Abstract
The thalamus plays a role in many different types of cognitive processes and is critical for communication between disparate cortical regions. Given its critical role in coordinating cognitive processes, it is important to understand how its function might be affected by aging. In the present study, we examined whether there are age differences in low-frequency fluctuations during rest in the thalamus. Across independent datasets, we found that the amplitude of low frequency (.01–.10 Hz) oscillations was greater in the thalamus among older than younger adults. Breaking this low frequency range down further revealed that this increase in amplitude with age in the thalamus was most pronounced at the low end of the frequency range (.010–.027 Hz), whereas in the higher low frequency range (.198–.250 Hz) younger adults showed greater amplitude than older adults. These shifts in thalamic low frequency oscillatory activity likely influence the complex dynamics of coordinated brain activity and influence cognitive performance.
Keywords: aging, thalamic oscillatory activity, fALFF
Introduction
Attempts at understanding the brain mechanisms underlying age-related cognitive processing declines have focused on declines in cortical regions. Less attention has been paid to subcortical regions such as the thalamus. Yet, the thalamus plays a central role in cognition; nearly all information into the cortex passes through the thalamus. Furthermore, the thalamus helps to coordinate activity among distant cortical regions.
The thalamus decreases in volume with age, with a linear course of change [1–3] that is associated with diminished cognitive speed [4], and thalamic white matter integrity also declines with age [5–8]. Despite the thalamic structural declines in aging and their association with cognitive speed, functional magnetic resonance imaging (fMRI) studies have found few age differences in overall thalamic activation levels during cognitive tasks [9]. However, one question not addressed by previous neuroimaging research contrasting activity during different cognitive tasks is whether thalamic oscillatory activity changes with age. Oscillatory behavior within the brain provides a mechanism for synchronizing activity across disparate brain regions [10].
In the current study, with two independent datasets, we examined how amplitudes of low frequency oscillations in the thalamus differ with age using as a measure the fractional amplitude of low frequency fluctuations [fALFF; 11]. This measure is the power within a specific frequency range divided by the total power in the entire detectable frequency range. Thus, fALFF provides a normalized measure of specific low-frequency oscillations relative to the rest of the available range. Its sensitivity and specificity to detect spontaneous brain activity is better than the non-normalized ALFF measure [11].
Methods
Subjects and data acquisition
University of Southern California (USC)
Twenty younger (12 males, Mage = 25.35, age range 19–37) and twenty older adults (10 males, Mage = 68.10, age range 61–78) participated after screening for right-handedness and medical, neurological, and psychiatric disorders. All participants provided written informed consent approved by the University of Southern California Institutional Review Board. Imaging data was collected at the USC Dana and David Dornsife Neuroimaging Center with a 3T Siemens MAGNETOM Trio scanner and a 12-channel matrix head coil. The resting-state fMRI data were acquired with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 25 ms, slice thickness = 3 mm, interslice gap = 0 mm, flip angle (FA) = 90°, and field of view (FOV) = 192 mm × 192 mm. During the 312 seconds of scanning, participants were instructed to close their eyes and relax, but not to fall asleep. A high-resolution structural image was also obtained from every participant.
International Consortium on Brain Mapping (ICBM)
Twenty-one younger (7 males, Mage = 22.71, age range 19–31) and twenty-one older adults (10 males, Mage = 66.81, age range 56–85) were obtained from a publicly available dataset of 86 participants’ resting-state fMRI and structural scans [12]. To match the USC dataset in sample size and age range, scans from participants between the ages of 32 and 55 were excluded. In addition, scans that did not have full brain coverage were discarded, leaving two 256-sec resting-state fMRI scans and one anatomical scan per person included in the analyses.
Data preprocessing
Resting-state fMRI scans were processed using scripts released by the 1000 Functional Connectomes Project [13]. Data preprocessing steps included: (1) three-dimensional motion correction, (2) skull-stripping, (3) time series de-spiking, (4) removal of linear trends, (5) spatial smoothing with a 6-mm full-width half-maximum Gaussian kernel, and (6) mean-based intensity normalization by scaling all volumes by a factor of 10,000. An estimate of the linear transformation (12 degrees of freedom) from individual functional space to the standard space (Montreal Neurological Institute’s 152-brain template; isotropic 2-mm voxel size) was also conducted for every resting-state scan using FSL FLIRT [14].
fALFF
For amplitude measures at each voxel we used the fast-Fourier-transform based fALFF, as implemented by Biswal et al. [13]. Using FSL commands, we first transformed the time series of each voxel to the frequency domain and obtaining the power spectrum. The low frequency band (.01–.10 Hz) was then extracted from the power spectrum. At every voxel, the amplitudes across the low frequency band were summed. Next, fALFF was calculated by dividing the value in each voxel by the sum of amplitudes across the whole frequency domain. Across the whole brain, these values yielded three-dimensional fALFF maps for each functional scan. For every functional scan, these three-dimensional maps were normalized to Z-scores and transformed into the standard MNI152 2-mm3 space. Age comparisons were conducted with group-level analyses using flameo (FSL) and the Z-score fALFF maps in standard space. Lastly, easythresh (FSL) was applied for multiple comparisons correction (Z>2.3, corrected cluster significance: p<.05).
fALFF in specific frequency bands
Based on categorizations used in previous research [e.g., 15,16], we subdivided the frequency range into the following bands: slow5 (.010–.027 Hz), slow4 (.027–.073 Hz), slow3 (.073–.198 Hz) and slow2 (.198–.250 Hz; the high frequency bound was constrained by the scan repetition time). With the exception of which frequency range was allowed through the band-pass filter, the procedures for obtaining the five slow fALFF Z-score maps followed that of the initial fALFF analysis covering the .01–.10 Hz range.
We conducted whole brain contrasts of younger and older adults’ fALFF for each of these frequency bands. To summarize the results of these five whole-brain contrasts, we used frequency counts of the voxels, for which younger adults showed significantly higher fALFF than older adults, and vice versa, for each frequency band within standard regions-of-interest (ROIs) of the thalamus. Group and frequency differences in thalamus fALFF were also examined within individually defined thalamus ROIs.
ROI delineation
Structurally defined standard masks of the thalamus were used for the frequency voxel counts. These standard ROIs of the thalamus were defined by the Harvard-Oxford atlas’s (http://www.cma.mgh.harvard.edu/fsl_atlas.html) probabilistic thalamus map at a 50% probability threshold.
Individually defined thalamus ROIs were created by first skull-stripping every anatomical brain using FSL BET [Brain Extraction Tool; 17]. Bilateral thalami were then segmented from each participant’s high resolution structural scan using FSL FIRST [18]. Post-segmentation, manual correction of thalamus masks involved removing erroneous voxels in non-thalamus regions such as ventricles, hippocampus, and brain stem [19]. The final thalamus masks were transformed into standard MNI152 2-mm3 space.
Results
Thalamus structural volume
Volumes of the individually segmented thalami were compared in a 2 (age: younger, older) × 2 (side: right, left) repeated-measures ANOVA. In both data sets, younger adults had significantly greater thalamic volume than older adults; for the USC group older M = 8908.4 mm3, younger M = 10235.2 mm3, F(1,38) = 22.12, p<.001, η2p=.37; for the ICBM group older M = 8895.2 mm3, younger M = 10202.3 mm3, F(1,40) = 30.52, p<.001, η2p=.43. Consistent with previous findings [e.g., 20], in both data sets, there also was significantly greater left than right thalamic volume; USC right M = 9434.8 mm3, left M = 9708.8 mm3, F(1,38) = 17.62, p<.001, η2p=.32; ICBM right M = 9393.5 mm3, left M = 9704.0 mm3, F(1,40) = 42.10, p<.001, η2p=.51. In neither data set did age and side interact significantly.
fALFF in whole-brain contrasts
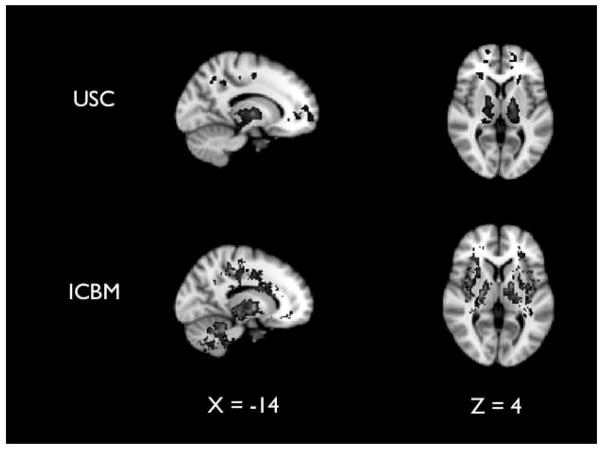
Across both datasets we analyzed, whole-brain analyses revealed greater fALFF values (.01–.10 Hz) in the thalamus for older adults than for younger adults (Fig. 1).
Fig. 1.
In both data sets (USC and ICBM), the fractional amplitude of low frequency fluctuations (the ratio of 0.01–0.1 Hz to the entire frequency range) was significantly greater in older than younger adults in the thalamus.
fALFF in specific frequency bands for whole-brain contrasts
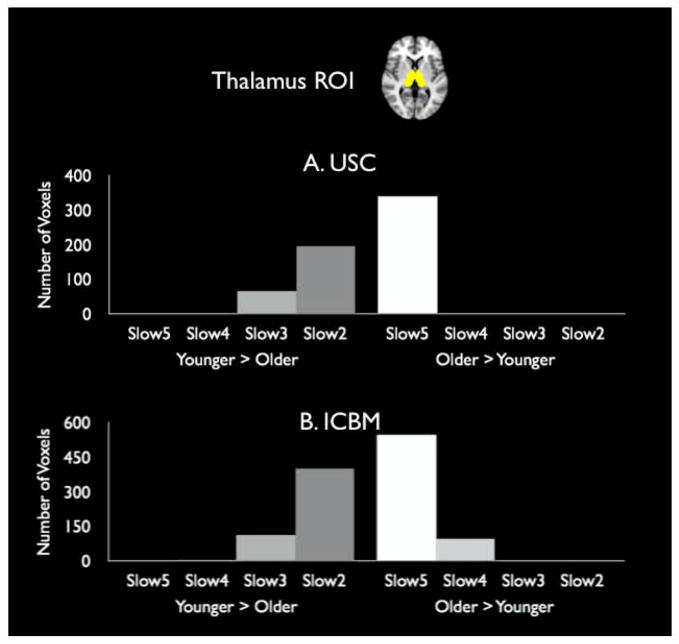
We conducted whole-brain contrasts of younger and older adults’ fALFF separately for four separate frequency bands (.010–.027 Hz [slow5], .027–.073 Hz [slow4], .073–.198 Hz [slow3], and .198–.250 Hz [slow2]). Then we used the bilateral atlas-based thalamus ROI to conduct frequency counts of the number of thalamus voxels that showed significant age differences in fALFF. In the slow5 frequency band, 340 voxels had significantly higher fALFF for older than for younger adults and none showed significantly higher fALFF for younger than for older adults (Fig. 2A). However, this age difference favoring older adults was only seen in this lowest frequency band, and actually reversed in the higher frequency bands, such that younger adults showed higher fALFF than older adults. A chi-square test of these frequency counts revealed that their distribution across frequency bands differed by age group, χ2 = 602.00, p<.001. The same pattern was found in the ICBM data, as well (Fig. 2B), χ2 = 1079.81, p<.001.
Fig. 2.
Number of voxels that showed significant age differences in whole-brain comparisons of fALFF by frequency band for data from A) USC and B) ICBM.
Individual fALFF averages within structural ROIs
The previous analyses compared fALFF values in each voxel across subjects, using a standard atlas-defined thalamus ROI. As older adults showed structural decline in the thalamus, we checked if the results would also be evident when using individually delineated ROIs. Thus, in a secondary analysis, we computed the fALFF for each frequency band averaged across the whole right and left thalamus ROI for each subject, using the thalamus ROIs delineated separately for each individual. We then compared these average fALFF values for younger and older participants. In both datasets, in the slow5 frequency band, older adults had significantly greater fALFF than younger adults (Table 1).
Table 1.
Average fALFF in individually delineated right and left thalamus ROIs for each frequency band for younger and older adults.
| USC Data | ||||
|---|---|---|---|---|
| Left Thalamus | Right Thalamus | |||
| Frequency Band | Younger | Older | Younger | Older |
| Slow5 | −.312 | −.201* | −.308 | −.207* |
| Slow4 | .023 | .051 | .054 | .064 |
| Slow3 | .237 | .165 | .223 | .179 |
| Slow2 | .046 | .002 | .047 | −.017 |
| ICBM Data | ||||
|---|---|---|---|---|
| Left Thalamus | Right Thalamus | |||
| Frequency Band | Younger | Older | Younger | Older |
| Slow5 | −1.044 | −.505* | −1.016 | −.575* |
| Slow4 | .141 | .456 | .186 | .371 |
| Slow3 | .613 | .600 | .594 | .600 |
| Slow2 | −.003* | −.601 | −.157* | −.633 |
Significantly higher fALFF values than the other age group, p<.05.
In addition, in the ICBM dataset, older adults had significantly lower fALFF in the slow2 frequency band. The middle frequency bands showed no significant age differences. Thus, consistent with the whole-brain analyses, older adults showed greater fALFF than younger adults in the lower frequency band but not in higher frequency bands. In addition, we checked whether the structural volume of the thalamus was associated with fALFF values. When controlling for age, there were no significant correlations between thalamus volume (either right or left) and the fALFF of the four frequency bands (of either the right or left thalamus). Thus, the age-related changes in fALFF were not accounted for by structural volume declines.
Discussion
In two independent data sets we found that low frequency (.01-.10 Hz) oscillations had greater amplitudes in older adults than in younger adults in the thalamus. When we examined the amplitude of oscillations separately for different frequency bands, a shift was evident across age within the thalamus, such that older adults had greater amplitudes than younger adults at the slowest frequency band categorization (the “slow5” band from .010–.027 Hz) whereas younger adults had greater amplitudes than older adults at the fastest frequency band categorization (the “slow2” band from .198-.250 Hz).
Low frequency oscillations have been shown in many studies to reveal functional brain networks [21,22]. The age differences we found in this study, in low frequency oscillations in the thalamus, suggest that older (compared to younger) adults will also show changes in functional connectivity with the thalamus. If so, these changes could have a broad impact on cognition, memory, and behavior, as the thalamus plays a key role in much of the communication between different cortical regions [21], including the hippocampal memory network [24].
Thus, questioning how functional connectivity with the thalamus might change with age is an important next question for this line of research. A typical approach for finding functional connectivity of an ROI is to aggregate the pattern of activity in that region and correlate the timeline of that activity with every other voxel in the brain. As can be seen in Fig. 1, the age differences in low frequency oscillations were evident in most voxels within the thalamus. However, as the thalamus is comprised of clearly delineated subregions that have distinct connections with different cortical regions [25], it will be important that future studies use approaches that do not assume the whole thalamus has similar patterns of functional connectivity.
Oscillatory behavior not only supports important functions including biasing input selection and replaying recent inputs to consolidate memory, but it also provides a mechanism for synchronizing diverse regional activities in the brain. When studying coordination of brain activity across different regions, a key region to inspect is the thalamus; the thalamus is also responsible for processing new perceptual inputs. Thus, our characterization of how oscillatory dynamics within the thalamus change with age is especially important for understanding age-related change in coordinated activity across disparate brain regions. The current findings reveal age-related changes in low frequency fluctuations in the thalamus. Future work should fully investigate potential age-related changes in oscillatory activity (by looking at both higher and lower frequencies) through simultaneous EEG and fMRI recordings of younger and older adults during resting state.
Acknowledgments
Source of funding: Grants RO1AG025340 and K02AG032309 from the National Institute on Aging.
Footnotes
Conflicts of interest: None declared.
References
- 1.Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 3.Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PAM, Vuurman E, Uylings HBM, et al. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging: A magnetic resonance imaging-based volumetric analysis. Cognitive Brain Research. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, et al. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF-suppression. J Magn Reson Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- 7.Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. Am J Roentgenol. 2000;175:425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- 8.Ota M, Obata T, Akine Y, Ito H, Matsumoto R, Ikehira H, et al. Laterality and aging of thalamic subregions measured by diffusion tensor imaging. NeuroReport. 2007;18:1071–1075. doi: 10.1097/WNR.0b013e3281c10e27. [DOI] [PubMed] [Google Scholar]
- 9.Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2011;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 11.Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans AC. ICBM data. 2009 Retrieved from http://wwwnitrcorg/frs/?group_id=296.
- 13.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 15.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 16.Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: Complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooteman W, Cretsinger K. Iowa Mental Health Clinical Research Center Tracing Guidelines. 2005. [Google Scholar]
- 20.Murphy DGM, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992;49:839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 21.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]