did cues predictive of neutral outcomes.
Figure 2.
Correct response rates in the cue-hat association memory test in Studies 1 and 2. Memory for cue-hat associations was better for cues that predicted negative outcomes than cues that predicted neutral outcomes. Error bars represent standard error.
Cue-valence contingency awareness
The accuracy in the contingency awareness test was not significantly different across the conditions (Mneg = .83 vs. Mneut = .84; p > .70) and performance was significantly better than chance for both emotional and neutral harbinger faces, with respective ts (39) = 8.63, 10.78, ps < .01.
Performance in the cue-learning phase
Participants viewed each face eight times during the cue-learning phase; these eight repetitions were categorized into 4 blocks. Prediction accuracy was significantly above chance in all blocks, ts (39) = 2.23, 2.56, 5.57, 7.31 for blocks 1–4, respectively, ps < .05, but it also improved across the blocks (Mblock1 = .54, Mblock2 = .58, Mblock3= .68, Mblock4 = .75), F (3, 117) = 20.11, ηp2 = .34, p < .01. At the initial block, participants showed a lower accuracy for faces paired with negative sounds than those paired with neutral sounds (Mneg = .43 vs. Mneut = .65), F (1, 117) = 16.77, p < .01. Thus, participants initially tended to predict neutral sounds for all faces, possibly due to the fact that all faces showed neutral expressions. At the final block, however, participants showed equally good prediction performance irrespective of valence (p > .40; Mneg = .73 vs. Mneut = .77). These results are in line with the results from the valence awareness memory test, suggesting that participants acquired equally strong memories about cue-valence contingencies both for emotional and neutral harbinger faces.
Performance in the association study phase
Participants were faster in reacting to the dot following faces previously associated with neutral sounds (M = 903 ms) than those associated with negative sounds (M = 998 ms), F (1, 39) = 6.88, p < .05. But they showed equally good accuracy in dot-color judgments, irrespective of valence (p > .30, Mneg = .96 vs. Mneut = .97). Performance was significantly better than chance for both emotional and neutral harbinger faces, with respective ts (39) = 35.83, 37.82, ps < .01.
Study 2
Consistent with our prediction, Study 1 revealed better association learning for emotional harbingers than for neutral harbingers when people were aware of the cue-outcome contingencies. Study 2 was designed to extend Study 1 by addressing several questions about Study 1.
The first question concerns the nature of emotional/neutral outcomes used in the cue-learning phase. Mather & Knight (2008) used emotional or neutral pictures, whereas Study 1 used emotional or neutral sound clips. In addition, we repeated each harbinger cue only 8 times in Study 1 (vs. 16 times in the previous study). To make these factors more comparable across studies, in Study 2, we employed emotional/neutral pictures (instead of sounds) and increased the number of repetitions for each harbinger face in the cue-learning phase. Thus, Study 2 examined whether the Study 1 pattern of results would be obtained with procedures similar to Mather and Knight’s previous studies.
Second, Study 1 did not tease apart the effects of general associations between cues and valence and the effects of specific cue-outcome associations. That is, participants in Study 1 might have learned specific associations between cue and outcome sounds (e.g., “this face was paired with a baby’s scream”), in addition to general associations between cues and valence (e.g., “this face was paired with negative sounds”). These specific memories might be more responsible for the enhanced association learning for emotional harbingers than for neutral harbingers. To address this possibility, we introduced an additional memory test to examine participants’ memory for specific cue-outcome pairs they encountered during initial learning.
Methods
Participants
Thirty-eight undergraduates (Mage = 19.82, SD = 1.33; 6 males) took part in the study for course credit.
Materials
We employed 128 matched picture pairs, in which each negative picture was yoked with a less arousing neutral picture which was similar in appearance, complexity, content and focus of interest. Those matched pictures involved pairs obtained from previous studies (Mather & Nesmith, 2008; Sakaki, Niki, & Mather, 2011) and those created for the current study using pictures from other resources (e.g., the Internet). Ten participants who did not take part in any of the studies in this paper rated each picture in terms of valence (1: extremely negative – 9: extremely positive) and arousal (1: least arousing – 9: most arousing). The average valence rating was 2.15 (SD = 0.45) for the negative and 5.21 (SD = 0.48) for the neutral version. The average arousal rating was 6.97 (SD = 0.73) for the negative and 1.96 (SD = 0.65) for the neutral version. The 128 pairs were grouped into two sets of 64 matched-pair pictures (i.e., Set A and Set B) that equated arousal and valence across the sets for the negative versions and also for the neutral versions. During the study, half of the participants were shown the negative versions from Set A and the neutral versions from Set B, while the other half were shown the neutral versions from Set A and the negative versions from Set B. Whether participants saw the negative or neutral version from each matched-picture pair was counterbalanced across participants.
We used the same eight faces as in Study 1. Half of the faces were paired with 16 different negative pictures (negative condition), while the other faces were paired with 16 neutral pictures (neutral condition). Whether a face was paired with negative or neutral pictures was counterbalanced across participants.
Procedures
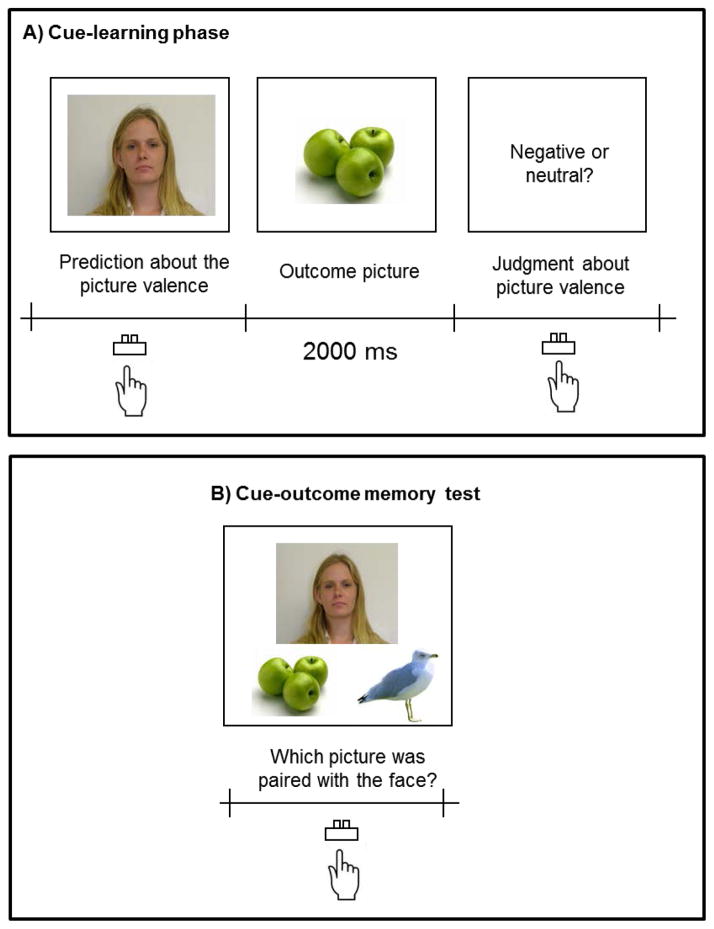
The procedures were similar to Study 1 with several modifications. First, we modified the procedure of the cue-learning phase (Figure 3A). During the cue-learning phase, participants used a key press to indicate their prediction about whether each face was followed by a negative or neutral picture, instead of a sound. Immediately after they pressed the key, they were shown an emotional or neutral picture for 2000 ms. We also increased the number of repetitions for each face from 8 to 16.
Figure 3.
Schematic representations of the cue-learning phase and the cue-outcome memory test in Study 2.
Second, we introduced an additional memory test at the end of the experiment (i.e., cue-outcome pair memory test; Figure 3B). During this memory test, each face was shown with two pictures: a picture paired with the face during the cue-learning phase, and another picture that had been paired with a different face in the same valence condition. Participants’ task was to select the picture paired with the face during the cue-learning phase. Since each face was paired with 16 different pictures in the cue-learning phase, each face appeared 16 times with different pairs of pictures during this memory test.
Results
Cue-hat associative memory
Memory for face-hat pairs was better for emotional harbinger faces (M = .88) than for neutral harbinger faces (M = .74; Figure 2), F (1, 37) = 8.51, ηp2 = .17, p < .01. Thus, Study 1’s findings were replicated with a design similar to the previous study (Mather & Knight, 2008).
Cue-valence contingency awareness
As in Study 1, performance in the contingency awareness test did not differ between emotional and neutral harbingers (p > .60); participants remembered the associated valence correctly for most of the faces irrespective of valence (Mneg = .91 vs. Mneut = .92), both of which were significantly better than chance, with respective ts (37) = 14.91, 16.75, ps < .01.
Cue-outcome pair memory
The accuracy in the final cue-outcome pair memory test did not significantly differ depending on the valence of outcome pictures (p > .40). Both negative and neutral harbinger faces yielded correct response rates significantly better than chance (Mneg = .55 vs. Mneut = .57), respective ts (37) = 3.68, 5.49, ps < .01.
To address the effects of strength of cue-outcome pair memory, participants were categorized into strong and weak pair memory groups based on a median split of the cue-outcome pair memory performance (Md = .54); the mean pair memory performance for each group was Mstrong = .62 vs. Mweak =.51. A 2 (valence: negative vs. neutral) × 2 (pair memory: strong vs. weak) analysis-of-variance (ANOVA) was then performed on the correct response rates in the cue-hat associative test. This ANOVA confirmed a significant effect of valence, F (1, 36) = 7.73, ηp2 = .16, p < .01, with no other significant effects (ps > .20). Thus, face-hat memory was better for emotional harbinger faces than for neutral harbinger faces, irrespective of the strength of initial cue-outcome associations (strong: Mneg = .82 vs. Mneu = .75; weak: Mneg = .93 vs. Mneu = .74).
Similar results were obtained from a general linear model analysis, where cue-outcome pair memory performance was treated as a continuous variable. The dependent variable was the mean correct response in the cue-hat associative test. Independent variables included the valence condition (negative or neutral), the mean accuracy for the face-outcome pair memory test, and an interaction between valence and pair memory performance. The results confirmed a significant effect of valence, F (1, 35) = 8.74, p < .01, but neither the main effect of the pair memory nor the interaction was significant (ps > .20).
Performance in the cue-learning phase
The 16 repetitions during the initial cue-learning phase were categorized into 4 blocks. Participants’ predictions were significantly better than chance in all blocks, with respective ts (37) = 2.19, 7.27, 9.26, 10.77, ps < .05. Their predictions also improved across the blocks (Mblock1 = .53, Mblock2 = .65, Mblock3 = .78, Mblock4 = .85), F (3, 111) = 72.06, ηp2 = .75, p < .01. Overall, participants tended to show better performance for negative than neutral faces (Mneg = .72 vs. Mneut= .69), F (1, 37) = 3.65, ηp2 = .09, p < .07, but by the last two blocks they showed equally good prediction performance irrespective of valence (block 3: Mneg = .78 vs. Mneut = .78; block 4: Mneg = .87 vs. Mneut = .83; ps > .20). Thus, it appears that participants acquired similarly strong memories about cue-valence contingencies for emotional and neutral harbinger faces.
Performance in the association study phase
Neither the reaction times to the dot (p > .50; Mneg = 995 ms vs. Mneut = 965 ms), nor the accuracy in dot-color judgments (p > .90, Mneg = .99 vs. Mneut = .99) showed significant valence effects. Performance accuracy was significantly better than chance for both emotional and neutral harbinger faces, with respective ts (37) = 54.46, 54.46, ps < .01.
Discussion
In Study 2, we used procedures similar to the previous study (Mather & Knight, 2008) and replicated the results from Study 1 that emotional harbingers produce better association learning than neutral harbingers when people are aware of cue-valence contingencies. Study 2 also examined the effects of cue-outcome pair memory and found that specific memories for cue-outcome pairs do not influence subsequent association learning for harbingers. Thus, the awareness of cue-valence contingency seems more critical than specific cue-item pair memories in facilitating association learning for emotional harbingers than for neutral harbingers.
Study 3
The main purpose of Study 3 was to confirm that the contingency awareness serves as a boundary condition between enhanced vs. impaired association learning for emotional harbingers than neutral harbingers by manipulating a prediction task during the cue-learning phase.
During the cue-learning phase, half of the participants were asked to predict whether an outcome picture was negative or neutral for each face (emotion learning condition; same as Studies 1 and 2). In contrast, the other participants saw the same face-picture pairs but were asked to predict a non-emotional aspect of outcome pictures (location learning condition). Following this cue-learning phase, all participants, irrespective of the conditions, learned face-hat associations for faces that had been paired with negative (old negative condition) or neutral pictures (old neutral condition). We expected that the prediction task manipulation should make participants in the emotion learning condition become more aware of the cue-valence contingencies than those in the location learning condition, allowing us to examine whether or not one’s awareness of cue-valence contingencies influences subsequent associative learning.
To confirm that the prediction task manipulation during the initial cue-learning phase did not change the overall memory performance in the subsequent association study phase, Study 3 also introduced another condition, in which participants learned face-hat associations for neutral faces that they never saw (new neutral condition).
In addition, Study 3 addressed a few remaining issues from Studies 1 and 2. The first question concerns the face-valence contingency awareness test. Participants in Studies 1 and 2 showed near ceiling performance in the final cue-valence contingency awareness test, in which they made a dichotomous judgment about whether each face was associated with negative or neutral outcomes. However, participants might not have felt confident about the contingencies even when they showed high accuracy in the dichotomous judgment task. To address this possibility, in Study 3, we asked participants to rate their confidence about a face-valence association/face-location association in addition to the dichotomous awareness memory test. This confidence rating score allowed us to check whether participants in the emotion learning condition were more confident about the face-valence associations than those in the location learning condition or not.
The valence confidence rating allowed us to further examine the effects of the contingency awareness by looking at the intra-individual effects of face-valence contingency awareness. If the cue-valence awareness is predictive of association learning for emotional harbingers, intra-individual variations in the valence confidence across trials should predict intra-individual differences in the cue-hat association performance for emotional harbingers than for neutral harbingers.
Second, the previous study (Mather & Knight, 2008) reported worse association learning for emotional than neutral harbingers, irrespective of whether hats were shown being worn or in a separate photograph next to the faces. In contrast, we presented hats being worn during the association learning phase both in Studies 1 and 2. In Study 3, hats were presented beside faces to confirm that the results are not specific to the spatial configuration used in Studies 1 and 2. Third, to improve the statistical power, we increased the number of harbinger faces to 10 faces for each condition (vs. 4 faces in Studies 1 and 2). Lastly, to confirm the generalizability of the results, a new set of faces and emotional/neutral pictures were also introduced.
Methods
Participants and design
Sixty undergraduates (Mage = 19.98, SD = 1.37; 9 males) took part in the study for course credit. They were randomly assigned to the emotion (N = 30) or location (N = 30) learning condition.
Materials
We used 30 faces of young females with neutral expressions obtained from the NimStim set (Raes, De Raedt, Fias, Koster, & Van Damme, 2009) and the FACES database (De Houwer & Tibboel, 2010). They were randomly assigned to one of the three conditions (old negative, old neutral and new neutral). The assignment was counterbalanced across participants. Thirty pictures of hats obtained from the Internet and commercial DVDs were randomly paired with one of the 30 faces.
In addition, 160 negative and 160 neutral pictures were selected from the International Affective Picture System (IAPS: Lang, Bradley, & Cuthbert, 2008). The IAPS includes standardized ratings of valence of each picture based on a scale ranging from 1 (most unpleasant) to 9 (most pleasant) and ratings of arousal level on a scale ranging from 1 (least arousing) to 9 (most arousing). The average valence of the images we employed was 2.74 for negative (SD = 0.71) and 5.52 (SD = 0.65) for neutral images. The average arousal level was 5.73 (SD = 0.79) for negative and 3.41 (SD = 0.74) for neutral pictures. To obtain an objective measure of visual complexity of each picture, we used Matlab’s Canny edge detector to compute the edge density of each image (Rosenholtz, Li, & Nakano, 2007). The Canny edge detector has three parameters: a low threshold, high threshold, and sigma. These thresholds were set to 0.11, 0.27, and 1 (Rosenholtz et al., 2007). The mean edge density was 0.056 (SD = 0.05) for negative and 0.030 (SD = 0.05) for neutral pictures; they were not significantly different (p > .40).
Faces assigned to the old negative condition were paired with 16 different negative pictures, while those assigned to the old neutral condition were paired with 16 different neutral pictures.
Procedures
Procedures in the emotion and location learning conditions were identical, except for the prediction task during the cue-learning phase. In both conditions, procedures were based on Study 2 with several modifications.
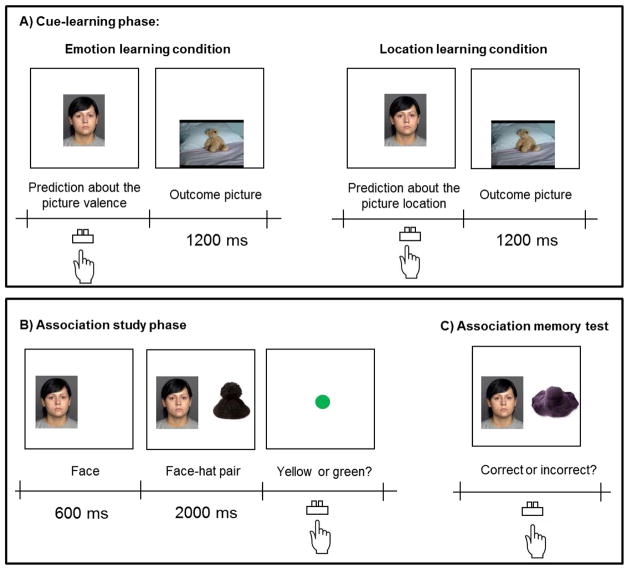
During the cue-learning phase (Figure 4A), participants in both conditions were shown a face and instructed to press a key to indicate their predictions. Participants in the emotion learning condition were asked to predict whether a subsequent picture was negative or neutral, whereas participants in the location learning condition were asked to predict whether a subsequent picture would appear on the top or the bottom of the computer screen. Immediately after they pressed a key, participants saw a negative or a neutral picture either on the top or the bottom of the screen for 1200 ms. Irrespective of the learning condition (emotion or location), half of the faces predicted negative pictures (i.e., old negative condition), while the other half predicted neutral pictures (i.e., old neutral condition). Half of the faces in the old negative condition predicted pictures on the top, while the other half predicted pictures on the bottom. Similarly, half of the faces in the old neutral condition predicted pictures on the top, while the other half predicted pictures on the bottom. Thus, the location of the outcome pictures was independent of the associated valence in both conditions. The intertrial interval was 800 ms. Faces assigned to the new neutral condition were not used in this phase.
Figure 4.
Schematic representations of procedures in Study 3.
Next, in the association study phase, participants learned face-hat associations (Figure 4B). Each trial started with presentation of a face (either old faces learned during the cue-learning phase or new faces that had not previously been seen). After 600 ms of the face, a hat appeared beside the face. Both the face and the hat remained on the screen for 2000 ms, which was followed by a yellow or a green dot. Participants were told to learn the face-hat pairing and to indicate the color of the dot by pressing keys.
The association study phase was immediately followed by the face-hat association memory test (Figure 4C). The procedures of this memory test were identical to Studies 1 and 2, except that hats were shown beside faces.
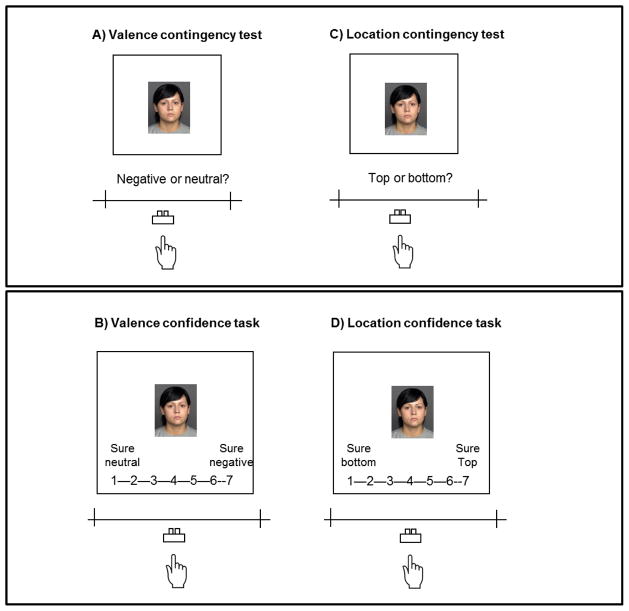
Finally, participants completed awareness tests and confidence rating tasks for face-valence contingencies and face-location contingencies. The valence contingency awareness test (Figure 5A) was the same as those in Studies 1 and 2. That is, for each old face, participants indicated whether it had been paired with negative or neutral pictures in the initial cue-learning phase. After the valence awareness test, participants viewed each of the old faces again and indicated how strongly they thought that the face was paired with negative or neutral pictures by using a 7-point scale (Figure 5B; 1: I’m sure the face was paired with neutral pictures, 2: I think it was paired with neutral pictures; 3: I guess it was paired with neutral pictures, 4: I have no idea; 5: I guess it was paired with negative pictures; 6: I think it was paired with negative pictures, 7: I’m sure it was paired with negative pictures).
Figure 5.
The valence/location awareness memory test and confidence rating task in Study 3.
In the location contingency awareness test, participants were shown old faces and told to indicate whether the face had predicted pictures on the top or bottom of the screen during the cue-learning phase (Figure 5C). This location awareness test was followed by a location confidence rating task (Figure 5D). In this confidence rating task, participants saw the faces and indicated their confidence about face-location associations by a 7-point scale (1: I’m sure the face was followed by pictures on the bottom of the screen, 2: I think it was followed by pictures on the bottom; 3: I guess it was followed by pictures on the bottom, 4: I have no idea; 5: I guess it was followed by pictures on the top; 6: I think it was followed by pictures on the top, 7: I’m sure it was followed by pictures on the top).
All participants completed an awareness test and a confidence rating task for the contingencies they were explicitly told to learn during the cue-learning phase, followed by another awareness test and a confidence rating task for the contingencies they were not explicitly told to learn. Thus, participants in the emotion learning condition completed the cue-valence contingency awareness test and the cue-valence confidence rating task first, followed by the awareness test and the confidence task for face-location associations. In contrast, participants in the location learning condition performed the awareness test and the confidence rating task for cue-location contingencies first.
Results and Discussion
First, we examined the effects of the prediction task manipulations on the accuracy in the face-location/face-valence contingency awareness memory tests to confirm that our manipulation worked as expected. Next, we examined the effects of face-valence contingency awareness on face-hat association learning performance. To address the effects of face-valence contingency awareness further, we then examined whether intra-individual variations of face-valence confidence ratings predicts those of face-hat association learning performance. Lastly, we describe results from the cue-learning phase and the association study phase.
Location and valence contingency awareness memory tests
One participant in the location learning condition showed a face-location awareness memory score far below chance (M = .35) with a low location confidence rating score (M = 3.9) which was not different from the score 4 corresponding to the option “I have no idea.” This participant also showed prediction performance in the initial cue-learning phase below chance even in the last block for negative harbinger faces (M = .38). These results indicate that this participant did not acquire face-location associations. Therefore, data from this participant were excluded from data analyses reported below.
The remaining participants showed the expected patterns; participants in the location learning condition were significantly more aware of face-location contingencies (Mneg = .76, Mneut= .81) than those in the emotion learning condition (Mneg = .51, Mneut= .46), F (1, 57) = 44.77, ηp2 = .44, p < .01. The location awareness score was not significantly different from chance in the emotion learning condition (ps > .15).
Next, we examined the awareness of face-valence contingencies. Overall, participants showed better valence awareness memory scores for negative than for neutral harbinger cues across conditions, F (1, 57) = 19.42, ηp2 = .25, p < .01. In addition, consistent with our prediction, participants showed better face-valence contingency awareness in the emotion learning condition (Mneg = .81, Mneut= .74) than in the location learning condition (Mneg = .62, Mneut= .46), F (1, 56) = 38.30, ηp2 = .40, p < .001.
However, even in the location learning condition, the valence contingency awareness score was significantly better than chance for emotional harbinger faces, t (29) = 4.38, p < .01. To address the effects of face-valence contingency awareness, therefore, participants in the location learning condition were split by the median valence awareness score (Md = .55) into two sub-groups: participants who were aware of face-valence contingencies (uninstructed-aware: M = .64) and those who were at chance in the face-valence awareness test (unaware: M = .46). In contrast, the valence awareness score was overall high in the emotion learning condition. Indeed, the median awareness score for emotional harbingers was .90 in this condition, suggesting that those who showed relatively lower awareness score were still highly aware of the face-valence contingencies in this condition. Since our primary focus was on the face-valence contingency awareness, we did not apply the median split for participants in the emotion learning condition. Subsequent analyses were performed based on these three groups (see Tabbert et al., 2011 for similar procedures).
A 2 (valence) × 3 (group) ANOVA on the face-valence contingency awareness score revealed a main effect of valence, F (1, 56) = 22.20, ηp2 = .28, p < .01, as in the previous ANOVA. In addition, the main effect of group was significant, F (2, 56) = 29.74, ηp2 = .52, p < .01, reflecting that the emotion learning condition showed better awareness than the uninstructed aware group, t (56) = 3.10, SE = 0.04, p < .01 (Tukey), which was better than unaware group, t (56) = 3.62, SE = 0.04, p < .01 (Tukey). The valence-by-group interaction was not significant (p > .20).
Effects of valence contingency awareness on cue-hat associative memory
The memory performance for new neutral faces did not significantly differ across groups (Memotion = .65, Muninstructed-aware = .58, Munaware = .62; p > .55), suggesting that the prediction task manipulation and the awareness of face-valence contingencies did not influence the overall memory performance in the associative learning phase. However, emotional and neutral harbinger faces showed different patterns depending on groups (Figure 6). In fact, a 3 (group: emotion, uninstructed-aware, vs. unaware) X 2 (cue type: old-negative vs. old-neutral) ANOVA on the correct response rate in the association memory test revealed a significant group-by-type interaction, F (2, 56) = 7.34, ηp2 = .21, p < .01. In the emotion learning condition, we replicated our results from Studies 1 and 2. That is, participants showed better face-hat memories for emotional harbinger cues than for neutral harbinger cues, F (1, 56) = 7.88, p < .01. A similar pattern was also observed for the uninstructed-aware group: better face-hat memory for emotional than for neutral harbinger faces, F (1, 56) = 4.56, p < .05. In contrast, participants who were not aware of the face-valence contingencies showed the opposite pattern; face-hat memory was worse for emotional than for neutral harbinger cues, F (1, 56) = 5.45, p < .05, the pattern seen previously in Mather and Knight’s (2008) studies. These results support our prediction that one’s awareness of cue-valence contingencies determines when emotional harbinger cues produce better and when they produce worse association learning than neutral harbinger cues.
Figure 6.
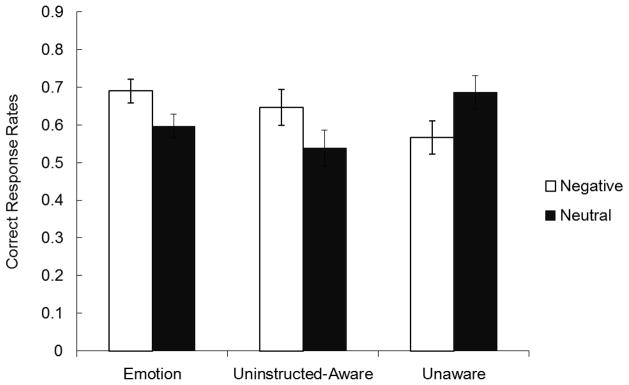
Correct response rates in the cue-hat association memory test in Study 3. Memory for cue-hat associations was modulated by awareness of contingencies between cue and emotional outcomes. Cues predictive of emotional outcomes produced better contextual memory than cues predictive of neutral outcomes when people were aware of associations between cues and emotional outcomes. In contrast, when people were not aware of the contingencies, cues that predicted negative outcomes produced worse contextual memory than cues that predicted neutral outcomes. Error bars represent standard error.
Location/valence confidence rating
Next, we examined the results from the face-location confidence task. The location confidence scores were re-coded so that a higher score meant stronger confidence for the correct location. Participants in the location learning condition (Mneg = 5.59 vs. Mneut= 5.62) showed higher confidence for the correct location than those in the emotion learning condition (Mneg = 3.95 vs. Mneut= 3.98), F (1, 57) = 71.86, ηp2 = .53, p < .01, with no significant effects of valence (ps > .60). The location confidence scores were not significantly different from the score 4 (“I have no idea”) in the emotion learning condition (ps > .30). The uninstructed-aware (Mneg = 5.96; Mneut = 5.76) and unaware subgroups (Mneg = 5.30; Mneut = 5.51) did not show any significant differences in the location confidence score (ps > .05). Thus, the results from the confidence rating task were consistent with those from the dichotomous awareness test and indicate that participants in the location learning condition learned the face-location association with higher confidence than those in the emotion learning condition.
A valence confidence rating score also provided results consistent with the valence awareness memory test. A 3 (group) × 2 (valence) ANOVA on the valence confidence score revealed a significant effect of valence, F (1, 56) = 21.75, ηp2 = .29, p < .01, reflecting higher scores for emotional than neutral harbingers; this is as expected given the scale we used (higher scores corresponded with more confidence towards negative, whereas lower scores corresponded with more confidence towards neutral). In addition, there was a significant condition-by-valence interaction, F (1, 56) = 25.71, ηp2 = .49, p < .01. Participants in the emotion learning condition differentiated emotional harbingers (M = 5.57) from neutral harbingers (M = 2.69), F (1, 29) = 58.74, p < .01. Participants in the uninstructed-aware group also showed a difference score for emotional harbingers (M = 4.47) than for neutral harbingers (M = 4.09), F (1, 12) = 7.10, p < .05. In contrast, the unaware group did not significantly differentiate emotional and neutral harbingers (p > .10; Mneg = 4.08; Mneut = 4.33).
Effects of confidence of face-valence contingency on cue-hat associative memory
Since the valence confidence rating was based on a continuous scale and given for each item for each individual, we examined the intra-individual effects of the confidence of face-valence contingencies. This approach allowed us to test the effects of the cue-valence awareness not only in the location learning condition, but also in the emotion learning condition which showed performance at ceiling in the dichotomous awareness memory test.
A hierarchical generalized linear model (Raudenbush & Bryk, 2002) was employed to examine whether intra-individual differences in the face-valence confidence rating predicted intra-individual differences in the cue-hat associative memory. Each trial was treated as a level-1 variable and each participant was treated as a level-2 variable. The dependent variable was a dichotomous variable indicating the cue-hat association test performance on each trial for each participant (1: correct, 0: incorrect). Level 1 predictors included valence (−1: neutral, 1: negative), a face-valence confidence rating, and a confidence-by-valence interaction. Level 2 predictors included condition (−1: emotion, 1: location). The model also included cross-level interactions, including a valence-by-condition interaction, a confidence-by-condition interaction, and a three-way interaction across confidence, condition and valence.
The results revealed a significant interaction between condition and valence, F (1, 1121) = 3.86, p < .05, reflecting better association learning for emotional harbingers than for neutral harbingers in the emotion learning condition than in the location learning condition (consistent with the results from the ANOVA described above). In addition, there was a significant interaction between confidence and valence, F (1, 1121) = 5.62, p < .05. This interaction reflects that higher confidence about face-valence contingencies predicted better association learning especially for emotional harbingers than for neutral harbingers (beta = 0.09, SE = 0.039). There were no other significant effects (p > .10). These results indicate that stronger confidence about face-valence contingencies predicts better association learning for emotional harbingers relative to neutral harbingers, irrespective of conditions. Thus, the results from this intra-individual difference approach also confirmed that the contingency awareness between cues and valence plays an important role in subsequent association learning. A similar analysis was performed with the location confidence score instead of the valence confidence score, but this analysis did not show any significant effects involving the location confidence (ps > .10).
Performance in the cue-learning phase
Next, we examined participants’ prediction performance in the cue-learning phase. The 16 repetitions during the cue-learning phase were categorized into 4 blocks. Prediction performance was significantly better than chance in the last three blocks both in the location learning condition, ts (28) = 4.91, 4.73, 6.70, ps < .01, and the emotion learning condition, ts (29) = 4.40, 6.89, 7.30, ps < .01. In addition, in both conditions, participants’ predictions improved across the 4 blocks (emotion: Mblock1 = .53, Mblock2 = .59, Mblock3 = .67, Mblock4 = .74; location: Mblock1 = .51, Mblock2 = .61, Mblock3 = .66, Mblock4 = .75), respective F (3, 87) = 35.47, ηp2 = .55, p < .01, F (3, 84) = 39.68, ηp2 = .59, p < .01. Prediction performance did not significantly differ between the two learning conditions (p > .90). In addition, neither the emotion (Mneg = .75; Mneut = .73) nor the location learning condition (Mneg = .74; Mneut = .77) showed significant valence effects during the last block (ps > .20). These results suggest that participants acquired similarly strong initial associations irrespective of valence and the learning conditions. There were no significant differences between the uninstructed-aware and unaware sub-groups in the location condition (p > .40).
Relation between prediction performance in the cue-learning phase and confidence rating
Since participants completed the confidence rating tasks and the awareness memory tests at the end of the session, it is possible that performance in these tasks did not reflect their contingency awareness during the initial cue-learning phase. To address this concern, for each individual for each valence condition, we obtained an across-face correlation coefficient between the confidence rating and the prediction performance in the 4th block during the cue-learning phase. Due to the near ceiling performance in the cue-learning phase in the last block, there were several participants who showed 100% accuracy in the prediction performance. Each condition also involved participants who did not have any variations in the confidence rating scores (e.g., showing the highest or lowest confidence for all items). These participants could not provide correlation measures and thus they were not included in this analysis.
Participants’ prediction performance was positively correlated with the face-location confidence ratings in the location learning condition (mean correlation for negative = .39; neutral = .27); both were significantly greater than zero, t (24) = 5.84, t (23) = 3.51, ps < .01. In contrast, the correlations between these two measures did not significantly differ from zero in the emotion learning condition (mean correlation for negative = −.05; neutral = −.02; ps > .50).
Next, we examined correlations between the prediction performance and the confidence ratings for face-valence associations. In the emotion learning condition, the better prediction performance was associated with a higher confidence score for emotional harbingers (mean correlation = .44) as well as a lower confidence score for neutral harbingers (mean correlation = −.45); both were significantly different from zero, t (27) = 8.07, t (25) = 6.60, ps < .01. In contrast, the correlations were not significantly different from zero in the location learning condition (mean correlation for negative = .007; neutral = .04; ps > .60). These results suggest that participants’ later confidence ratings for face-valence contingencies reflect their initial awareness.
Performance in the association study phase
The accuracy of the dot-color judgment did not differ by the type of cues, nor the groups (ps > .40; emotion: Mneg= .94, Mneut = .95, Mnew = .95; uninstructed- aware: Mneg= .99, Mneut = .98, Mnew = .96; unaware: Mneg= .95, Mneut = .98, Mnew = .95). Similarly, there were no significant effects of cue type and group in the reaction times to the dot (ps > .50; emotion: Mneg= 886 ms, Mneut = 856 ms, Mnew = 890 ms; uninstructed-aware: Mneg= 920 ms, Mneut = 922 ms, Mnew = 871 ms; unaware: Mneg= 882 ms, Mneut = 819 ms, Mnew = 850 ms).
General Discussion
Previous research indicated that it is harder to learn new associations for cues previously predictive of emotional outcomes (emotional harbingers) than for cues previously predictive of neutral outcomes (neutral harbingers; Mather & Knight, 2008). In the current study, we examined whether people’s awareness of cue-valence contingencies alters this impaired association learning for emotional harbinger cues.
In Studies 1 and 2, we explicitly asked and encouraged participants to learn cue-valence contingencies during the initial cue learning phase to increase their awareness of contingencies between cues and emotional outcomes. As expected, participants showed near ceiling performance in the final cue-valence contingency awareness test. In addition, with this intensive initial learning, we found the opposite pattern from that shown in previous research: emotional harbingers produced better association learning than neutral harbingers. The strength of specific associations between cues and outcomes did not modulate the results, which suggests that general memories about cue-valence associations plays a more crucial role than specific memories for cue-outcome pairs for the enhanced association learning for emotional harbingers.
Study 3 supported the idea that one’s awareness for cue-valence associations serves as a boundary condition predicting the switch between memory facilitation vs. memory impairment for emotional harbingers. In Study 3, we found that emotional harbingers produced worse association learning than neutral harbingers when participants were not aware of the cue-valence contingency, but emotional harbingers produced better association learning when participants were aware of the contingency. These results suggest that the awareness of the cue-valence contingency determines when it is easier and when it is harder to learn new associations for emotional harbinger cues than for neutral harbinger cues. Ample research on fear and evaluative conditioning has shown that contingency awareness increases emotional reactions to cues predictive of emotional outcomes (Carter et al., 2003; Dawson, 1973; Dawson et al., 2007; Hofmann et al., 2010; Klucken et al., 2009; Pleyers et al., 2007; Pleyers et al., 2009; Tabbert et al., 2011). The current results extend these past findings and indicate that subsequent cognitive processing of harbinger cues is also influenced by the contingency awareness.
Next, we turn to the question of the underlying mechanisms by which one’s awareness of cue-valence contingency influences subsequent association learning for emotional harbingers. One possibility is that contingency awareness modulates attentional priority of emotional harbinger cues relative to outcomes, which influences memory strength of the cues and subsequent association learning (cf. Madan, Caplan, Lau, & Fujiwara, 2012). When people are aware of the cue-valence contingencies, emotional harbinger cues should have high attentional priority because they predict something emotionally important (Blask et al., 2012; Carlsson et al., 2006; Koster et al., 2005; Kruschke, 2003; Le Pelley, 2004; Mackintosh, 1975). In fact, previous research shows that contingency awareness diminishes emotional reactions to outcomes while increasing reactions to cues (Donegan, 1981; Dunsmoor, Bandettini, & Knight, 2008; Marcos & Redondo, 1999), suggesting that people pay attention to emotional harbingers more than emotional outcomes when they are aware of the contingency. This increased attention to emotional harbingers might lead to stronger memory representations for emotional than neutral harbingers. Consistent with this idea, previous research indicates that emotional arousal induced by an item in a sequence facilitates memory for neutral stimuli just before the emotional item when people pay attention to the neutral stimuli (Anderson, Wais, & Gabrieli, 2006; M. Knight & Mather, 2009; Nielson & Arentsen, 2012; Nielson & Powless, 2007). Taken together, it appears that awareness of the cue-valence contingencies enhances attentional priority of emotional harbinger cues, and creates stronger memory representations for emotional harbingers than for neutral harbingers; the stronger memory representations should lead to subsequent better association learning for emotional harbingers.
In contrast, when people are not aware of cue-valence contingencies, they might not pay attention much to cues. Instead, the lack of the contingency awareness should increase attention to emotional outcomes. Indeed, previous research shows the strongest emotional reactions to emotional outcomes when people cannot predict those outcomes (Carlsson et al., 2006; Dunsmoor et al., 2008; Grupe & Nitschke, 2011; D. C. Knight, Waters, King, & Bandettini, 2010; Oka et al., 2010; Sarinopoulos et al., 2010). Thus, emotional outcomes should gain more attentional priority than harbinger cues under this situation. Furthermore, previous findings indicate that when people do not focus on neutral items before emotional items, memory for the neutral items is impaired by the subsequent emotional items (Hurlemann et al., 2005; Hurlemann et al., 2007; M. Knight & Mather, 2009; Strange, Hurlemann, & Dolan, 2003; Strange, Kroes, Fan, & Dolan, 2010). Thus, the lack of attentional focus on the harbinger cues should produce impaired memory for emotional harbingers relative to neutral harbingers. Taken together, these results suggest that when people are not aware of the cue-valence contingencies, emotional harbingers do not have attentional priority and have impaired memory representations relative to neutral harbingers. This impaired memory should result in worse performance in subsequent learning for emotional harbingers compared with neutral harbingers.
In summary, awareness about the cue-valence contingencies should influence the attentional priority of the harbinger cues, which is critical for determining whether emotion enhances or impairs memory strength for that cue (Mather & Sutherland, 2011). The enhanced vs. impaired memory strength of emotional harbingers might have resulted in whether emotional harbingers produce better or worse association learning in a subsequent session. Further research with independent manipulations of the contingency awareness and cue memory strength is needed to test this possibility. In addition, in the current study, we did not obtain attention measures during the cue-learning phase as well as the association learning phase. Future research which measures or/and manipulates participants’ attentional priority is also needed to address the potential mechanisms described above.
Another question for future research concerns the way we measured participants’ awareness of cue-valence contingencies. In the current study, we measured participants’ awareness of cue-valence contingencies at the end of the session. Thus, it is possible that our awareness measures do not reflect participants’ initial awareness of cue-valence contingencies. However, in Study 3, we found that the confidence rating for cue-valence associations was correlated with the prediction accuracy in the initial phase in the emotion learning condition. These results suggest that the confidence rating reported at the end of the session reflects participants’ initial confidence awareness. But we did not obtain an initial awareness measure for face-valence associations in the location learning condition. Future work with online measures about the contingency awareness during the initial learning phase should help to confirm the role of the contingency awareness.
Another question concerns the effects of positive emotion. In the current study, we examined association learning for harbinger cues that predicted negative emotional outcomes. Previous research indicates similar impaired association learning for emotional harbingers irrespective of whether outcomes are positive or negative (Mather & Knight, 2008). This suggests that arousal plays a more crucial role in subsequent association learning for harbinger cues than valence (i.e., positive or negative). However, positive and negative emotions sometimes impact cognitive processing differently (Sakaki, Gorlick, & Mather, 2011; Sakaki & Niki, 2011; Schmitz, De Rosa, & Anderson, 2009). Future work is needed to test the effects of contingency awareness for cues that predict emotionally positive outcomes.
In conclusion, the current study revealed that the awareness of the contingency between harbinger cues and emotional outcomes modulates subsequent association learning for the cues. Across three studies, we found that people were better able to learn new associations to cues that previously predicted emotional outcomes when they were aware of the cue-outcome contingencies. In contrast, the opposite pattern was observed when people were not aware of the contingencies. As seen in other experimental contexts (see Mather & Sutherland, 2011 for a review), whether memory for inherently neutral information is enhanced or impaired by its interaction with something emotionally arousing depends on the nature of the attentional focus on that neutral information.
Acknowledgments
We thank Ching-Yu Wang, Chris Peterka, Nicole Samii, Gladys Leung, Rohit Jayakar, and Wing Kwan for their assistance with data and stimuli collection. This work was supported by grants from the National Institute on Aging (R01AG025340 and K02AG032309).
References
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134(2):258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Anan Y, De Houwer J, Nosek BA. Evaluative conditioning and conscious knowledge of contingencies: A correlational investigation with large samples. The Quarterly Journal of Experimental Psychology. 2010;63(12):2313–2335. doi: 10.1080/17470211003802442. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Mogg K, Bradley BP. Emotional conditioning to masked stimuli and modulation of visuospatial attention. Emotion. 2005;5:67–79. doi: 10.1037/1528-3542.5.1.67. [DOI] [PubMed] [Google Scholar]
- Beesley T, Le Pelley ME. The effect of predictive history on the learning of sub-sequence contingencies. The Quarterly Journal of Experimental Psychology. 2010;63(1):108–135. doi: 10.1080/17470210902831767. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, et al. Attentional modulation of emotional stimulus processing: An fMRI study using emotional expectancy. Human Brain Mapping. 2006;27(8):662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask K, Walther E, Halbeisen G, Weil R. At the crossroads: Attention, contingency awareness, and evaluative conditioning. Learning and Motivation. 2012;43(3):99–106. doi: 10.1016/j.lmot.2012.03.004. [DOI] [Google Scholar]
- Bradley MM, Lang PJ. Tech Rep No B-2. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Öhman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32(4):1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Carter RM, Hofstötter C, Tsuchiya N, Koch C. Working memory and fear conditioning. Proceedings of the National Academy of Sciences. 2003;100(3):1399–1404. doi: 10.1073/pnas.0334049100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4:173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- Dawson ME. Can classical conditioning occur without contingency learning? A review and evaluation of the evidence. Psychophysiology. 1973;10(1):82–86. doi: 10.1111/j.1469-8986.1973.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Rissling AJ, Schell AM, Wilcox R. Under what conditions can human affective conditioning occur without contingency awareness? Test of the evaluative conditioning paradigm. Emotion. 2007;7(4):755–766. doi: 10.1037/1528-3542.7.4.755. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Tibboel H. Stop what you are not doing! Emotional pictures interfere with the task not to respond. Psychonomic Bulletin & Review. 2010;17(5):699–703. doi: 10.3758/pbr.17.5.699. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proceedings of the National Academy of Sciences. 2010;107(13):6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298(5596):1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Denkova E. Neural correlates of encoding emotional memories: a review of functional neuroimaging evidence. Cell Science Reviews. 2008;5:78–122. [Google Scholar]
- Donegan NH. Priming-produced facilitation or diminution of responding to a Pavlovian unconditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7(4):295–312. doi: 10.1037/0097-7403.7.4.295. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 2011;11(2):413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5(9):394–400. doi: 10.1016/S1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hofmann W, De Houwer J, Perugini M, Baeyens F, Crombez G. Evaluative conditioning in humans: A meta-analysis. Psychological Bulletin. 2010;136(3):390–421. doi: 10.1037/a0018916. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Wagner M, Hawellek B, Reich H, Pieperhoff P, Amunts K, et al. Amygdala control of emotion-induced forgetting and remembering: Evidence from Urbach-Wiethe disease. Neuropsychologia. 2007;45(5):877–884. doi: 10.1016/j.neuropsychologia.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Kattner F. Revisiting the relation between contingency awareness and attention: Evaluative conditioning relies on a contingency focus. Cognition & Emotion. 2011;26(1):166–175. doi: 10.1080/02699931.2011.565036. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP. Observational fear conditioning in the acquisition and extinction of attentional bias for threat: An experimental evaluation. Emotion. 2007;7(2):324–335. doi: 10.1037/1528-3542.7.2.324. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31(8):1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routs to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Science. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005. [DOI] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: Neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45(13):2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Klucken T, Kagerer S, Schweckendiek J, Tabbert K, Vaitl D, Stark R. Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture–picture conditioning paradigm. Neuroscience. 2009;158(2):721–731. doi: 10.1016/j.neuroscience.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. NeuroImage. 2010;49(1):843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Mather M. Reconciling findings of emotion-induced memory enhancement and impairment of preceding items. Emotion. 2009;9(6):763–781. doi: 10.1037/a0017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7(4):705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, De Houwer J. Signals for threat modulate attentional capture and holding: Fear-conditioning and extinction during the exogenous cueing task. Cognition & Emotion. 2005;19(5):771– 780. [Google Scholar]
- Kruschke JK. Attention in learning. Current Directions in Psychological Science. 2003;12(5):171–175. doi: 10.2307/20182870. [DOI] [Google Scholar]
- Kruschke JK, Blair NJ. Blocking and backward blocking involve learned inattention. Psychonomic Bulletin & Review. 2000;7(4):636–645. doi: 10.3758/bf03213001. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Review Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. The Quarterly Journal of Experimental Psychology Section B. 2004;57(3):193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME, McLaren IPL. Learned associability and associative change in human causal learning. The Quarterly Journal of Experimental Psychology Section B. 2003;56(1):68–79. doi: 10.1080/02724990244000179. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences. 2006;103(38):14200. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82(4):276–298. doi: 10.1037/h0076778. [DOI] [Google Scholar]
- Madan CR, Caplan JB, Lau CSM, Fujiwara E. Emotional arousal does not enhance association-memory. Journal of Memory and Language. 2012;66(4):695–716. doi: 10.1016/j.jml.2012.04.001. [DOI] [Google Scholar]
- Maddox GB, Naveh-Benjamin M, Old S, Kilb A. The role of attention in the associative binding of emotionally arousing words. Psychonomic Bulletin & Review. 2012;19(6):1128–1134. doi: 10.3758/s13423-012-0315-x. [DOI] [PubMed] [Google Scholar]
- Marcos JL, Redondo J. Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological Psychology. 1999;50(2):89–102. doi: 10.1016/S0301-0511(99)00007-1. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24(1):897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2(1):33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. The emotional harbinger effect: Poor context memory for cues that previously predicted something arousing. Emotion. 2008;8(6):850–860. doi: 10.1037/a0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61(1):54–57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58(2):449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland M. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review. 2005;12(4):654–661. doi: 10.3758/BF03196754. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Mather M. The effect of emotional arousal on memory binding in normal aging and Alzheimer’s disease. The American journal of psychology. 2011;124(3):301–312. doi: 10.5406/amerjpsyc.124.3.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Arentsen TJ. Memory modulation in the classroom: Selective enhancement of college examination performance by arousal induced after lecture. Neurobiology of Learning and Memory. 2012;98(1):12–16. doi: 10.1016/j.nlm.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Powless M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 min after learning. Neurobiology of Learning and Memory. 2007;88(1):40–47. doi: 10.1016/j.nlm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Notebaert L, Crombez G, Van Damme S, De Houwer J, Theeuwes J. Signals of threat do not capture, but prioritize, attention: A conditioning approach. Emotion. 2011;11(1):81–89. doi: 10.1037/a0021286. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80(3):381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Oka S, Chapman CR, Kim B, Shimizu O, Noma N, Takeichi O, et al. Predictability of painful stimulation modulates subjective and physiological responses. The Journal of Pain. 2010;11(3):239–246. doi: 10.1016/j.jpain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby C, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pierce BH, Kensinger EA. Effects of emotion on associative recognition: Valence and retention interval matter. Emotion. 2011;11(1):139–144. doi: 10.1037/a0021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleyers G, Corneille O, Luminet O, Yzerbyt V. Aware and (Dis)Liking: Item-Based Analyses Reveal That Valence Acquisition via Evaluative Conditioning Emerges Only When There Is Contingency Awareness. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33(1):130–144. doi: 10.1037/0278-7393.33.1.130. [DOI] [PubMed] [Google Scholar]
- Pleyers G, Corneille O, Yzerbyt V, Luminet O. Evaluative conditioning may incur attentional costs. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35(2):279–285. doi: 10.1037/a0013429. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology. 2012;92:492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Prados J, Redhead ES, Pearce JM. Active preexposure enhances attention to the landmarks surrounding a Morris swimming pool. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25(4):451–460. [Google Scholar]
- Raes AK, De Raedt R, Fias W, Koster EHW, Van Damme S. Does contingency awareness mediate the influence of emotional learning on the cueing of visual attention? Psychological Research. 2009;73(1):107–113. doi: 10.1007/s00426-008-0141-y. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models (Second edition) Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Reid LS. The development of noncontinuity behavior through continuity learning. Journal of Experimental Psychology. 1953;46(2):107–112. doi: 10.1037/h0062488. [DOI] [PubMed] [Google Scholar]
- Rosenholtz R, Li Y, Nakano L. Measuring visual clutter. Journal of Vision. 2007;7(2) doi: 10.1167/7.2.17. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Gorlick AM, Mather M. Differential interference effects of negative emotional states on subsequent semantic and perceptual processing. 2011 doi: 10.1037/a0026329. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Niki K. Effects of the brief viewing of emotional stimuli on understanding of insight solutions. Cognitive, Affective, & Behavioral Neuroscience. 2011;11:1–15. doi: 10.3758/s13415-011-0051-0. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Niki K, Mather M. Updating existing emotional memories involves teh frontopolar/orbitofrontal cortex in ways that acquiring enw emotional memories does not. Journal of Cognitive Neuroscience. 2011;23:3498–3514. doi: 10.1162/jocn_a_00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Niki K, Mather M. Beyond arousal and valence: The importance of the biological versus social relevance of emotional stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(1):115–139. doi: 10.3758/s13415-011-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2010;20(4):929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack U. Attentional interference effects of emotional pictures: Threat, negativity, or arousal? Emotion. 2005;5(1):55–66. doi: 10.1037/1528-3542.5.1.55. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, De Rosa E, Anderson AK. Opposing influences of affective state valence on visual cortical encoding. Journal of Neuroscience. 2009;29(22):7199–7207. doi: 10.1523/jneurosci.5387-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, Zald DH. An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion. 2006;6(3):523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Kroes MC, Fan J, Dolan RJ. Emotion causes targeted forgetting of established memories. Frontiers in Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, et al. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Social Cognitive and Affective Neuroscience. 2011;6(4):495–506. doi: 10.1093/scan/nsq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobalon JB, Miguelez D, McLaren IPL, Mackintosh NJ. Intradimensional and extradimensional shifts in spatial learning. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29(2):143–152. doi: 10.1037/0097-7403.23.1.110900886510.1037/0097-7403.23.1.1101997-02158-009. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Hermans D, Koster EHW, Eccleston C. The role of extinction and reinstatement in attentional bias to threat: A conditioning approach. Behaviour Research and Therapy. 2006;44(11):1555–1563. doi: 10.1016/j.brat.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Notebaert L. Attentional bias to threat: A perceptual accuracy approach. Emotion. 2008;8(6):820–827. doi: 10.1037/a0014149. [DOI] [PubMed] [Google Scholar]
- Verona E, Patrick CJ, Curtin JJ, Bradley MM, Lang PJ. Psychopathy and physiological response to emotionally evocative sounds. Journal of abnormal psychology. 2004;113(1):99–108. doi: 10.1037/0021-843X.113.1.99. [DOI] [PubMed] [Google Scholar]
- Walther E, Nagengast B. Evaluative conditioning and the awareness issue: Assessing contingency awareness with the Four-Picture Recognition Test. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32(4):454–459. doi: 10.1037/0097-7403.32.4.454. [DOI] [PubMed] [Google Scholar]