Summary
Bariatric surgery is the most effective method for promoting dramatic and durable weight loss in morbidly obese subjects. Furthermore, type 2 diabetes is resolved in over 80% of patients. The mechanisms behind the amelioration in metabolic abnormalities are largely unknown but may be due to changes in energy metabolism, gut peptides and food preference. The goal of this meeting was to review the latest research to better understand the mechanisms behind the ‘magic’ of bariatric surgery. Replication of these effects in a non-surgical manner remains one of the ultimate challenges for the treatment of obesity and diabetes. Promising data on energy metabolism, gastrointestinal physiology, hedonic response and food intake were reviewed and discussed.
Keywords: Bariatric surgery, gut hormones, weight loss
The Pennington Scientific Symposium on ‘Bariatric surgery: do the mechanisms hold the key for novel therapies?’ was held in Baton Rouge, Louisiana on 6–7 December, 2010. The aim of the symposium was to gather leaders in the field of bariatric surgery research to discuss the latest findings and identify emerging areas of research interest and clinical need with the overall aim of a greater understanding of the mechanisms behind the spectacular metabolic improvements and weight loss after bariatric surgery (Fig. 1).
Figure 1.
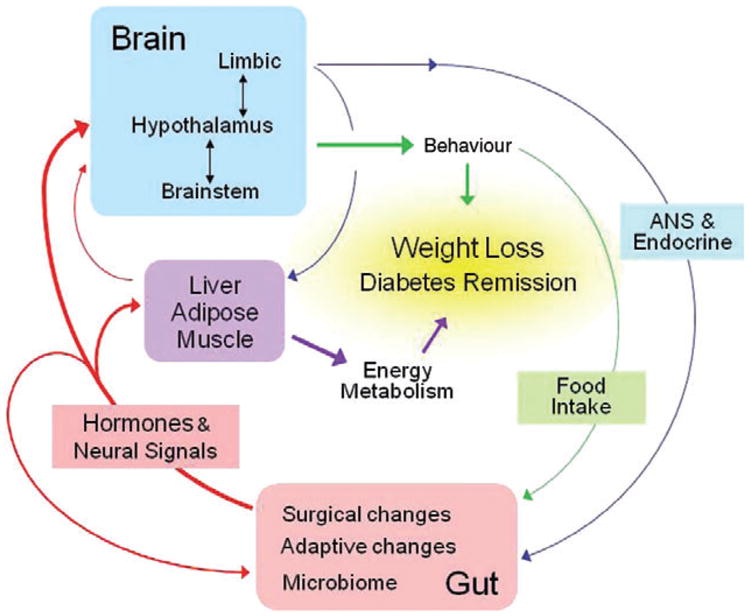
Schematic diagram showing flow of information potentially involved in the physiological and behavioural consequences of gastric bypass surgery. Changes in hormonal and neural signals generated by the surgery and its adaptive consequences affecting other peripheral organs and the brain are shown in red (Berthoud, Holst, Kaplan, Poires, Strader, Seeley, Hajnal) Behavioural changes such as food intake and food preference resulting from altered signalling to the brain are shown by green lines (Berthoud, Bueter, Chakravarthy, Geleibter, Seeley). Changes in autonomic and endocrine functions that feed back to the gut and other peripheral organs are shown by blue lines (Raybould). The contribution of changed energy metabolism and energy expenditure is shown in purple (Kaplan, Ravussin). Note that the arrangement allows learning to take place, as ingestion of different foods produces different consequences in the altered gut that are in turn sensed by the brain.
The overall programme and list of speakers (Appendix 1) encompassed the physiological, mechanistic and hedonic aspects of bariatric surgery research. The first session focused on energy balance and gastrointestinal (GI) physiology. The second session focused on the role of gut hormones in the effects of bariatric surgery. The third session discussed the latest research in animal models of bariatric surgery and the fourth session focused on the role of hedonics in animal and human studies after bariatric surgery. The programme ended with an engaging round-table discussion of the questions that were raised by the meeting and identified current and novel research directions.
Session 1: Energy balance and gastrointestinal physiology
Eric Ravussin began the symposium with an overview on energy balance regulation, with an emphasis on metabolic adaptation with bariatric surgery. Energy balance is achieved when energy intake (calories consumed) is equal to energy intake (calories burned). This is not a static relationship because both the energy content of weight change and the fraction of weight loss as fat-free mass vs. fat mass is not constant and energy expenditure changes in response to weight loss. Therefore, accurately predicting the time course of body weight change in response to energy imbalance is a real challenge and common dieting advice based on an extrapolation of the cumulative negative energy balance to a ubiquitous 7.7 kcal g−1 of weight loss drastically overestimates expected weight loss. Ravussin argued that extrapolating an energy gap over time should be avoided and that dividing VO2 by body weight is erroneous as it introduces a mathematical artefact. Web-based body weight simulators may assist clinicians and researchers in prescribing individual weight loss plans with a new model proposed by Hall at the National Institute of Diabetes and Digestive and Kidney Diseases (http://bwsimulator.niddk.nih.gov/) as well as Thomas at Montclair State University (http://pages.csam.montclair.edu/∼thomasd/BodePlot.html).
Metabolic adaptation in response to weight loss is usually defined as a decrease in energy expenditure not explained by the loss of tissue mass. Compared with other weight loss interventions (1), the magnitude of metabolic adaptation appears to be blunted with bariatric surgery. In 20 obese women, measured resting energy expenditure was significantly lower than predicted 3 months after Roux-en-Y gastric bypass (RYGB) although this metabolic adaptation disappeared by months 6 and 12 (2). Similarly, Carrasco et al. reported that resting energy expenditure expressed per kilogram of fat-free mass significantly decreased from 33.4 to 30.1 kcal kg−1 and respiratory quotient from 0.86 to 0.82 6 months after RYGB in 31 subjects (3). Finally, van Gemert et al. reported that total daily energy expenditure and sleeping metabolic rate were significantly decreased 3 months after vertical banded gastroplasty, which persisted at 12 months and paralleled increases in lipid oxidation (4). In general, the data in humans are not as obvious as those in rodents. However, the findings suggest that gastric bypass causes a decrease in magnitude of the metabolic adaptation with a concomitant increase in lipid oxidation. Ravussin concludes by speculating that a blunted metabolic adaptation response and increased fat oxidation may contribute to long-term maintenance of weight loss observed in bariatric surgery patients, compared with individuals who undergo lifestyle interventions. This hypothesis needs to be further examined in larger cohorts of subjects undergoing different types of bariatric procedures.
Next, Helen Raybould presented an overview of GI physiology with an emphasis on the gut-brain axis. Raybould hypothesized that (i) the GI tract, the largest immune and endocrine system in the body, is the source of inflammation associated with high-fat diets (HFD) and obesity and (ii) changes in gut epithelial function lead to alterations in nutrient detection and plasticity of vagal afferent neuron pathways. During her talk, Raybould systematically discussed the lines of evidence supporting these hypotheses.
First, the link between gut microbiota and energy homeostasis is well established by studies showing that germ-free mice have less body fat and are resistant to diet-induced obesity (DIO). Obesity is also associated with altered gut microbiota and gut bacteria metabolize indigestible polysaccharides to generate short-chain fatty acids and monosaccharides thus promoting their absorption and storage as fat. Ding et al. recently showed that HFD in normal animals increases tumour necrosis factor-α mRNA levels in the gut and nuclear factor kappa BEGFP reporter genes activation in epithelial cells, immune cells and endothelial cells of the small intestine (5); these changes were not seen in germ-free mice. These proinflammatory changes in the small intestine precede weight gain and obesity and show strong and significant associations with progression of obesity and the development of obesity. At the same time, DIO results in a chronic low increase in circulating lipopolysaccharide, described as ‘metabolic endotoxemia’ (6), with antibiotic treatment reversing this metabolic inflammation (7). Raybould further shows that in HFD fed Sprague Dawley rats, there is a decrease in total cecal bacterial density and a bloom in Clostridiales order regardless of whether the rats become obese on the HFD or remain lean. However, HFD-induced obese rats have increased plasma levels of lipopolysaccharide and activation of toll-like receptor 4 in the gut wall (8). Toll-like receptor 4 has previously been shown to alter tight junction and increase intestinal permeability. These data suggest that HFD-induced changes in gut microflora alone do not induce changes in food intake and body weight. Instead, the response of the host to the change in gut microflora is crucial and may depend on induction of gut inflammation.
Next, Raybould examined the phenotypeof vagal afferent neurons in DIO compared with diet-restricted or low-fat fed controls. Vagal afferent neurons represent the major pathway by which information about ingested nutrients reaches the central nervous system and influences both GI function and feeding behaviour. Cholecystokinin (CCK) is the master regulator of the vagal afferent neuron pathway resulting in reflex changes in GI function and induction of satiation. In humans and rodents, exogenous CCK or per-fusion of the small intestine with nutrients terminates feeding via a CCK1R vagal afferent pathway. Rats deficient in CCK1R are hyperphagic and obese; CCK1R null mice eat significantly longer and larger meals compared with wild-type mice. Under fasting conditions in low-fat fed rats, mRNA and protein levels of orexigenic peptides (cannab-inoids and melanin concentrating hormone) and their receptors are increased. At the same time, there is a decrease in the expression of anorexigenic PYY3-36 receptor and the anor-exigenic peptide, cocaine- and amphetamine-related transcript. Feeding or exogenous CCK reverses this phenotype. Thus, CCK acts to decrease expression of peptides and receptors associated with stimulation of appetite. Raybould presented evidence that HFD induces phenotypic changes in vagal afferent nerve function, which may play a role in hyperphagia and obesity in response to an HFD.
Raybould also presented data on change in the glucose sensing role of the intestine in a model of type 2 diabetes (T2D). Her laboratory used the UC-Davis T2D rat model to examine glucose sensing in these animals, particularly the role of endocrine cells. They found decreased activation of endocrine cells in response to glucose in diabetic rats, particularly in cells expressing 5-HT, glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic peptide (GIP), compared with pre-diabetic controls. These data suggest that there are significant changes in the physiology of gut nutrient sensing mechanisms in T2D, which may result in altered enteric, brain and hormonal function normally seen in response to intestinal glucose. To close, Raybould summarized that DIO is associated with changes in gut microbiota, increased gut inflammation, intestinal permeability and plasma lipopolysaccharide and alterations in vagal afferent neuron phenotypes.
Session 2: Gut hormones and bariatric surgery
Jens Holst started the second session with an overview of gut hormones, and highlighted findings from meal studies to elucidate patterns of gut hormone secretion in patients before and at various time-points after bariatric surgery. In recent years, several studies have demonstrated that levels of incretins, gut hormones that stimulate insulin secretion, are perturbed following weight loss surgery and are dependent on the type of surgery performed (9). Korner et al. compared GLP-1 and GIP levels in women who had undergone adjustable gastric banding (BND) or RYGB and age-, body mass index (BMI)-matched controls through out a 3-h period after a liquid meal. GLP-1 levels at 30 min were over threefold higher in RYGB compared with BND and controls, and correlated with insulin levels. GIP levels at 30 min were lower in RYGB compared with BND and controls. This may be due to different paths of nutrient flow described as the ‘upper vs. lower intestinal hypothesis’. Glucagon levels were not different between the three groups (9).
The Hvidore Meal Study was conducted to examine the effects of three different test meals (25 or 50 g glucose or a mixed liquid meal) on insulin, C-peptide and gut hormones responses before and after gastric bypass in subjects with normal glucose tolerance. The unique aspect of this study is the early window of testing, 3–14 d after the operation. Post bypass, the mixed meal test induced a rapid increase, followed by a sharp decrease in glucose, insulin and C-peptide levels. There was a massive increase in GLP-1 and GLP-2, but no change in GIP. There was an early decrease in ghrelin, increased CKK and a dramatic increase in peptide YY. Interestingly, gastric bypass resulted in lower gastrin levels during the meal test. Similar patterns in gut hormones have also been observed in subjects with T2D during a 200 mL liquid meal test post RYGB. Homeostatic model of assessment of insulin resistance was significantly improved 3–4 d after RYGB, with early massive increases in GLP-1, which were further increased 3 months post RYGB. In a recently published study, Isbell et al. reported similar improvements in insulin sensitivity after RYGB but also in a matched control group subjected to the same degree of energy restriction (10).
The mechanism behind the massive increase in GLP-1 is not established although rapid gastric emptying, as seen in subjects with reactive hypoglycaemia after total gastrectomy (11). After RYGB there is virtually no retention of nutrients resulting in accelerated delivery of nutrients to L cells in the distal small intestine where GLP-1 is primarily produced, thus enhancing GLP-1 secretion (12). The case for altered nutrient delivery is further supported by a unique case where a standardized liquid meal administered via gastrostomy tube into the remnant stomach (vs. orally) completely reversed neuroglycopenic symptoms (13). Similarly, a test meal taken orally revealed normal glucose tolerance whereas the same meal delivered on a consecutive day by a gastrostomy tube resulted in overtly diabetic postprandial glucose levels (14).
GLP-1 has enormous therapeutic potential due to its wide-ranging physiological actions (15). GLP-1 is released in response to meal intake and acts to stimulate insulin secretion, inhibit glucagon secretion, inhibit GI motility and secretion and regulate appetite and food intake (16). Indeed, glucose tolerance is restored by i.v. GLP-1 infusion in T2D (17) and a meta-analysis of five studies reported that GLP-1 infusion reduced energy intake in a dose-dependent manner in 115 lean and overweight subjects (18), possibly because of interaction with nuclei in the brainstem, hypothalamus and amygdale. Early clinical studies with liraglutide or exanatide are promising with reductions in HbA1c and plasma glucose and improvements in cardiovascular risk factors. Because of their action on appetite regulation, both of these GLP-1 analogues are weight neutral with low doses and cause clinically meaningful weight loss at higher doses (19).
In the following talk, Walter Pories gave a very informative overview of the developments and changes in bariatric surgery over the past 30 years with an emphasis on the use of bariatric surgery to examine mechanisms of T2D remission. Similar to findings from the Swedish Obesity Study 15 years after bariatric surgery (20), data from East Carolina University (ECU) (n = 831) show that mean weight loss 16 years after RYGB is 106 pounds, i.e. 55% of excess body weight. Notably, even after RYGB, most patients are still overweight or obese and eat more than the average person. Mortality rates are tremendously improved, decreasing by 78% 9 years after RYGB (1% per year vs. 4.5% per year in patients that refused the operation due to personal or insurance reasons) in the ECU cohort. This is supported by mortality data from the Swedish Obesity Study (20) and a study in Canada showing 89% reduction in mortality rates 5 years after RYGB (21). However, the most significant outcome of bariatric surgery is the drastic improvement if not remission/of comorbidities of obesity (T2D, hypertension, obstructive sleep apnoea, non-alcoholic steatohepatitis, stress incontinence, asthma, etc.) (22). For example, at ECU, there was 83% remission of T2D and 99% remission of impaired glucose tolerance 9.4 years after RYGB. These improvements following bariatric surgery lead to massive reductions in medication usage and annual health costs in patients with T2D. Surgery resulted in the elimination of diabetes medication therapy in 75% of patients at 6 months, 81% at 1 year and 85% 2 years after surgery. As such, the costs of diabetes medication massively plummeted from $10 572 per year before surgery to $1878 per year, 2 years after surgery (23).
Session 3: Animal models of bariatric surgery
The 3rd session of this symposium highlighted the latest research using animal models of bariatric surgery. Lee Kaplan started this session by introducing the idea of a body's ‘set point’ or better ‘settling point’. After forced dietary manipulation, from overfeeding or food restriction, mice return to their natural body weight when returned to an ad libitum diet. As such, although long considered to act through restriction of food intake and malabsorption of ingested nutrients, it may be that RYGB exerts its therapeutic effects by altering the physiological regulation of energy balance and glucose homeostasis. Like human patients, animals with T2D exhibit dramatic improvements in fasting blood glucose and glucose tolerance after RYGB (24). RYGB induces weight loss by decreasing food intake and increasing resting energy expenditure (24). The alterations in food intake are associated with decreased appetitive drive and altered food preferences. Late after surgery, these animals exhibit a change in preference from a high-fat, high-sugar diet to a normal chow diet. The RYGB-associated increase in energy expenditure results from stimulation of diet-induced thermogenesis, demonstrated by increased 18F-Fluorodeoxyglucose uptake by PET scan and UCP1 staining and protein levels, suggesting that this operation works in part by enhancing the normal ther-mogenic response to ingested nutrients possibly via brown adipose tissue activation (BAT).
MC4R−/− mice have impaired diet-induced thermogenesis, elevated respiratory quotient and impaired leptininduced BAT activation. When MC4R mice are compared with C57BL6 mice 1 year after RYGB, they have less weight loss and little change in %fat. In addition, RYGB induces hypothalamic POMC expression in C57BL6 mice. In summary, RYGB stimulates resting energy expenditure in rat and mouse models by stimulating central pathways of thermogenesis, activation of BAT and increasing core temperature. Stimulating hypothalamic POMC activation and MC4R signalling is imperative for these processes.
RYGB is a complex operation that can be conveniently divided into five distinct components including (i) isolation of the gastric cardia; (ii) exclusion of the distal stomach; (iii) exclusion of the duodenum and proximal jejunum; (iv) enhanced exposure of the mid and distal jejunum to undigested nutrients and (v) partial vagotomy. Comparison of each of these procedures on body weight, food intake, resting energy expenditure, fasting glucose and glucose tolerance revealed that manipulation of the stomach can account for much of the reduction in food intake after RYGB. In contrast, exclusion of the duodenum and jejunum with increased exposure of the distal small bowel to undigested nutrients appears to account for the stimulation of resting energy expenditure with a concomitant independent improvement in insulin secretion after RYGB (25). Kaplan concludes that regulation of energy expenditure and glucose homeostasis, but not food intake, by the GI tract appears to share key mechanisms. Food intake appears to be largely mediated through gastric signalling.
In the next talk, Marco Bueter described findings from his model of RYGB in rats and the transferability of animal findings to human physiology. A unique aspect of Bueter's RYGB model is that the gastric pouch created is the smallest one reported in the literature (2–5% of the original size compared with >20% for Kaplan's group) (24,26,27). Similar to humans, RYGB in rats induces significant reductions in body weight and food intake (27) and increases PYY and GLP-1 levels, with and without preservation of the vagal nerve during the procedure (26). RYGB rats have higher energy expenditure and diet-induced thermogenesis compared with sham-operated and weight-matched groups (27). When examining GI anatomy, Bueter showed that the small bowel of RYGB rats was 72% heavier and demonstrated distinct histological features (increased muscle thickness, mucosal and villus height and crypt depth) compared with corresponding sections of the duodenum, jejunum and ileum of sham-operated, ad libitum fed rats (27).
To investigate the potential role of mechanical restriction through gastric volume reduction for the success of RYGB, Bueter et al. food-restricted a group of RYGB rats such that they received less than half of their normal food intake over 14 d with subsequent ad libitum access to solid, normal chow. RYGB rats increased their food intake as soon as ad libitum food was available. Instead of returning to the level of food intake seen in the ad libitum fed RYGB rats, the food-restricted RYGB rats ate significantly more and even exceeded sham-operated ad libitum fed rats indicating that mechanical restriction is unlikely to be a major factor of a reduced food intake after RYGB (Bueter, unpublished).
Taste preference is uniquely altered after RYGB in animals and humans. One year after RYGB, patients reported consuming less sweet foods (candy, dessert, cake, cookies) and more fruits and vegetables compared with patients who had vertical banded gastroplasty (28). Similarly, when Wistar rats were given the choice of low- or high-fat chow after RYGB, there was an 11% increase in preference for low-fat chow, although RYGB rats still ingested more calories from high-fat than from low-fat chow (Bueter, unpublished). To assess sucrose preference in the context of natural feeding and drinking, the two-bottle (distilled water vs. sucrose) preference test using seven ascending concentrations of sucrose (1–1000 nM) was performed. Sham-operated rats preferred higher concentrations of glucose and had greater intake of sucrose compared with RYGB rats (Bueter, unpublished). These findings are in accordance with recent publications (29,30) where rats showed reduced consummatory behaviour (number of licks) when exposed to sucrose in brief access tests.
Finally, Bueter addressed general differences between RYGB studies in animals and humans. For instance, although GLP-1 and PYY levels are raised postprandially in both humans and rats, fasting levels are not increased in humans (31,32). Mortality rates were significantly different in rat studies using different rat strains (10–50%) even with a similar protocol, operation and surgeon. Gut hypertrophy occurs in rat RYGB studies (27), but not in humans after RYGB. In his concluding remarks, Bueter emphasized the need for standardization and the reporting of methods used in RYGB rat models. Differences between models include pouch size, animal strain and sex and diets before and after surgery. As such, although rat models assist in understanding the physiology of RYGB, we need to exercise caution when extrapolating animal findings to humans.
In the next talk, Hans-Rudolf Berthoud discussed his laboratory's animal model of RYGB and its utility for examining basic mechanisms leading to the beneficial effects of RYGB surgeries in obese humans. Berthoud's RYGB rat model induces body weight and fat mass losses and normalizes obesity-induced glucose intolerance similar to that reported in human studies (30,33). Two weeks after RYGB, rats have 50% reduction in intake of liquid diet because of significantly smaller meal sizes with only partial compensation in meal frequency. Similar findings were seen with solid food 6 weeks after RYGB. Furthermore, using two-choice liquid or solid foods, RYGB rats preferred the low-fat choice, or showed decreased acceptance for the high-fat choice (30). These findings show that RYGB changes not only food and energy intake but also food choice, with lower acceptance of fatty foods. Compared with sham-operated (obese) and age-matched lean control rats, RYGB rats of both models exhibited more positive orofacial responses to low concentrations of sucrose but fewer to high concentrations. These changes in ‘liking’ by RYGB rats were translated into a shift of the concentration–response curve in the brief access test, with more vigorous licking of low concentrations of sucrose and corn oil, but less licking of the highest concentrations. Furthermore, the reduced ‘wanting’ of a palatable reward in the incentive runway seen in sham-operated obese Sprague Dawley rats was fully restored after RYGB to the level found in lean control rats. Thus, RYGB leads to a shift in hedonic evaluation, favouring low- over high-calorie foods and restores obesity-induced alterations in ‘liking’ and ‘wanting’. It remains to be determined whether these effects are simply due to weight loss or specific changes in gut-brain communication. Given the emerging evidence for modulation of cortico-limbic brain structures involved in reward mechanisms by gut hormones, RYGB-induced changes in the secretion of these hormones could potentially be mediating these effects.
Using chronically implanted jugular catheters in rats, Berthoud's group showed postprandial increases in GLP-1, PYY and amylin, as well as suppressed ghrelin levels 3–4 months after RYGB, replicating most of the hormonal changes reported in obese subjects after RYGB (33). The increased plasma amylin concentrations are interesting in view of recent reports suggesting that amylin and leptin synergize in their anorexigenic effects (34). In a 24-week double-blind RCT in overweight/obese subjects, coadministration of recombinant human leptin and the amylin analogue pramlintide elicited 12.7% mean weight loss, significantly more than was observed with either treatment alone (35,36).
Next, April Strader introduced the model of ileal interposition in the rat, which has been shown to improve glucose homeostasis in rat models of diabetes and improve T2D in humans. With this model, a portion of the lower intestine (ileum) is relocated to a region of the jejunum and is therefore prematurely exposed to higher concentrations of nutrients and biliopancreatic secretions. Physiologically, ileal interposition results in enhanced secretion of GLP-1, PYY and glucagon with no change in GIP (37,38). These changes in glucose tolerance and gut hormones are independent of changes in body weight (37). Similar effects of ileal interposition were seen in the University of California-T2D rat model, a polygenic obese animal model of T2D, which further showed that ileal interposition delayed diabetes onset by 3–4 months (39).
Strader hypothesizes that lower intestinal stimulation is essential for post-surgical metabolic improvements in glucose homeostasis. To explore this, Strader uses a new diabetic rat model induced by an HFD and low-dose streptozotocin treatment and examines mRNA expression of gut hormones in different sections of the intestine using realtime polymerase chain reaction. Streptozotocin treatment reliably results in a large amount of beta cell mass loss and consequently causes severe hyperglycaemia in rats. Ileal interposition in this model resulted in decreased glucose and insulin levels (improved insulin sensitivity), massive increases in pre-proglucagon PYY, PEPCK, GLUT2, APOAIV, and a decrease in ASBT (bile transporter) mRNA. During ileal interposition, it appeared that mRNA levels of ileum-specific gut hormones were up-regulated by other parts of the intestine including the portion of ileum that remains and the colon. This finding lead Strader to hypothesize that it may not be the ileum itself, but rather the remaining and lower intestinal segments overcompensating for the relocation of the interposed ileum that may be contributing to improved glucose homeostasis. The ileum is not only an important site of hormone synthesis and secretion but is also the key site for bile acid uptake. As the ileum is in a more proximal location following ileal transposition, Strader hypothesized that this results in a higher absorptive capacity for bile acids. Indeed, rats with ileal interposition have higher plasma levels of bile acids (37) and alterations in the bile acid transporters. Interestingly, apical sodium bile transporter mRNA expression is reduced by 95% while the cytosolic bile transporter (ILBABP) is increased. However, the lower and remaining segments of ileum and colon show extremely high expression of these transporters in rats that have had ileal interposition. Furthermore, bacteria produce hydroxysteroid dehydrogenases that change the composition of the bile salt pool. Total bacteria are increased in feces of rats fed an HFD for 1 week compared with low-fat diet and ileal interposition results in a large increase in the Genus Clostridium, the primary genus involved in 12-alpha hydroxylation. The important role of biliary diversion was observed as early as 1985, with Manfredini showing that internal biliary diversion improved glucose tolerance, which was maintained 3 weeks and 9 months after the operation (40). Similar results were demonstrated by Ermini et al. (41) and Strader 1 week after internal biliary diversion and with ligation of the bile duct. Strader concluded her talk by raising the question of whether improvements in glucose homeostasis depend on bile contact with the ileum or whether simply relocating or delivering bile to a lower region of small intestine is sufficient to achieve glycaemic improvement.
In the last talk of the session on animal models of bariatric surgery, Randy Seeley discussed pre-clinical models of vertical sleeve gastrectomy (VSG). Sleeve gastrectomy is a relatively new bariatric procedure, which involves the creation of a reduced stomach lumen (<80% of original) because of removal of the gastric fundus; the intestine itself remains intact. In contrast to common thought, Seeley hypothesized that VSG is not simply a restrictive procedure and that VSG rats would actively defend their new body weight. In rats, VSG resulted in sustained losses in weight and fat mass and preservation of lean tissue (42). Food intake was transiently decreased 7 d after VSG but then gradually increased to pre-VSG levels by day 15. This was due to greater meal frequency but smaller meal size. Interesting, a chronic food restriction challenge in VSG rats resulted in pre-restriction, rather than pre-VSG body weights. Similar to other animal studies in RYGB animal models, VSG rats when given the choice to eat carbohydrate, protein or fat have decreased fat intake and reduced preference for HFD.
Next, Seeley showed data from VSG rat confirming that VSG qualifies as a metabolic surgery. VSG rats have significantly decreased glucose levels compared with sham and naïve rats and have comparable results with what has previous been shown for RYGB rats: the same massive increases in GLP-1 secretion, increased glucose disposal rate during a euglycaemic hyperinsulinaemic clamp, increased glucose clearance and hepatic glucose production and reversal of raised triglycerides. As RYGB and VSG show similar results, these findings dispel the idea of a ‘foregut hindgut hypothesis’.
Finally, Seeley discussed the critical role of bile acids in lipid reabsorption in the ileum. Kohli et al. reported that plasma bile acids are significantly higher in rats after ileal interposition (43). Seeley concluded his talk by highlighting the need for studies that isolate different aspects of bileopancreatic diversion with the aim of replicating the dramatic results, without the malabsorptive effects.
Session 4: Role of hedonics: animal and human studies
Andras Hajnal started this session by discussing his research on the role of taste and reward in rat models of RYGB surgery and summarized the response of the gustatory reward system with weight loss. Dietary restriction increases appetite and cravings for palatable foods and increases food reward. In contrast, RYGB appears to reduce appetite and the appeal of savoury meals. To examine this phenomenon, Hajnal's laboratory investigated the effects of RYGB on taste and food reward functions in various rat models of obesity, although he only presented results from OLETF (rats lacking the CCK-1 receptor) and DIO in his talk. As expected, RYGB rats had significantly reduced body weight, decreased insulin levels and improved glucose tolerance up to 20 weeks post surgery. Interestingly, RYGB DIO rats have increased food and caloric intake compared with sham controls although they exhibit a reduced two-bottle preference and decreased lick response to high concentration of sucrose as early as 3 weeks post RYGB compared with sham-operated controls. In the genetic obese OLETF rats RYGB reversed avidity to palatable sweet solutions (44). In both DIO and OLETF RYGB rats, similar responses to sucrose were seen for other sweet compounds including fructose, Na-saccharin and alanine. No differences were seen for NaCl, MSG and Quinine-HCL.
Interestingly, DIO rats have a distinct pattern of sucrose licking during the brief access taste preference test with overall decreased licking 3 weeks post RYGB. To test if this altered orosensory preference (‘liking’) for sucrose also affected incentive motivation (‘wanting’), Hajnal performed operant tests using fixed ratio and progressive ratio schedules of reinforcement licking tasks for sucrose in HF-DIO rats in both the absence and presence of dopamine receptor agonists. They found higher sucrose reward licks across all concentrations tested (0.03–1.0 M). In order to discern orosensory (palatability) factors from other possible factors that may be driving the conditioned procurement, they analysed lick patterns elicited to sucrose on the reward spout. When less effort was required (fixed ratio vs. progressive ratio), this effect only happened at lower sucrose concentrations. This may suggest that anticipatory and not consummatory reward is increased in the RYGB rats. Nevertheless, when initial 5-min continuous access was analysed, RYGB DIO emitted less burst lick (measure of ‘liking’) to 1.0 M sucrose relative to surgical controls and not more than they did to 0.1 M sucrose. This finding is very similar to the shorter (10-s) access gustometer findings. It suggests that despite the effects of RYGB in increasing the willingness to work harder for sucrose solutions (particularly to the lower concentrations), hedonic responses to high concentrations (1.0 M and above) sucrose are reduced after the surgery.
Next, Hajnal discussed his laboratory's recent studies investigating alcohol preferences after RYGB. They found that despite no difference in short-term preference, chronically exposed RYGB rats drank nearly twice as much alcohol (g [kg of body weight]−1 d−1) than HF-DIO ad libitum or pair-fed sham-operated controls, and 50% more than normal diet lean control rats when given access to 2–8% alcohol drinks. Similarly the intake of 8% alcohol during reinstatement after 2-week abstinence in RYGB rats matched the intake of normal diet lean controls. In contrast, chronic HFD in this rat model appears to reduce ethanol intake and preference. These findings suggest that RYGB increases sensitivity to alcohol reward, which may be blunted in HF-DIO rats.
Collectively, the sucrose and alcohol studies demonstrate that RYGB in obese rats may reset regulation of taste and reward functions. Hajnal hypothesized that the underlying mechanism may be improved GI signalling after RYGB. GLP-1 administration has been shown to dose-dependently decrease sucrose preferences (brief lick responses to palatable solutions) in rats. Preliminary studies in the Hajnal lab using in vivo microiontophoresis show that GLP-1 directly modulates taste and dopamine neurons.
Hajnal concludes that RYGB alleviates gustatory reward deficits in both genetic and dietary obese rats evidenced by (i) reduced intake and preference for higher concentrations of sucrose solutions, (ii) blunted reward sensitivity and reduced incentive motivation in rats to work for sucrose rewards at lower concentrations and (iii) resets altered coding for sweet in taste neurons.
In the next talk, Allan Geliebter discussed neuroimaging before and after bariatric surgery. He started by summarizing his earlier work using gastric balloons to study gastric distension signals. A reduced gastric capacity resulting from balloon placement, besides physically limiting food intake, can also enhance gastric distention mediated satiety signals, following even small amounts of food. When a gastric balloon was covertly filled via an oral tube to different volumes (0–800 mL) in lean and obese individuals, prior to ingestion of a liquid test meal, intake was reduced by about 40% of the balloon volume (45). When the gastric balloon was filled to 800 mL and then quickly emptied prior to ingestion, intake was similar to that following a balloon volume at 0 mL, consistent with a short-acting neural distension signal (45). In another study, when balloon volume was gradually increased beyond 800 mL, gastric capacity, as reflected in maximum tolerated volume and measures of gastric compliance (volume/pressure), was significantly greater in obese than in lean participants (46).
A similar protocol was used to distend a gastric balloon in participants while lying inside a functional magnetic resonance imaging (fMRI) scanner. fMRI measures changes in blood flow related to neural activity in the brain and is safer, more cost-effective, and has greater specificity than PET. Both the amygdala (involved with emotion and reward) and the insula (involved with visceral perception) responded to various levels of gastric distension. Moreover, as BMI increased, activation in the amygdala to gastric distension was reduced, possibly because of greater gastric capacity in those of higher BMI (47).
Functional magnetic resonance imaging was also used to compare lean and obese women with and without binge eating behaviour, while viewing high energy dense foods, typically consumed during binge episodes (e.g. cookies, cakes) and low-energy-dense foods (e.g. asparagus, broccoli) (48). The only brain area activated for all members of a group was the oral premotor area in the obese binge eaters in response to viewing binge-type foods. The premotor area is involved in planning of motor behaviour, and activation there may reflect thoughts about ingesting the binge-type foods. In a separate group analysis, obese as compared with lean individuals had greater responses to high-energy-dense foods in the hippocampus, ventral pallidum, and ventral tegmental area, areas associated with the mesolimbic dopaminergic reward pathway (Geliebter et al. submitted).
In the next set of studies, fMRI was used to study neural responses to food cues before and after RYGB. Patients scanned 1-month post RYGB showed reduced activation of areas within the mesolimbic pathway, including the ventral tegmental area, ventral striatum, putamen, posterior cingulate and dorsomedial prefrontal cortex (49,50). Activation post surgery was significantly reduced in response to the higher, compared with the lower-energy-dense food cues. As such, brain activation changes may be another potential mechanism for weight loss produced by RYGB. Theoretically, these results may aid in the development of new medications to treat obesity that mimic these neural effects.
Next, Manu Chakravarthy discussed the exploration of fMRI for probing eating behaviour. He presented a study where they used Blood Oxygen Level Dependent contrast and Arterial-Spin Labeling-derived regional Cerebral Blood Flow to determine whether these signals can reliably distinguish between hunger and satiety.
First, they investigated whether these measures are modulated by sibutramine, a drug known to decrease food intake after a single dose. In this randomized, double-blind, placebo-controlled, three period (two placebo/one sibutramine 30 mg single dose) cross-over study, 15 obese subjects underwent fMRI scanning sessions in the resting state with eyes closed, and while viewing images of food, non-food animals and blurred objects before and after a standard meal. The primary endpoint was a pre-specified hunger-satiety matrix region of interest (ROI) derived from the amygdala, insula, hippocampus, dorsal striatum, anterior cingulate cortex and orbitofrontal cortex. Results showed that food and nonfood images robustly activated the visual cortex in all subjects demonstrating alertness and task engagement. The Blood Oxygen Level Dependent signal had low sensitivity (treatment effect size between −0.6 and 0) and poor reproducibility (intraclass correlation coefficient 0.2– 0.4) within the six pre-specified ROIs. By contrast, Arterial-Spin Labeling-derived regional Cerebral Blood Flow measurements had better reproducibility (intraclass correlation coefficient ∼0.6) in these six ROIs. The increased regional Cerebral Blood Flow observed in fed vs. fasted state in the placebo group was significantly attenuated by sibutramine in the dorsal striatum, anterior cingulate and orbitofrontal cortex (effect size ∼0.9). This modulation was restricted to hunger-satiety matrix regions and not observed in the visual- or motor-cortex. An exploratory voxel-wise analysis of the whole-brain indicated modest treatment effects in both activation and deactivation patterns in some of the hunger-satiety matrix ROIs (orbitofrontal cortex), as well as within the Default Mode Network (medial temporal lobe, posterior cingulate cortex, precuneus and inferior parietal cortex).
Taken together, these data not only provide reproducibility metrics for specific fMRI endpoints in the context of normal physiology (hunger/satiety), but also suggest for the first time, a single-dose effect of an anorectic agent on neuronal activity within discrete limbic areas of the human brain associated with feeding behaviour.
Summary and conclusions
The Pennington Scientific Symposium on Bariatric Surgery was a productive forum for presenting the latest research (pre-clinical and clinical) on the potential mechanisms behind the metabolic improvements and weight reductions in patients having bariatric surgery. Clearly, the specific mechanism(s) bringing about the lasting benefits of bariatric surgeries have not yet been identified. Given the likely very complex and temporally variable interactions of multiple signalling pathways at the molecular, tissue and systems levels, it is perhaps unrealistic to rapidly find ‘the smoking gun’. However, research in humans and animal models has already directed our attention to a number of mechanisms that have great potential for the development of therapeutic strategies.
The meeting concluded with a round-table discussion lead by Kaplan and Seeley of the questions that had been raised during the meeting and the identification of knowledge gaps for future research:
Gut hormones
In terms of the role of gut hormones after bariatric surgery, there are no definitive answers, although there is a lot of correlation data linking gut hormone levels and weight loss. Pre-clinical studies that block GLP-1 post bariatric surgery will be the next step forward. Also, we need to measure local levels of gut hormones, as plasma levels may not be reflective of the local milieu. Of course, there may be undiscovered hormones that could potentially mediate major physiological effects. Peptidomic technology studies to find Factor X are underway.
Mechanisms related to GI physiology
An approach of excising tissues from different sections of the intestine and examining different peptides that are secreted from each one should be considered. An important area that is largely unknown is the mechanisms by which expression and secretion of GI hormones are altered after surgery. Research to-date has mainly focused on the delivery of nutrients, but we still have little clue about other factors such as the roles of the enteric and autonomic nervous systems as well as changes in the gut microbiome. At the other end of gut hormone signalling, we still have a lot to learn about the specific sites of action in the periphery and brain, and their respective downstream pathways leading to changes in energy balance and glucose homeostasis.
Animal models of bariatric surgery
Researchers commented on the need to report the details of their sham procedures in an effort standardize surgical techniques in animals. Although, similar findings have been reported for gut hormones, results for brief access tests differ and differences in surgical techniques may be relevant.
This meeting report has presented the latest pre-clinical and clinical research in energy balance and gut physiology, hedonics and animal models related to bariatric surgery. There is clearly a vast array of unanswered questions ranging from the mechanisms driving alterations in gut hormones to the changes in energy balance, which occur post surgery. Understanding the mechanisms behind the ‘magic’ of bariatric surgery and hence replicating these effects in a non-surgical manner will be one of the ultimate challenges for the treatment for obesity.
Appendix 1.
Bariatric surgery: do the mechanisms hold the key for novel therapies?
| Monday, December 6 | |
| SESSION 1: Energy balance and GI physiology – Eric Ravussin, Chair | |
| 8:30–9:15 | Eric Ravussin, PhD Pennington Biomedical Research Center Energy balance regulation |
| 9:15–10:00 | Helen Raybould, PhD UC Davis Gastrointestinal physiology and the gut-brain axis |
| 10:00–10:15 | BREAK |
| SESSION 2: Gut hormones and bariatric surgery – Walter Pories, Chair | |
| 10:15–11:00 | Jens Holst, MD, PhD University of Copenhagen The physiology of gut hormones |
| 11:00–11:45 | Walter Pories, MD, FACS Brody School of Medicine, East Carolina University The wonderful puzzle: the physiology of gut hormones |
| 11:45–1:00 | LUNCH – Lod Cook Alumni Center, Cook Room |
| SESSION 3: Animal models of bariatric surgery – Hans-Rudi Berthoud, Chair | |
| 1:00–1:45 | Lee Kaplan, MD, PhD Massachusetts General Hospital The physiology of bariatric surgery |
| 1:45–2:30 | Marco Bueter, MD Hammersmith Hospital, Imperial College London, UK Gastric bypass in rodents – what does it tell us about human physiology? |
| 2:30–3:15 | Hans-Rudolf Berthoud, PhD Pennington Biomedical Research Center A rat model of bariatric surgery |
| 3:15–3:30 | BREAK |
| 3:30–4:15 | April Strader, PhD Southern Illinois University School of Medicine Bile diversion and diabetes cure |
| 4:15–5:00 | Randy Seeley, PhD University of Cincinnati Sleeve gastrectomy |
| 5:00 | Hospitality Suite Open |
| 7:00 | Dinner – Lod Cook Alumni Center, Cook Room |
| 9:00 | Hospitality Suite Open |
| Tuesday, December 7 | |
| SESSION 4: Role of hedonics: animal and human studies – Lee Kaplan, Chair | |
| 8:30–9:15 | Andras Hajnal, MD, PhD Penn State University Role of taste and reward in rat models |
| 9:15–10:00 | Allan Geliebter, PhD Columbia University Neuroimaging before and after bariatric surgery |
| 10:00–10:15 | BREAK |
| 10:15–10:30 | Manu Chakravarthy, MD, PhD Merck Research Laboratories Probing eating behaviour with functional MRI |
| 10:30–11:00 | Lee Kaplan and Randy Seeley Future and perspectives |
| 11:00–12:00 | Lunch & Roundtable Discussion for consensus paper draft All Chairs |
Footnotes
Disclosures: This meeting was sponsored by Ajinomoto Co, Inc., Amylin/Lilly Alliance, Covidien, Ethicon Endo Surgery, Inc., GlaxoSmithKline, PepsiCo and The Pennington Bio-medical Research Center Foundation.
Conflict of Interest Statement: No conflict of interest was declared.
References
- 1.Schwartz A, Doucet E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obes Rev. 2010;11:531–547. doi: 10.1111/j.1467-789X.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- 2.Bobbioni-Harsch E, Morel P, Huber O, Assimacopoulos-Jeannet F, Chassot G, Lehmann T, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 4.van Gemert WG, Westerterp KR, van Acker BA, Wagenmakers AJ, Halliday D, Greve JM, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 5.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 8.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36:1361–1370. doi: 10.1007/BF01296800. [DOI] [PubMed] [Google Scholar]
- 12.Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1).Association with inhibition of gastric acid secretion in humans. Dig Dis Sci. 1995;40:1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 14.Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ, et al. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 17.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 18.Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt WE. Early clinical studies with liraglutide. Int J Clin Pract Suppl. 2010;64:12–20. doi: 10.1111/j.1742-1241.2010.02500.x. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 21.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Clarke JM, Shore AD, Magnuson TH, Richards T, Bass EB, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg. 2010;145:726–731. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 24.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre V, Stylopoulos N, Grinbaum R, Kaplan LM. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity (Silver Spring) 2008;16:2585–2592. doi: 10.1038/oby.2008.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 32.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Rouxen-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab. 2010;21:473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmaco-therapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strader AD, Clausen TR, Goodin SZ, Wendt D. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg. 2009;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 38.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 39.Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manfredini G, Ermini M, Scopsi L, Bonaguidi F, Ferrannini E. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol. 1985;249:G519–G527. doi: 10.1152/ajpgi.1985.249.4.G519. [DOI] [PubMed] [Google Scholar]
- 41.Ermini M, Iaconis E, Mori A. The effects of bilio-jejunal diversion on streptozotocin diabetes in the rat. Acta Diabetol Lat. 1991;28:79–89. doi: 10.1007/BF02732117. [DOI] [PubMed] [Google Scholar]
- 42.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1675–R1686. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geliebter A, Westreich S, Gage D. Gastric distention by balloon and test-meal intake in obese and lean subjects. Am J Clin Nutr. 1988;48:592–594. doi: 10.1093/ajcn/48.3.592. [DOI] [PubMed] [Google Scholar]
- 46.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochner CN, Gibson C, Carnell S, Dambkowski C, Geliebter A. The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol Behav. 2010;100:549–559. doi: 10.1016/j.physbeh.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


