Abstract
Objective
The effects of excess alcohol consumption (alcohol misuse) on outcomes in patients with acute lung injury (ALI) have been inconsistent, and there are no studies examining this association in the era of low tidal volume ventilation and a fluid conservative strategy. We sought to determine whether validated scores on the Alcohol Use Disorders Identification Test (AUDIT) that correspond to past year abstinence (zone 1), low-risk drinking (zone 2), mild to moderate alcohol misuse (zone 3), and severe alcohol misuse (zone 4) are associated with poor outcomes in patients with ALI.
Design
Secondary analysis.
Setting
The Acute Respiratory Distress Syndrome (ARDS) network, a consortium of 12 university centers (44 hospitals) dedicated to the conduct of multi-center clinical trials in patients with acute lung injury.
Subjects
Patients meeting consensus criteria for ALI enrolled in one of three recent ARDS network clinical trials.
Interventions
None
Measurements and Main Results
Of 1,133 patients enrolled in one of three ARDS network studies, 1,037 patients had an AUDIT score available for analysis. Alcohol misuse was common with 70 (7%) of patients having AUDIT scores in zone 3 and 129 (12%) patients in zone 4. There was a u-shaped association between validated AUDIT zones and death or persistent hospitalization at 90 days (34% in zone 1, 26% in zone 2, 27% in zone 3, 36% in zone 4; p < 0.05 for comparison of zone 1 to zone 2 and zone 4 to zone 2). In a multiple logistic regression model, there was a significantly higher odds of death or persistent hospitalization in patients in AUDIT zone 4 when compared to those in zone 2 (adjusted OR 1.70; 95% CI 1.00, 2.87; p = 0.048).
Conclusions
Severe, but not mild to moderate alcohol misuse is independently associated with an increased risk of death or persistent hospitalization at 90 days in ALI patients.
Keywords: alcohol use disorders identification test, acute lung injury, alcohol use disorder, alcohol misuse, unhealthy alcohol use
INTRODUCTION
Alcohol misuse is defined as consumption of alcohol in excess of recommended limits and refers to a spectrum of unhealthy drinking (1). At the milder end of the alcohol misuse spectrum, at-risk drinking is present when consumption exceeds recommended limits but there are no adverse medical, legal, or social consequences (2-3). When drinking leads to medical, legal, or social consequences, by the criteria established in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), either alcohol abuse or dependence is present (4). Validated questionnaires are frequently used as a sensitive screening tool to identify alcohol misuse (5). Although higher scores on alcohol screening questionnaires do not establish the presence of alcohol abuse or dependence, generally accepted cutoffs denote an increasing severity of alcohol misuse and a higher probability of alcohol abuse or dependence (5-7).
An estimated 190,600 cases of acute lung injury (ALI) occur each year in the U.S., and despite improvements in treatment, mortality in observational studies continues to approach 40% (8). Several prior investigations have determined that a history of alcohol abuse independently increases the risk of developing ALI in diverse patient populations. A prospective observational study of 351 critically ill patients demonstrated that a history of alcohol abuse was associated with a two-fold increase in the risk of developing ALI (9). The association between alcohol abuse and the development of ALI was subsequently confirmed in a multi-center prospective cohort study of patients with septic shock in the US and in a national survey in the Netherlands (10-11). Alcohol abuse has also been identified as a risk factor for transfusion-related acute lung injury (12) and the development of ALI in patients undergoing surgery for lung cancer (13). However, the relationship between alcohol misuse and outcome in patients with ALI is not clear with a higher mortality in patients with alcohol misuse in one prior observational study and no difference in mortality between patients with and without alcohol misuse in another (9, 11). Both of these studies were conducted prior to important advancements in ALI including low tidal volume ventilation and a fluid conservative strategy.(14-15). Furthermore, both of these observational studies focused on short-term outcomes and neither examined the correlation between the severity of alcohol misuse and outcomes in patients with ALI.
Given the disparate findings regarding the relationship of alcohol misuse and mortality in ALI and the inconsistent definitions regarding alcohol misuse in prior studies, we sought to further clarify the relationship of alcohol misuse with hospital outcomes. We utilized information from large, multicenter investigations of patients with ALI conducted by the ARDS network between 2007 and 2011. Specifically, we sought to determine whether higher alcohol screening scores on the extensively validated Alcohol Use Disorder Identification Test (AUDIT) were independently associated with a combined primary endpoint of mortality or persistent hospitalization at 90 days in patients with ALI. We hypothesized that patients with severe alcohol misuse would have a higher rate of death or persistent hospitalization at 90 days when compared to those patients with low-risk alcohol use.
MATERIALS AND METHODS
We performed a secondary analysis of three recently completed multi-center, randomized, double-blind, placebo-controlled trials in patients with ALI. All trials were funded by the National Heart Lung and Blood Institute. Institutional Review Boards (IRB) at each of the participating institutions for these investigations reviewed and approved study protocols, and informed consent was obtained from patients or their appropriate surrogate prior to enrollment in the parent studies, including later use of collected data. All data was de-identified prior to our analyses.
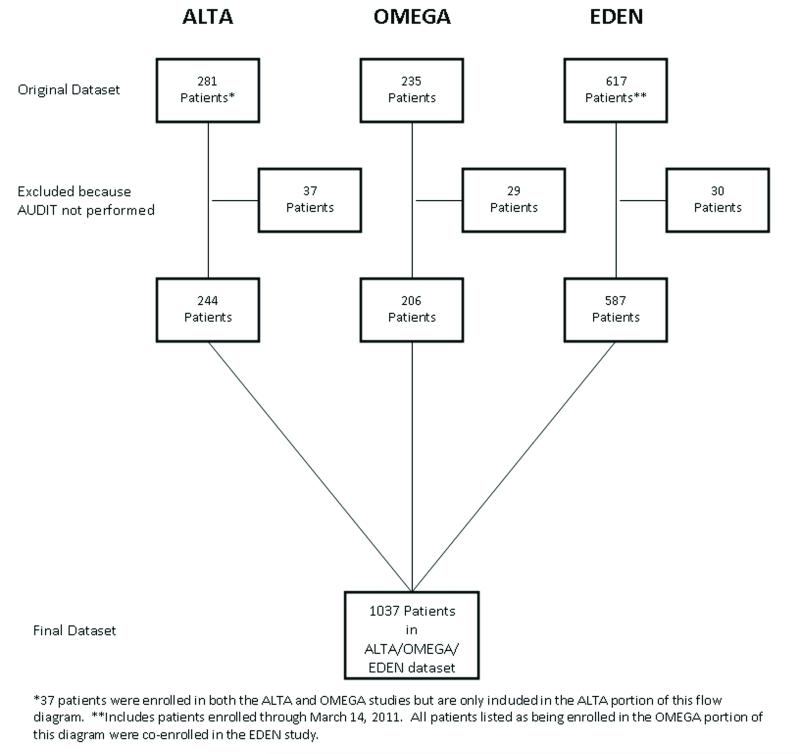
Data from the three trials was obtained from the ARDS network, a consortium of 12 university centers (44 hospitals) dedicated to the conduct of multi-center clinical trials in patients with ALI. The full inclusion and exclusion criteria for each study are described in the parent manuscripts (16-18). Importantly, patients with a Child-Pugh score of 11 or higher were excluded from these studies. The first ARDSNet trial, known as the Albuterol to Treat Acute Lung Injury (ALTA) study, enrolled patients who met American-European Consensus Conference (AECC) criteria for ALI and required mechanical ventilation (18-19). Patients were recruited between August 2007 and July 2008 and randomized to blinded treatment with either aerosolized albuterol or placebo. The second trial, the Omega-3 Fatty Acid/Antioxidant Supplementation (Omega) Study, enrolled ALI patients between January 2008 and February 2009, randomizing them to treatment with either enterally administrated omega-3 fatty acids or placebo. The third study, the Early Versus Delayed Enteral Feeding (EDEN) trial, randomized ALI patients to minimal versus full enteral feeding and enrolled patients between January 2008 and April 2011. Patients in the ALTA, OMEGA, and EDEN studies received protocolized standard of care incorporating strategies determined to improve in-hospital outcomes, including a fluid conservative and low tidal volume ventilation strategy (14-15). There was no significant difference in the primary outcomes between the treatment and placebo arms in the ALTA, OMEGA, or EDEN studies (16-18). For the purpose of investigating our outcome variables, data from patients enrolled in the ALTA, OMEGA, and EDEN studies were combined to form a single data set for analysis (Figure 1).
Figure 1.
Data from the Albuerol Treatement for Acute Lung Injury (ALTA, Omega-3 Fatty Add/Antioxidant Supplementation Study (OMEGA), and Early versus Delayed Enteral Nutrition (EDEN) studies were combined to form a single database.
*37 patients were enrolled in both the ALTA and OMEGA studies but are only induded in the ALTA portion of this flow diagram. **Includes patients enrolled through March 14, 2011. All patients listed as being enrolled in the OMEGA portion of this diagram were co-enrolled in the EDEN study.
Independent Variable
Alcohol Screening Scores
At the time of enrollment, in all three studies, alcohol use was assessed by administering the Alcohol Use Disorders Identification Test (AUDIT) to either the patient or a surrogate. The AUDIT was developed by the World Health Organization and has been validated in several healthcare settings, including medical inpatients and surrogate decision makers (20-21). The AUDIT contains 10 questions assessing alcohol consumption, dependence symptoms, and harmful alcohol use. Each question is scored from 0 to 4 with total scores ranging from 0 (indicating abstinence) to 40. AUDIT scores clinically correlate to abstinence, low-risk drinking, mild to moderate alcohol misuse with a lower risk of alcohol dependence, and severe alcohol misuse (5, 22-23). Numerous studies have demonstrated that the established WHO AUDIT cutpoints should be lowered for women(5, 24-25). Accordingly, a priori, we used validated, gender-specific AUDIT cutoffs to categorize patients into one of four zones (Table 1) (7, 24).
Table 1.
Validated, gender-specific cutoffs were used to categorize patients into AUDIT zones. Patients in zones 3 and 4 have alcohol misuse (consumption of alcohol in excess of recommended limits). Scores in zone 3 generally correlate with mild to moderate alcohol misuse in which there are fewer (or no) alcohol-related consequences and there is a low prevalence of alcohol abuse or dependence. Scores in zone 4 correlate with more alcohol-related consequences and a higher prevalence of alcohol abuse or dependence (severe alcohol misuse).
| AUDIT Score | Description of Category | ||
|---|---|---|---|
| Men | Women | ||
| Zone 1 | 0 | 0 | Abstinence |
| Zone 2 | 1-7 | 1-4 | Low-risk alcohol use |
| Zone 3 | 8-15 | 5-12 | Mild to moderate alcohol misuse |
| Zone 4 | ≥ 16 | ≥ 13 | Severe alcohol misuse |
Outcome Variables
For the primary outcome variable, we chose a combined endpoint of mortality or persistent hospitalization at 90 days. Patients who were not home with unassisted breathing at the 90 day follow-up were considered to be persistently hospitalized. This combined endpoint was chosen because survivors of mechanical ventilation who are not discharged home with unassisted breathing are known to have poor outcomes (26-28). Similar composite outcomes have been used in prior studies of critical illness (29). Secondary outcomes included the number of ventilator, and ICU-free days out of the 28 days following study entry. As previously described, patients who died prior to study day 28 were considered to have 0 ventilator-free days (VFD) or ICU free days (ICUFD) (15, 30).
Statistical Analysis
Differences between patients in each of the AUDIT zones were compared using analysis of variance or the t-test for normally distributed variables. The Wilcoxon rank sum test was used to compare continuous variables that were not normally distributed. Categorical variables were compared using the chi-square test. To determine the association between AUDIT zone and our primary outcome variable, stepwise logistic regression was performed with AUDIT zone as the predictor variable and mortality or persistent hospitalization at 90 days as the outcome variable. Age, severity of illness measured by APACHE III scores, and smoking status (current, former, and never) were chosen a priori to include in the model as confounders. Although age is included in APACHE III scores, age and APACHE III were only weakly associated (r2 = 0.05) and therefore, both were included as covariates in our models. Other variables included in the model were baseline differences in comorbidities (diabetes(31), immunosuppression, cirrhosis, and CHF),ALI risk factors, and body mass index. Body mass index was classified as < 20 kg/m2, 20-29 kg/m2, or 30-39, or > 39 kg/m2 as previously described (32). Given that hypoalbuminemia is associated with severe alcohol misuse and poor outcomes in the setting of critical illness, we tested the presence of hypoalbuminemia as a mediator once our final model was obtained. As previously described, patients were dichotomized into the presence or absence of hypoalbuminemia based on a cut off of 2mg/dL (33). The Hosmer Lemeshow goodness of fit statistic was used to ensure adequate model fit.
To test the hypothesis that proper stratification of alcohol use would alter our results, we used a similar logistic regression model to determine whether our results persisted when alcohol screening scores were dichotomized into the absence (zone 1 and zone 2) or presence (zone 3 and zone 4) of alcohol misuse. To analyze the association between AUDIT zone and secondary outcomes, stepwise multiple linear regression was performed using AUDIT category as the predictor variable and ICU or ventilator-free days as the outcome variable while adjusting for age, severity of illness, gender, smoking status, body mass index, and baseline differences in ALI risk factors and co-morbidities. A two-sided p value of <0.05 was considered to be significant.
Selection of the control group
The relationship between alcohol consumption and adverse health-related outcomes has been reported to have a “J-shaped” association with mortality, with abstinent individuals having a higher mortality than low-risk drinkers. Individuals who abstain from alcohol are more likely to be older, have greater comorbidity, and poorer health status when compared with low-risk drinkers (34-35). Therefore, as in multiple prior studies assessing the association between alcohol screening scores and adverse outcomes, we chose a priori to use AUDIT zone 2 (low-risk drinkers) as the referent group for our primary analysis (36-38).
RESULTS
Of the 1133 total patients enrolled in the ALTA, OMEGA, and EDEN studies, 1037 (92%) had an AUDIT score performed and were included in the analysis (Figure 1). There was no significant difference in race, age, ALI risk factor, smoking status, APACHE III score, or the primary outcome variable between patients who did and did not have an AUDIT score recorded. However, patients who did not have an AUDIT score recorded were more likely to be male than those who did have one available (63% vs 51%, p = 0.03). Overall, 70 (7%) and 129 (12%) of patients included in the analysis were in zones 3 and 4, respectively (Table 2). Notably, patients in zone 1 were more likely to have co-morbidities including immunosuppression, diabetes, and CHF than patients in zones 2-4. Compared to patients in zone 2, those in zone 4 were younger, more likely to be male, more frequently had cirrhosis (Child’s Class A or B), less likely to be diabetic, were more likely to be a current smoker, and more likely to have aspiration as an ALI risk factor. Overall, patients in AUDIT zones 2 and 3 were similar with respect to their demographic factors, ALI risk factors, and co-morbidities, though patients in AUDIT zone 3 were more likely to be current smokers.
Table 2.
Characteristics of patients with abstinence, low-risk alcohol use, and unhealthy alcohol use who were enrolled in the ALTA, OMEGA, and EDEN studies.
|
AUDIT
Zone 1 (n = 534) |
AUDIT
Zone 2 (n = 304) |
AUDIT
Zone 3 (n = 70) |
AUDIT
Zone 4 (n = 129) |
P-Value | |
|---|---|---|---|---|---|
| Age (median, IQR) | 54 [44, 67] | 51 [38, 63] | 51 [42, 56] | 50 [42, 56] | < 0.01 |
| Gender (% male) | 41 | 55 | 60 | 75 | < 0.01 |
| Body Mass Index (kg/m2) | 29 [24, 36] | 28 [24, 34] | 27 [23, 32] | 25 [22, 30] | <0.01 |
| APACHE III score | 91 [73, 100] | 88 [69, 111] | 95 [72, 110] | 87 [70, 107] | 0.60 |
| Race (%) | 0.14 | ||||
| Caucasian, Non-Hispanic | 68 | 72 | 71 | 64 | |
| African American | 22 | 16 | 16 | 27 | |
| Other | 9 | 12 | 13 | 9 | |
| ALI Risk Factor (%)* | |||||
| Trauma | 4 | 10 | 10 | 6 | 0.02 |
| Sepsis | 58 | 58 | 47 | 62 | 0.29 |
| Transfusion | 4 | 4 | 3 | 2 | 0.79 |
| Aspiration | 18 | 14 | 33 | 35 | < 0.01 |
| Pneumonia | 70 | 69 | 70 | 74 | 0.79 |
| Other | 12 | 20 | 7 | 14 | <0.01 |
| Co-morbidities | |||||
| Immunosuppression | 14 | 8 | 5 | 1 | <0.01 |
| Cirrhosis | 3 | 4 | 5 | 14 | < 0.01 |
| Diabetes | 32 | 25 | 29 | 13 | <0.01 |
| CHF | 9 | 3 | 5 | 2 | < 0.01 |
| Smoking Status | <0.01 | ||||
| Never | 54 | 39 | 19 | 13 | |
| Former | 22 | 25 | 17 | 15 | |
| Current | 24 | 35 | 64 | 72 |
Some patients had more than 1 ALI risk factor. Thus, the sum of percentages is greater than 100. See table 1 for a description of the AUDIT zones.
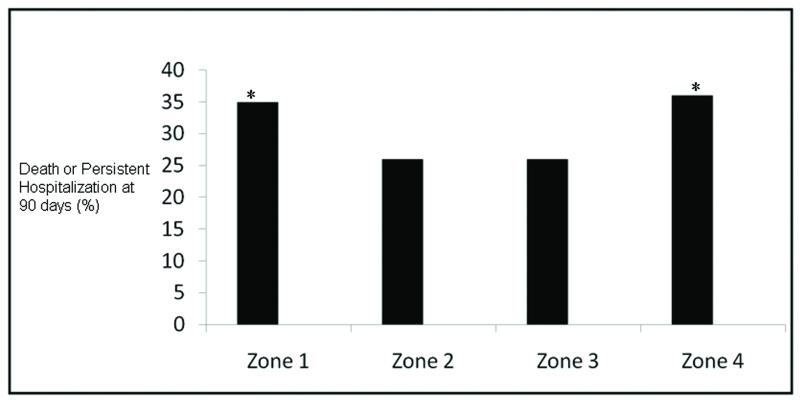
On univariate analysis, patients in zone 4 were significantly more likely than those in zone 2 to die or be persistently hospitalized at 90 days (36% vs. 26%, p = 0.04). Patients in zone 1, who were abstinent from alcohol, were also significantly more likely than those in zone 2 to die or be persistently hospitalized at 90 days (34% vs 26%, p = 0.01). Rates of death or persistent hospitalization at 90 days were similar in patients in zone 3 (27%) and zone 2 (26%) (p = 0.88) (Figure 2). Adjusting for age, gender, severity of illness, smoking status, and baseline differences in ALI risk factor and comorbidities, the significantly higher rate of death or persistent hospitalization in patients in zone 4 as compared to zone 2 persisted (adjusted OR 1.78; 95% CI 1.07, 2.94; p = 0.03; ). When hypoalbuminemia was added to this model, there was not a substantial change in the adjusted odds ratio, though hypoalbuminemia was significantly associated with a higher rate of death or persistent hospitalization (Table 3). In a simple logistic regression model, there was not a higher odds of hypoalbuminemia in patients in zone 4 when compared to patients in zone 2 (OR 1.43; 95% CI 0.93, 1.86; p = 0.10). In this model as well as the model without hypoalbuminemia, the higher odds of death or persistent hospitalization in patients in zone 1 when compared to zone 2 was no longer statistically significant (p = 0.10 and p = 0.06, respectively).. Patients with trauma as an ALI risk factor, as compared to those without, also had a higher odds of death or persistent hospitalization (adjust OR 2.24; 95% CI 1.22, 4.10; p < 0.01).
Figure 2.
Unadjusted rates of death or persistent hospitalization at 90 days in AUDIT zones 1-4. *p < 0.05 for comparison with zone 2.
Table 3.
Final logistic regression model demonstrating a higher odds of mortality or persistent hospitalization for patients in AUDIT Zone 4 when compared to AUDIT zone 2 after adjusting for age, gender, severity of illness, smoking status, baseline differences in ALI risk factors, hypoalbuminemia, and co-morbidities.
| Odds Ratio |
95% Confidence Interval |
P-value | |
|---|---|---|---|
| AUDIT Score | |||
| AUDIT Zone 1 | 1.35 | 0.94, 1.96 | 0.10 |
| AUDIT Zone 2 | 1.00 | -- | -- |
| AUDIT Zone 3 | 1.09 | 0.56, 2.04 | 0.80 |
| AUDIT Zone 4 | 1.70 | 1.00, 2.87 | 0.048 |
| Other Covariates | |||
| Age (continuous) | 1.03 | 1.02, 1.04 | < 0.01 |
| APACHE (continuous) | 1.02 | 1.02, 1.03 | < 0.01 |
| No trauma as an ALI risk factor | 1.00 | -- | -- |
| Trauma as an ALI risk factor | 2.09 | 1.13, 3.80 | 0.02 |
| Male | 1.00 | -- | -- |
| Female | 0.77 | 0.55, 1.05 | 0.10 |
| Smoking Status | |||
| Never | 1.00 | -- | -- |
| Current | 0.72 | 0.48, 1.08 | 0.12 |
| Former | 0.89 | 0.61, 1.29 | 0.54 |
| Albumin (< 2 mg/dL) | 1.53 | 1.11, 2.10 | < 0.01 |
When AUDIT scores were dichotomized into the presence or absence of alcohol misuse, on univariate analysis there was no significant difference in the rate death or persistent hospitalization between patients with alcohol misuse compared to those without (33% vs 31%, p = 0.71). In multivariate analysis adjusting for age, severity of illness, and baseline differences, the odds of death or persistent hospitalization were similar between those with and without alcohol misuse (adjusted OR 1.22; 95% CI 0.0.82, 1.80; p = 0.32).
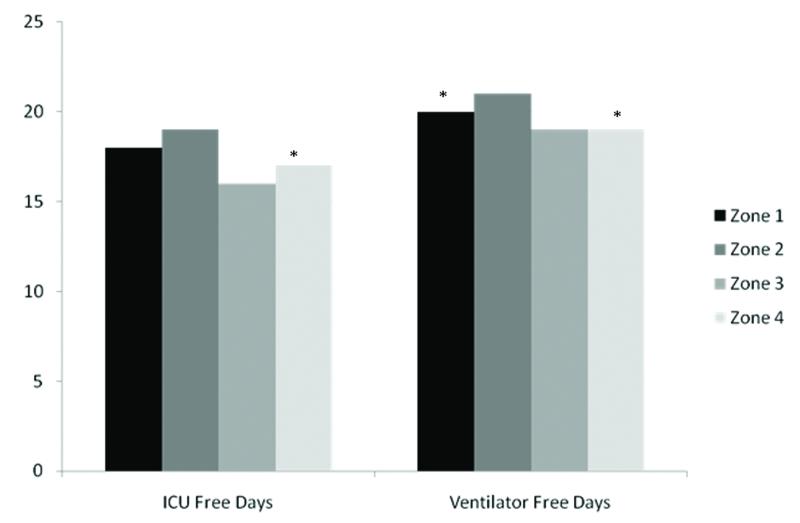
In comparing the average number of VFD out of the first 28 after enrollment, patients in zone 4 had significantly fewer VFD than those in zone 2 (Figure 3). On average, patients in zone 4 had 19 [IQR 0,24] VFD, patients in zone 3 had 19 [IQR 6,23] VFD, and patients in zone 2 had 21 [IQR 5, 25] VFD (p < 0.05 for zone 4 vs zone 2; p = 0.07 for comparison of zone 3 to zone 2). However, after adjusting for age, gender, severity of illness, smoking status, body mass index, co-morbidities, and ALI risk factor, the difference in VFD across AUDIT zones was no longer significant. Paralleling what was observed for VFD, on average, patients in zone 4 had significantly fewer ICUFD than those in zone 2 (17 [IQR 0, 23] vs. 19 [IQR 6, 24], p = 0.03) while patients in zone 3 (median ICUFD 16 [IQR 7, 22]) and zone 1 (median ICUFD 18, [IQR 1, 23]) did not have significantly fewer ICUFD when compared to patients in zone 2 (p = 0.08 and p = 0.05, respectively). Differences in ICUFD no longer significantly varied by zone of alcohol consumption after adjusting for age, gender, severity of illness, smoking status, body mass index, and baseline differences in co-morbidities and ALI risk factors.
Figure 3.
Univariate comparison of ventilator and ICU free days in AUDIT zones 1-4. See table 1 for a definition of the AUDIT zones. *p <0.05 when compared to Zone 2.
DISCUSSION
In this secondary analysis of over 1000 patients with ALI enrolled in 3 randomized controlled trials at 12 academic centers and 44 hospitals across the U.S., using a validated screening approach, we found that nearly 20% of patients have AUDIT scores that are consistent with alcohol misuse. Further, we observed an independent association between severe alcohol misuse and a combined outcome of mortality or persistent hospitalization at 90 days. This finding is consistent with the initial observation that the presence alcohol of abuse is associated with higher mortality among patients with ALI (65% vs. 35%, p = 0.003) (9). The lack of an association between alcohol abuse and mortality in the 2003 prospective cohort study may be explained by the fact that low-risk drinkers were grouped with patients with abstinence in the control group and the screening tool used in that study failed to identify a group of patients with severe alcohol misuse (11). Supporting this, the association between alcohol use and poor outcomes in this study was no longer present when AUDIT scores were dichotomized into the presence or absence of alcohol misuse. Consistent with the reasoning for our a priori selection of low-risk alcohol use as the proper control group, patients with abstinence were more likely than to have immunosuppression, diabetes, and congestive heart failure. Overall, our findings suggest that the AUDIT, an easily administered screening tool, can be used to identify a subpopulation of ALI patients with a higher risk of adverse outcomes.
Several potential biological mechanisms may explain the relationship we observed between severe alcohol misuse and death or persistent hospitalization. Chronic excess alcohol consumption alters expression levels of pro-inflammatory cytokines (39-40), including IL-6 and IL-8 that have been associated with poorer outcomes in the setting of ALI (41). Moreover, chronic excess alcohol consumption has also been associated with impaired alveolar-capillary permeability both in otherwise healthy people with chronic heavy alcohol consumption (42), as well as in the setting of sepsis (43). These permeability effects may be related to decreased alveolar lining concentrations of the antioxidant glutathione(44). Importantly, low intrapulmonary glutathione is a cardinal feature of ALI (45). These alcohol-associated effects, alone or in combination, could contribute to protracted illness and poor outcomes among ALI patients with the highest AUDIT scores. Alternatively, the poor outcomes observed in the group with severe alcohol misuse could be secondary to alcohol withdrawal delirium which is associated with an increase in mortality and longer duration of mechanical ventilation in some critically ill patient populations (46-49).
Our findings extend the association between AUDIT scores consistent with severe alcohol misuse and adverse medical outcomes previously reported in outpatients and surgical inpatients to the critical care setting (37-38, 50). These findings are consistent with prior studies that used administrative databases to demonstrate an association between alcohol dependence and the development of sepsis and septic shock as well as the need for mechanical ventilation in hospitalized patients (46, 51). Our findings demonstrate the adverse effects of chronic heavy alcohol consumption in a group of patients that is cared for exclusively in the intensive care unit.
The current study has limitations. First, this study is a secondary analysis of three clinical trials in patients with ALI that were not specifically designed to test our hypothesis. However, use of data from these three clinical trials allowed us to establish the largest cohort to date of well-characterized ALI patients with alcohol use history collected in a validated consistent fashion to answer our study question. Second, the observed mortality and effect size are smaller than previous observational studies (8-9, 11), possibly due to overall improvements in care. However, selection bias should be considered as another potential explanation since the present cohort was comprised of patients enrolled in a clinical trial that excluded moribund patients and those with severe chronic lung or liver disease, who would be most likely to have poor outcomes. Finally, although our primary analysis demonstrated an independent positive association between the highest AUDIT scores and the odds of death or persistent hospitalization at 90 days, our secondary analyses failed to show significant differences in ventilator, or ICU free days between AUDIT groups in multivariable analyses. This latter finding may suggest that the clinical outcomes for patients in AUDIT zone 4 significantly diverge from those in AUDIT zone 2 only after day 28 of the onset of ALI; alternatively, the number of subjects examined may have had inadequate power to detect differences in these outcome variables.
While it is clear that exposure to cigarette smoke increases the risk of developing ALI in patients with severe chest trauma, the association between smoking and mortality in ALI has not been examined (52). Experimental studies provide potential mechanisms by which cigarette smoking may lead to poor outcomes in ALI (53-56). We did include smoking status in our multiviariate models. However, given recent data that serum biomarkers may more accurately measure exposure to cigarette smoke than a clinical assessment of smoking and that severe alcohol misuse is associated with more severe tobacco dependence, it is possible that our findings may also be due to residual confounding from smoking (57-58). Alternatively, exposure to cigarette smoke may moderate the association between severe alcohol misuse and poor outcomes.
CONCLUSIONS
The administration of the AUDIT questionnaire is feasible in the setting of large, multi-center clinical trials enrolling critically ill patients with ALI and alcohol misuse in such patients remains a significant co-morbidity. The presence of severe alcohol misuse according to AUDIT scores in patients with ALI may aid clinicians by identifying individuals who are at higher risk of death or persistent hospitalization at 90 days. Future studies should aim to clarify our understanding of the potential mechanisms that explain these associations.
Acknowledgments
Supported by National Institutes of Health grants K24-HL-089223, R24-AA-019661-01A1 and National Institutes of Health contract N01 HR56167. Dr. Cecere is funded by a VA HSR&D fellowship (TPM 61-037)
Footnotes
The authors have not disclosed any potential conflicts of interest
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352(6):596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 2.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80(1):44–50. [PubMed] [Google Scholar]
- 3.National Institute on Alcohol Abuse and Alcoholism [September 12, 2012];Helping Patients Who Drink Too Much. 2005 Accessed at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
- 4.Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 5.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 6.Donovan DM, Kivlahan DR, Doyle SR, et al. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction. 2006;101(12):1696–1704. doi: 10.1111/j.1360-0443.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 7.Rubinsky AD, Kivlahan DR, Volk RJ, et al. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108(1-2):29–36. doi: 10.1016/j.drugalcdep.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 9.Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 10.Wind J, Versteegt J, Twisk J, et al. Epidemiology of acute lung injury and acute respiratory distress syndrome in The Netherlands: a survey. Respir Med. 2007;101(10):2091–2098. doi: 10.1016/j.rmed.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 12.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97(6):1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 14.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 15.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The National Heart L. Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Initial Trophic vs Full Enteral Feeding in Patients with Acute Lung Injury. JAMA. 2012 [Google Scholar]
- 18.Matthay MA, Brower RG, Carson S, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Donovan DM, Dunn CW, Rivara FP, et al. Comparison of trauma center patient self-reports and proxy reports on the Alcohol Use Identification Test (AUDIT) J Trauma. 2004;56(4):873–882. doi: 10.1097/01.ta.0000086650.27490.4b. [DOI] [PubMed] [Google Scholar]
- 21.MacKenzie D, Langa A, Brown TM. Identifying hazardous or harmful alcohol use in medical admissions: a comparison of audit, cage and brief mast. Alcohol Alcohol. 1996;31(6):591–599. doi: 10.1093/oxfordjournals.alcalc.a008195. [DOI] [PubMed] [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol-Use Disorders Identification Test (Audit) - Who Collaborative Project on Early Detection of Persons with Harmful Alcohol-Consumption .2. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 23.Babor TF, Higgins-Biddle JC, Saunders JB, et al. The Alcohol Use Disorders Identification Test. [cited September 28, 2010]. 2001. p. 2. [Available from:
- 24.Neumann T, Neuner B, Gentilello LM, et al. Gender differences in the performance of a computerized version of the alcohol use disorders identification test in subcritically injured patients who are admitted to the emergency department. Alcohol Clin Exp Res. 2004;28(11):1693–1701. doi: 10.1097/01.alc.0000145696.58084.08. [DOI] [PubMed] [Google Scholar]
- 25.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 26.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153(3):167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoller JK, Xu M, Mascha E, et al. Long-term outcomes for patients discharged from a long-term hospital-based weaning unit. Chest. 2003;124(5):1892–1899. doi: 10.1378/chest.124.5.1892. [DOI] [PubMed] [Google Scholar]
- 28.Carson SS, Bach PB, Brzozowski L, et al. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 29.Daly BJ, Douglas SL, Gordon NH, et al. Composite outcomes of chronically critically ill patients 4 months after hospital discharge. Am J Crit Care. 2009;18(5):456–464. doi: 10.4037/ajcc2009580. quiz 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Siegelaar SE, Hickmann M, Hoekstra JB, et al. The effect of diabetes on mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2011;15(5):R205. doi: 10.1186/cc10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien JM, Jr., Philips GS, Ali NA, et al. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med. 2004;140(5):338–345. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 33.Freire AX, Bridges L, Umpierrez GE, et al. Admission hyperglycemia and other risk factors as predictors of hospital mortality in a medical ICU population. Chest. 2005;128(5):3109–16. doi: 10.1378/chest.128.5.3109. [DOI] [PubMed] [Google Scholar]
- 34.Kinder LS, Bryson CL, Sun H, et al. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs. 2009;70(2):253–260. doi: 10.15288/jsad.2009.70.253. [DOI] [PubMed] [Google Scholar]
- 35.Bradley KA, Maynard C, Kivlahan DR, et al. The relationship between alcohol screening questionnaires and mortality among male veteran outpatients. J Stud Alcohol. 2001;62(6):826–833. doi: 10.15288/jsa.2001.62.826. [DOI] [PubMed] [Google Scholar]
- 36.Di Castelnuovo A, Costanzo S, Bagnardi V, et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 37.Lembke A, Bradley KA, Henderson P, et al. Alcohol screening scores and the risk of new-onset gastrointestinal illness or related hospitalization. J Gen Intern Med. 2011;26(7):777–782. doi: 10.1007/s11606-011-1688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinsky AD, Sun H, Blough DK, et al. AUDIT-C Alcohol Screening Results and Postoperative Inpatient Health Care Use. J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Deviere J, Content J, Denys C, et al. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 40.Laso FJ, Vaquero JM, Almeida J, et al. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31(5):846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 41.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-232. [DOI] [PubMed] [Google Scholar]
- 42.Burnham EL, Halkar R, Burks M, et al. The effects of alcohol abuse on pulmonary alveolar-capillary barrier function in humans. Alcohol Alcohol. 2009;44(1):8–12. doi: 10.1093/alcalc/agn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkowitz DM, Danai PA, Eaton S, et al. Alcohol abuse enhances pulmonary edema in acute respiratory distress syndrome. Alcohol Clin Exp Res. 2009;33(10):1690–1696. doi: 10.1111/j.1530-0277.2009.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss M, Guidot DM, Wong-Lambertina M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161(2 Pt 1):414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 45.Pacht ER, Timerman AP, Lykens MG, et al. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991;100(5):1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 46.de Wit M, Best AM, Gennings C, et al. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007;31(7):1224–1230. doi: 10.1111/j.1530-0277.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 47.Bard MR, Goettler CE, Toschlog EA, et al. Alcohol withdrawal syndrome: Turning minor injuries into a major problem. J Trauma. 2006;61(6):1441–1445. doi: 10.1097/01.ta.0000245981.22931.43. discussion 1445-1446. [DOI] [PubMed] [Google Scholar]
- 48.Spies CD, Nordmann A, Brummer G, et al. Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesthesiol Scand. 1996;40(6):649–656. doi: 10.1111/j.1399-6576.1996.tb04505.x. [DOI] [PubMed] [Google Scholar]
- 49.Lukan JK, Reed DN, Jr., Looney SW, et al. Risk factors for delirium tremens in trauma patients. J Trauma. 2002;53(5):901–906. doi: 10.1097/00005373-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Chew RB, Bryson CL, Au DH, et al. Are smoking and alcohol misuse associated with subsequent hospitalizations for ambulatory care sensitive conditions? J Behav Health Serv Res. 2011;38(1):3–15. doi: 10.1007/s11414-010-9215-x. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien JM, Jr., Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 52.Calfee CS, Matthay MA, Eisner MD, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones JG, Minty BD, Lawler P, et al. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1(8159):66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 54.Mason GR, Uszler JM, Effros RM, et al. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest. 1983;83(1):6–11. doi: 10.1378/chest.83.1.6. [DOI] [PubMed] [Google Scholar]
- 55.Li XY, Rahman I, Donaldson K, et al. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax. 1996;51(5):465–471. doi: 10.1136/thx.51.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blann AD, McCollum CN. Adverse influence of cigarette smoking on the endothelium. Thromb Haemost. 1993;70(4):707–711. [PubMed] [Google Scholar]
- 57.Hsieh SJ, Ware LB, Eisner MD, et al. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Crit Care Med. 2011;39(1):40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.John U, Meyer C, Rumpf HJ, et al. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38(6):606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]