Abstract
Purpose of review
Focal therapy for prostate cancer is emerging as a management option between active surveillance and radical treatments. In this article we present two of the most important imaging modalities in focal therapy, multiparametric MRI and Ultrasonography. We review recent advances within these two platforms.
Recent findings
State-of-the-art imaging in all phases of focal therapy is essential for treatment safety. In patient selection, treatment guidance and follow-up, different aspects of imaging are important. mpMRI is an imaging technology with high imaging resolution and contrast. This makes it an excellent technology for patient selection and treatment planning and follow-up. Ultrasound has the unique property of real time image acquisition. This makes it an excellent technology for real time treatment guidance. There are multiple novelties in these two platforms that have increased the accuracy considerably. Examples in ultrasound are: CEUS, elastography, shear-wave elastography and histoscanning. In mpMRI these advantages consist of multiple sequences combined to one image and MR thermometry.
Summary
Standardization of mpTRUS and mpMRI is of paramount importance. For targeted treatment and follow-up, a good negative predictive value of the test is important. There is much to gain both of these developing fields and imaging accuracy of the two platforms is comparable. Standardization in conduct and interpretation, 3D reconstruction and fusion of the two platforms can make focal therapy for prostate cancer standard of care.
Keywords: Prostate cancer, Focal Therapy, MRI, Ultrasonography
1. Introduction
Following the discovery of Prostate Specific Antigen (PSA), the incidence of prostate cancer increased drastically, whereas cancer specific mortality rate remained unchanged at 5% [1]. Concerns have especially been raised about the increasing detection of low- and intermediate risk prostate cancer, since active treatment may not improve disease related survival but on the other hand may impair quality of life significantly. Consequently, deferred treatment such as active surveillance or watchful waiting are appealing management solutions which maximize quality of life [2]. However, these management options have their disadvantages. Biopsy sampling errors, particularly those leading to missed anterior tumors, and consequently undergrading of the tumor, is the biggest limitation of active surveillance[3]. Furthermore, repeat biopsies can result in increased costs, pain and infections associated with increased hospitalizations [4]. A cancer diagnosis is stressful for the patient and not actively treating the disease may, in susceptible individuals, cause additional anxiety [5]. Finally, compliance is also an issue, with some studies showing as few as 53% of patients in specific subgroups comply with protocol mandated biopsy at 1 year [6]. Focal therapy can offer an alternative in patients with low and intermediate risk prostate cancer, since it offers cancer control on the one side without the side effects of radical treatments on the other side [7][8][9]**[10]*.
For focal therapy, high end, imaging is of paramount importance. The clinician has to be positive that the targeted tumor is low- or intermediate risk prostate cancer and that there is no extra capsular extension. During treatment, the clinician should be certain that the targeted tumor is being totally ablated and that outside the targeted area, no significant tumor resides. In the follow-up period, recurrences or residual tumor should be clearly recognized on imaging. The two most important imaging modalities for imaging in focal therapy are mpMRI and mpUS [11].
In focal therapy, roughly 3 phases can be distinguished, each demanding different aspect from imaging modalities. These phases include: 1) Patient selection and treatment planning, 2) Treatment guidance and 3) follow-up after focal therapy. Definitions of these phases differ among literature, therefore we defined the definitions as used in this review:
Patient selection: Selection of a patient with low or intermediate prostate cancer (≤T2, Gleason Score ≤ 4+3, and PSA < 20ng/mL), with a target lesion confined to one lobe of the prostate.
Treatment guidance: Real time guidance of the focal treatment modality to the targeted lesion.
Follow-up: After treatment control that there is no residual tumor, or after a period of time, no recurrent tumors.
The review focusses on imaging for focal therapy in these three different phases of this treatment form.
2. Imaging for patient selection
The requirements of imaging in patient selection are to identify patients with low-to intermediate risk prostate carcinoma. Moreover, the targeted area should be identifiable (the tumor should be clearly visible on imaging). Furthermore, outside of the targeted area should not be a significant other lesion. It is therefore essential to have a high-resolution 3D imaging technology available that identifies in high accuracy the location, size, grade and stage of the tumor. Moreover, negative predictive value of the non-affected prostate lobe should be high.
Magnetic resonance imaging (MRI) has the potential to fulfill the strong imaging requirements for patient selection in focal therapy. MRI works with a magnetic field varying between 0.5 and 7 Tesla (T). Most systems for prostate scanning use 1.5 T or 3T. The technology relies on detection of a radiofrequency signal emitted by excited hydrogen atoms in the body, using the energy from an oscillating magnetic field applied at the appropriate resonant frequency [12]. Until recently, the diagnostic performance of MRI was too low to implement the technology into the diagnostic workup leading to focal therapy of prostate cancer. However, evidence coming from centers of excellence, support an accurate diagnostic performance of MRI, provided that multiple sequences are used and that their outputs are combined in a multiparametric MRI (mpMRI). These sequences include T2 weighted (T2w) imaging, dynamic contrast enhanced (DCE) imaging, diffusion weighted imaging (DWI), and sometimes proton spectroscopic imaging (MRSI) (figure 1). Disagreement among experts in the field of uro-radiology about the conduct, interpretation and reporting of mpMRI have prohibited the formation of uniformly comparable literature on diagnostic accuracy and hence the formation of guidelines. Various consensus projects have therefore been initiated to standardize the conduct and reporting of mpMRI in prostate cancer [13][14][15][16]*.
Figure 1. 70 year old patient with Gleason 3+3 prostate carcinoma.
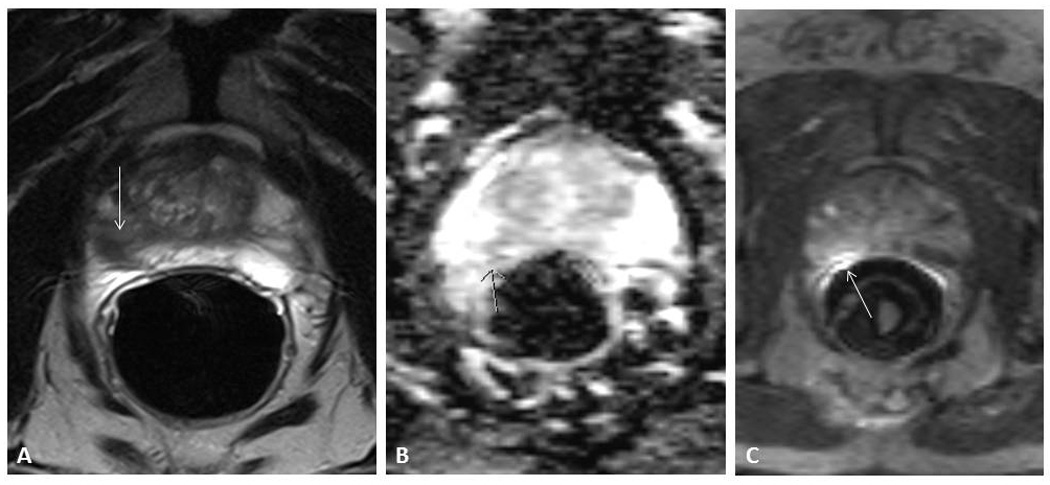
A: T2 Weighted image, with hypodense lesion (arrow) B: Diffusion Weighted Image. The arrow indicates an area with diffusion restriction C: Dynamic Contrast Enhanced Image. The arrow indicates an enhancing lesion.
The key difficulty in medical imaging research is the comparison of images to histopathology. It can be challenging to relate the images on MRI to the histopathology specimens, because of shrinkage and free hand slicing. Several solutions have been posed to overcome these challenges. Orzyck et al. developed deformable histopathology mpMRI fusion computer software based on 22 landmarks in the specimens. They managed to reach an improvement in matching accuracy of 32% over rigid comparison methods [17]. Trivedi et al. developed a 3D histopathology analysis tool using 3D printed customized moulds based on pre-operative mpMRI data, corrected for shrinkage, for slicing of the histopathological specimens in exactly the same plane as the mpMRI scanning [18]. Using this methods, Turkbey et al. determined the diagnostic accuracy for the separate sequences used in mpMRI (T2w, DWI, DCE, MRS) in a prospective study including 45 patients [19]. The results are depicted in table 1.
Table 1. Diagnostic accuracies of mpMRI sequences and Ultrasound modalities (adapted from [19]).
T2W: T2 Weighted MR imaging, ADC: Apparent Diffusion coefficient, used in Diffusion Weighted Imaging (DWI), MRS: Magnetic Resonance Spectroscopy, DCE: Dynamic Contrast Enhanced MRI, NPV: Negative predictive value, PPV: positive predictive value. This table is based on different studies with different amounts of patients and different endpoints.
| MRI | Ultrasound | ||
|---|---|---|---|
| Sensitivity (p value) | Sensitivity | ||
| T2W | 0.58 (0.04)[19] | CEUS | 0.731 [20] |
| ADC | 0.53 (0.04)[19] | Histoscanning | 0.9 [21] |
| MRS | 0.16 (0.04)[19] | Elastography | 0.608 [22] |
| DCE | 0.38 (0.05)[19] | Shear Wave Elastography | 0.962 [23] |
| Specificity (p value) | Specificity | ||
| T2W | 0.93 (0.01)[19] | CEUS | 0.873 [20] |
| ADC | 0.95 (0.01)[19] | Histoscanning | 0.7 [21] |
| MRS | 1 (0)[19] | Elastography | 0.684 [22] |
| DCE | 0.98 (0.01)[19] | Shear Wave Elastography | 0.962 [23] |
| NPV (p value) | NPV | ||
| T2W | 0.9 (0.01)[19] | CEUS | 0.904 [20] |
| ADC | 0.89 (0.01)[19] | Histoscanning | 0.82 [21] |
| MRS | 0.83 (0.01)[19] | Elastography | 0.878 [22] |
| DCE | 0.87 (0.01)[19] | Shear Wave Elastography | 0.996 [23] |
| PPV (p value) | PPV | ||
| T2W | 0.7 (0.05)[19] | CEUS | 0.664 [20] |
| ADC | 0.73 (0.04)[19] | Histoscanning | 0.83 [21] |
| MRS | 0.93 (0.04)[19] | Elastography | 0.324 [22] |
| DCE | 0.86 (0.04)[19] | Shear Wave Elastography | 0.694 [23] |
By using advanced histopathology matching, the validity of mpMRI accuracy research improved substantially. The same research group demonstrated that 3T mpMRI could accurately estimate tumor volume independent of Gleason score, using this method, in 135 patients. These results are valuable in focal therapy treatment planning as well as follow-up after therapy [24]*. Another way to improve the diagnostic performance of MRI is to increase MRI field strength. In the prostate, only few studies have yet been done regarding 7T mpMRI. The advantage of a higher field strength is a higher signal to noise ratio. Rosenkrantz et al. demonstrated the feasibility of 7T MRI in 2 patients with biopsy proven prostate cancer. They demonstrated a signal to noise ratio gain of 2.1 at 7T MRI vs 3T MRI and images showed excellent visual correlation with the radical prostatectomy specimens [25]. Several studies demonstrated the safety and feasibility of MR spectrocopic imaging at 7T in patients with biopsy proven prostate cancer. 7 T MRI rendered a safe and promising technology [26][27]. Another promising novelty is Correlated Diffusion Imaging (CDI). CDI takes the advantage of multiple gradient pulse strengths and timings. This not only reduces dependency on the way diffusion gradient pulses are applied, but it also improves delineation between cancerous and healthy tissue. One study demonstrated an impressive improvement in area under the ROC curve for CDI over normal diffusion weighted imaging (0.9789 vs 0.9183) in 20 patients with prostate cancer [28]**. The future of mpMRI lies also in standardization of conduct and interpretation and quantification of the results. These aspects will make the technology more comparable, more reproducible and less operator dependent. Maas et al. demonstrated good performance with quantitative evaluation of computed high b value diffusion weighted magnetic resonance imaging of the prostate [29]. Artan et al. proposed the first mechanism of automated prostate cancer localization on multiparametric MRI. The results in the first 8 patients look promising, but have to be investigated in larger trials [30].
Ultrasonography (US) works with oscillating sound pressure waves that move through the body and reflect on surfaces in between dense and soft structures. In the past decennia there were multiple advances in the field of ultrasound, which increased sensitivity and specificity of the technology for the detection of prostate carcinoma [31]*. The major benefit of ultrasound it that the technology is real-time, so that information can be interpreted during the investigation. Frequency varies between 2mHz and 60MHz with as a general rule: the higher the frequency, that higher the imaging resolution. However, penetration depth decreases with increasing frequency. In the field of ultrasound, similar developments as with MRI are emerging. Detection rates of prostate cancer in grey-scale ultrasound are low, but when the outputs of several sequences are combined, the accuracy would presumably go up. Advances in the field of ultrasound imaging of the prostate include: Contrast Enhanced Ultrasonography (CEUS) (figure 2), Elastography, Shear Wave Elastography, Power Doppler and Histoscanning. A consensus meeting about the use of Ultrasound modalities in focal therapy concluded that the ultrasonography alone was insufficiently accurate for patient selection in 2011 [32]. In the past years however, much research has been done into different modalities of ultrasound, which gives the technology a multiparametric character.
Figure 2. CEUS in the apex of the prostate.
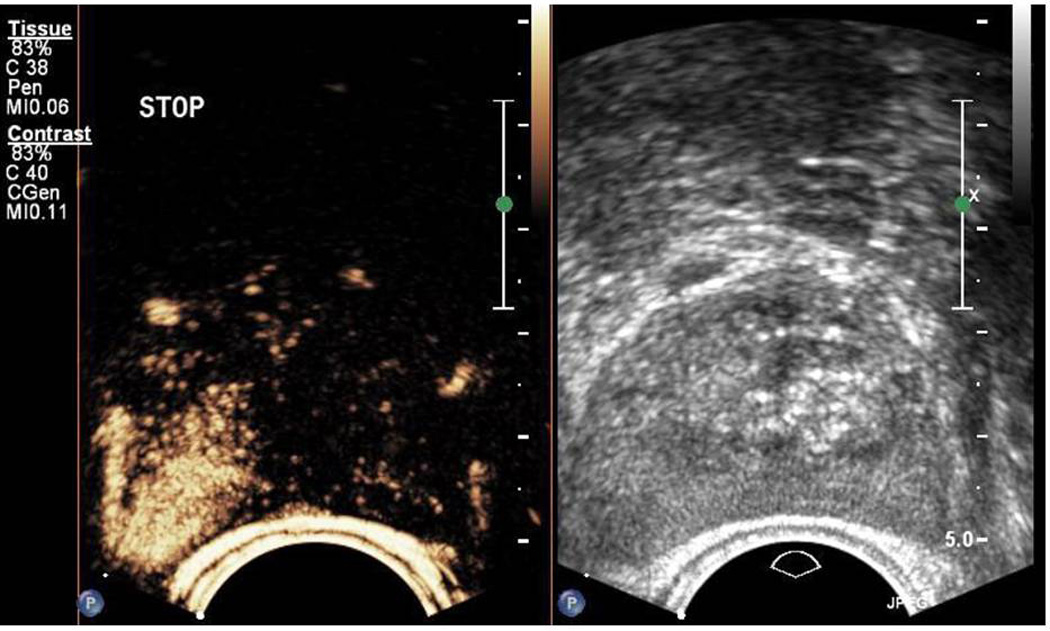
On the right we can see the grey-scale TRUS image. On the left, the contrast enhanced picture is visible, 28 seconds after injection of contrast medium. An area of enhancement is seen on the right apex indicating suspicion for tumor.
In the past it has been suggested that end fire US probes have a higher detection rate than side fire probes. A recent article by Rom et al. 2012 showed that the cancer detection rate in TRUS does not depend on the type of ultrasound probe you use (end-fire 34.3% or side fire probe 34.4%) in 300 patients [33]. Contrast Enhanced Ultrasonography with contrast tuned imaging technology has a greater sensitivity (73.1%) and accuracy (83.7%) than conventional grey scale ultrasound (50.9% and 78.8%), and power Doppler (48.3% and 77.7%). This study was performed in 150 patients with targeted biopsy as an endpoint [20]. Another prospective study investigated the hypothesis whether pre-treatment with dutasteride would improve the detection rate of CEUS guided biopsies. Receiver Operator Characteristics (ROC)-analysis showed that CEUS increased detection of cancer compared to grey scale ultrasound (sensitivity went up from 0.60 to 0.64 for all lesions). For high-grade lesions however, sensitivity improved from 0.74 to 0.80. These numbers were even higher for high-grade lesions with more than 50% core involvement (0.83 to 0.90). Dutasteride pre-treatment did not improve the detection rate [34]. Zalesky et al. described an area under the ROC curve of 0.776 for power Doppler compared to 0.67 for normal grey scale, indicating that the sensitivity and specificity for power Doppler is significantly higher than grey scale ultrasound. This prospective study consisted of 146 patients [35]. Brock et al. described in 2012 in a prospective study with 353 patients that real time elastography-guided biopsies revealed more prostate cancer (60.8 and 68.4 sensitivity and specificity) than grey scale ultrasound guided biopsies (15 and 92.3 sensitivity and specificity) [22]. Simmons et al. described the diagnostic accuracy of Histoscanning. 31 patients were included and results were compared to radical prostatectomy. Sensitivity of 93% was found [21]. A novelty in the field of elastography is the invention of shear wave elastography. In shear wave elastography, the ultrasound probe automatically generates standardized pressure waves, instead of depending on the pressure wave manually generated by the physician. In a prospective study of 53 patients Barr et al. demonstrated a sensitivity of 96.2% with a specificity of 96.2% PPV of 69.4, NPV of 99.6 in patients with elevated PSA and/or abnormal DRE [23]**. Combining the outputs of these different ultrasound modalities into a standardized, automated multiparametric TRUS, can be very promising for the diagnostic accuracy of ultrasound. Combined data on sensitivity and specificities of the different ultrasonography modalities can be found in table 1.
3. Imaging for treatment guidance
The goal of imaging during treatment guidance is to get the modality of focal therapy to the targeted area. For this, real time orientation is essential. An ideal combination would be the high resolution and contrast of multiparametric MRI with the real time feedback of ultrasound.
There have been some recent advances in the field
Traditionally, ultrasound is the technology most used as a treatment modality in focal therapy. However, MRI guided technologies are used more and more frequently. In 2012 Lindner et al. described the first case of MRI guided High Frequency Focused Ultrasound (HIFU) ablation of prostate cancer. The major advantage of the procedure is that the tumor can be accurately targeted with small margins on MRI and the ablation zone can be accurately monitored during treatment. Disadvantage is that the technique is time consuming [36]. Bovers et al. provided an overview of MRI guided focal technologies in 2012. Conclusions of this review article were: currently MRI-guided laser ablation and MRI guided HIFU are the most promising options for focal treatment of the prostate in patients with prostate cancer. Other techniques- that is, cryosurgery, microwave ablation, and radiofrequency ablation – are, for several and different reasons, less suitable for MRI guided focal therapy of the prostate [37]*.
A relatively new technology for image guided thermal focal therapy is MR thermometry. With this technology, the temperature of tissue can be exactly monitored during ablative therapy. Partanen et al. investigated the technology in dogs using transurethral focused ultrasound monitored by MR thermometry. The technology rendered safe and promising for use in humans [38].
In 2013, Kuru et al. demonstrated the accuracy of TRUS-MRI fused transperineal prostate biopsies. They proved a high detection rate of clinically significant tumors using this technology [39]. A TRUS-MRI elastic fusion technology with excellent accuracy was also demonstrated by Ukimura et al. in 2012 [40]. It is expected that TRUS-MRI fusion will gain field not only in targeted biopsy, but also in focal therapy treatment guidance in future. Results of the first use of TRUS-MRI fusion in focal therapy guidance are already emerging [41].
4. Imaging for follow-up
Imaging for follow-up after focal therapy should ideally be fast, accurate, insensitive to treatment artefacts and should be able to distinguish ablated area from tumorous tissue. The images should be comparable to previous imaging, to accurately detect recurrent/residual lesions. Ideally, the technology is minimally invasive, because imaging for follow-up has to be done repetitively.
There is little evidence about imaging in the follow up after focal therapy of the prostate
Last year a consensus project was set up along a 45 member panel of urologists and radiologists, and it was decided that for follow-up of focal therapy should start with a mpMRI after 6 months, followed by a yearly mpMRI. mpMRI findings should be confirmed by targeted biopsy before re-treatment [16]*.
Few studies have been done on imaging modalities in the follow-up after focal therapy. One of these studies is a retrospective work by Punwani et al. in 2012, in which 26 patients had a DCE-MRI and targeted biopsy of the prostate after whole gland High Intensity focused Ultrasound treatment. Sensitivity ranged between 73% and 87% between 3 readers, whereas specificity ranged between 73% and 82%. These results were comparable with pre-biopsy PSA levels [42].
A retrospective study with MRSI showed that most recurrences after focal radiation therapy occur at the same site as the dominant primary tumor at baseline. This suggest that supplementary focal therapy aimed at enhancing lesions would be a rational addition to management [43].
Vescovo et al. evaluated DCE-MRI in a prospective study in 25 patients. In all patients a rim of enhancement alongside the targeted area was observed on DCE-MRI, one month after treatment, which typically disappeared on later DCE MRIs. 4 months after treatment, the prostate had significantly reduced volume (average 61% volume reduction) [44]*. Decision support systems (DSS) for mpMRI of the prostate are being designed to make reading more standard and less objective. The DSS provides a probability map for peripheral zone prostate tumors based on endorectal mpMRI. These probability maps may aid in reaching a higher accuracy and more comparable outcomes in the follow-up of focal therapies [45].
5. Conclusions and future perspectives
State-of-the-art imaging in all phases of focal therapy is essential for treatment safety. In patient selection, treatment guidance and follow-up, different aspects of imaging are important. Not only a high PPV is required, but also a high NPV to rule out significant cancer in other parts of the prostate. mpMRI is an imaging technology with high imaging resolution and contrast. This makes it an excellent technology for patient selection and treatment planning and follow-up. Ultrasound has the unique property of real time image acquisition. This makes it an excellent technology for real time treatment guidance.
There are multiple novelties in these two platforms that have increased the accuracy considerably. Examples in ultrasound are: CEUS, elastography, shear-wave elastography and Histoscanning. In mpMRI these advantages consist of multiple sequences combined to one image (mpMRI) and MR thermometry. Standardization of mpTRUS and mpMRI is of paramount importance. There is much to gain in these two fields and imaging accuracy of the two platforms is comparable. Standardization in conduct and interpretation, 3D reconstruction and fusion of the two platforms can make focal therapy a step closer to standard of care.
Key points.
Multiparametric MRI is the standard of imaging in patient selection for focal therapy
Real time treatment guidance in focal therapy is often performed with Ultrasound
Novelties as Histoscanning, Contrast Enhanced Ultrasound, Real-Time Elastography and Shear Wave Elastography, significantly improve cancer detection rates over grey scale ultrasonography.
Apart from a high positive predictive value, a high negative predictive value is needed to rule out prostate cancer in the part of the prostate that is not targeted.
Standardization and automation in conduct and interpretation of imaging will lead to a higher accuracy.
Acknowledgments
Disclosures:
PA Pinto receives an unrestricted research grant from National Institutes of Health (NIH)
BG Muller and W van den Bos receive an unrestricted research grant from the “Cure for Cancer Foundation”
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA. Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid H-P, van der Kwast T, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L. Active surveillance for prostate cancer: a review. Curr. Urol. Rep. 2010;11:165–171. doi: 10.1007/s11934-010-0110-z. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J. Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Bergh RCN, Essink-Bot M-L, Roobol MJ, Wolters T, Schröder FH, Bangma CH, Steyerberg EW. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115:3868–3878. doi: 10.1002/cncr.24446. [DOI] [PubMed] [Google Scholar]
- 6.Marberger M, Barentsz J, Emberton M, Hugosson J, Loeb S, Klotz L, Koch M, Shariat SF, Vickers A. Novel approaches to improve prostate cancer diagnosis and management in early-stage disease. BJU Int. 2012;109(Suppl):1–7. doi: 10.1111/j.1464-410X.2011.10870.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed HU, Emberton M. Active surveillance and radical therapy in prostate cancer: can focal therapy offer the middle way? World J. Urol. 2008;26:457–467. doi: 10.1007/s00345-008-0317-5. [DOI] [PubMed] [Google Scholar]
- 8.Valerio M, Ahmed HU, Emberton M, Lawrentschuk N, Lazzeri M, Montironi R, Nguyen PL, Trachtenberg J, Polascik TJ. The Role of Focal Therapy in the Management of Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2013;44:1–20. doi: 10.1016/j.eururo.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickinson L, Ahmed HU, Kirkham aP, Allen C, Freeman A, Barber J, Hindley RG, Leslie T, Ogden C, Persad R, Winkler MH, Emberton M. A multi-centre prospective development study evaluating focal therapy using high intensity focused ultrasound for localised prostate cancer: The INDEX study. Contemp. Clin. Trials. 2013;36:68–80. doi: 10.1016/j.cct.2013.06.005. The first multicenter, prospective clinical trial with medium term follow-up in cancer control and quality of life. Important for validation of focal therapy as standard of care.
- 10. Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, Scott R, Allen C, Van der Meulen J, Emberton M. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13:622–632. doi: 10.1016/S1470-2045(12)70121-3. The first extensive trial to prove focal therapy a safe treatment option with little side effects.
- 11.Hoang AN, Volkin D, Yerram NK, Vourganti S, Nix J, Linehan WM, Wood B, Pinto Pa. Image guidance in the focal treatment of prostate cancer. Curr. Opin. Urol. 2012;22:328–335. doi: 10.1097/MOU.0b013e32835482cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur. Radiol. 2005;15:946–959. doi: 10.1007/s00330-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham aPS, Haslam P, Keanie JY, McCafferty I, Padhani aR, Punwani S, Richenberg J, Rottenberg G, Sohaib A, Thompson P, Turnbull LW, Kurban L, Sahdev A, Clements R, Carey BM, Allen C. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin. Radiol. 2013;68:1016–1023. doi: 10.1016/j.crad.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, Heijmink SW, Hoskin PJ, Kirkham A, Padhani AR, Persad R, Puech P, Punwani S, Sohaib AS, Tombal B, Villers A, van der Meulen J, Emberton M. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur. Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 16. Muller B, van den Bos W, Brausi M, Cornud F, Gontero P, Kirkham A, Pinto P, Polascik TJ, Rastinehad AR, de Reijke T, de la Rosette J, Ukimura O, Villers A, Walz J, Wijkstra H, Marberger M. The Role of Multiparametric Magnetic Resonance Imaging in Focal Therapy for Prostate Cancer: A Delphi Consensus Project. BJU Int. 2013:1–24. doi: 10.1111/bju.12548. Structured consensus meeting about mpMRI of the prostate in focal therapy.
- 17.Orczyk C, Rusinek H, Rosenkrantz aB, Mikheev A, Deng F-M, Melamed J, Taneja SS. Preliminary experience with a novel method of three-dimensional co-registration of prostate cancer digital histology and in vivo multiparametric MRI. Clin. Radiol. 2013;68:e652–e658. doi: 10.1016/j.crad.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi H, Turkbey B, Rastinehad AR, Benjamin CJ, Bernardo M, Pohida T, Shah V, Merino MJ, Wood BJ, Linehan WM, Venkatesan AM, Choyke PL, Pinto Pa. Use of patient-specific MRI-based prostate mold for validation of multiparametric MRI in localization of prostate cancer. Urology. 2012;79:233–239. doi: 10.1016/j.urology.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, Pang Y, Daar D, Benjamin C, McKinney YL, Trivedi H, Chua C, Bratslavsky G, Shih JH, Linehan WM, Merino MJ, Choyke PL, Pinto Pa. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J. Urol. 2011;186:1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie SW, Li HL, Du J, Xia JG, Guo YF, Xin M, Li FH. Contrast-enhanced ultrasonography with contrast-tuned imaging technology for the detection of prostate cancer: comparison with conventional ultrasonography. BJU Int. 2012;109:1620–1626. doi: 10.1111/j.1464-410X.2011.10577.x. [DOI] [PubMed] [Google Scholar]
- 21.Simmons LaM, Autier P, Zát’ura F, Braeckman J, Peltier A, Romic I, Stenzl A, Treurnicht K, Walker T, Nir D, Moore CM, Emberton M. Detection, localisation and characterisation of prostate cancer by prostate HistoScanning(™) BJU Int. 2012;110:28–35. doi: 10.1111/j.1464-410X.2011.10734.x. [DOI] [PubMed] [Google Scholar]
- 22.Brock M, von Bodman C, Palisaar RJ, Löppenberg B, Sommerer F, Deix T, Noldus J, Eggert T. The impact of real-time elastography guiding a systematic prostate biopsy to improve cancer detection rate: a prospective study of 353 patients. J. Urol. 2012;187:2039–2043. doi: 10.1016/j.juro.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 23. Barr RG, Memo R, Schaub CR. Shear wave ultrasound elastography of the prostate: initial results. Ultrasound Q. 2012;28:13–20. doi: 10.1097/RUQ.0b013e318249f594. First trial on shear-wave elastography in the prostate. By using a standardized device generated pressure wave, the accuracy of shear-wave elastography is higher than real time elastography, making it an ultrasound modality with very high accuracy.
- 24. Turkbey B, Mani H, Aras O, Rastinehad AR, Shah V, Bernardo M, Pohida T, Daar D, Benjamin C, McKinney YL, Linehan WM, Wood BJ, Merino MJ, Choyke PL, Pinto Pa. Correlation of magnetic resonance imaging tumor volume with histopathology. J. Urol. 2012;188:1157–1163. doi: 10.1016/j.juro.2012.06.011. Trial that proves mpMRI can accurately estimate index tumor volume.
- 25.Rosenkrantz AB, Zhang B, Ben-Eliezer N, Le Nobin J, Melamed J, Deng F-M, Taneja SS, Wiggins GC. T2-weighted prostate MRI at 7 tesla using a simplified external transmit-receive coil array: Correlation with radical prostatectomy findings in two prostate cancer patients. J. Magn. Reson. Imaging. 2013;00:1–7. doi: 10.1002/jmri.24511. [DOI] [PubMed] [Google Scholar]
- 26.Kobus T, Bitz AK, van Uden MJ, Lagemaat MW, Rothgang E, Orzada S, Heerschap A, Scheenen TWJ. In vivo 31P MR spectroscopic imaging of the human prostate at 7 T: safety and feasibility. Magn. Reson. Med. 2012;68:1683–1695. doi: 10.1002/mrm.24175. [DOI] [PubMed] [Google Scholar]
- 27.Arteaga de Castro CS, van den Bergen B, Luijten PR, van der Heide Ua, van Vulpen M, Klomp DWJ. Improving SNR and B1 transmit field for an endorectal coil in 7 T MRI and MRS of prostate cancer. Magn. Reson. Med. 2012;68:311–318. doi: 10.1002/mrm.23200. [DOI] [PubMed] [Google Scholar]
- 28. Wong A, Glaister J, Cameron A, Haider M. Correlated diffusion imaging. BMC Med. Imaging. 2013;13:26. doi: 10.1186/1471-2342-13-26. First trial being conducted on Correlated Diffusion imaging in the prostate. CDI improves the accuracy of DWI.
- 29.Maas MC, Fütterer JJ, Scheenen TWJ. Quantitative evaluation of computed high B value diffusion-weighted magnetic resonance imaging of the prostate. Invest. Radiol. 2013;48:779–786. doi: 10.1097/RLI.0b013e31829705bb. [DOI] [PubMed] [Google Scholar]
- 30.Artan Y, Oto A, Yetik IS. Cross-device automated prostate cancer localization with multiparametric MRI. IEEE Trans. Image Process. 2013;22:5385–5394. doi: 10.1109/TIP.2013.2285626. [DOI] [PubMed] [Google Scholar]
- 31. Ghai S, Toi A. Role of transrectal ultrasonography in prostate cancer. Radiol. Clin. North Am. 2012;50:1061–1073. doi: 10.1016/j.rcl.2012.08.007. Overview of studies up to 2012 about ultrasound modalities for detection of prostate cancer.
- 32.Smeenge M, Barentsz J, Cosgrove D, de la Rosette J, de Reijke T, Eggener S, Frauscher F, Kovacs G, Matin SF, Mischi M, Pinto P, Rastinehad A, Rouviere O, Salomon G, Polascik T, Walz J, Wijkstra H, Marberger M. Role of transrectal ultrasonography (TRUS) in focal therapy of prostate cancer: report from a Consensus Panel. BJU Int. 2012;110:942–948. doi: 10.1111/j.1464-410X.2012.11072.x. [DOI] [PubMed] [Google Scholar]
- 33.Rom M, Pycha A, Wiunig C, Reissigl A, Waldert M, Klatte T, Remzi M, Seitz C. Prospective randomized multicenter study comparing prostate cancer detection rates of end-fire and side-fire transrectal ultrasound probe configuration. Urology. 2012;80:15–18. doi: 10.1016/j.urology.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 34.Halpern EJ, Gomella LG, Forsberg F, McCue Pa, Trabulsi EJ. Contrast enhanced transrectal ultrasound for the detection of prostate cancer: a randomized, double-blind trial of dutasteride pretreatment. J. Urol. 2012;188:1739–1745. doi: 10.1016/j.juro.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalesky M, Urban M, Smerhovský Z, Zachoval R, Lukes M, Heracek J. Value of power Doppler sonography with 3D reconstruction in preoperative diagnostics of extraprostatic tumor extension in clinically localized prostate cancer. Int. J. Urol. 2008;15:68–75. doi: 10.1111/j.1442-2042.2007.01926.x. discussion 75. [DOI] [PubMed] [Google Scholar]
- 36.Lindner U, Ghai S, Spensieri P, Hlasny E, Van Der Kwast TH, McCluskey SA, Haider MA, Kucharczyk W, Trachtenberg J. Focal magnetic resonance guided focused ultrasound for prostate cancer: Initial North American experience. Can. Urol. Assoc. J. 2012;6:E283–E286. doi: 10.5489/cuaj.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bomers JGR, Sedelaar JPM, Barentsz JO, Fütterer JJ. MRI-guided interventions for the treatment of prostate cancer. AJR. Am. J. Roentgenol. 2012;199:714–720. doi: 10.2214/AJR.12.8725. Overview up to 2012 of all studies regarding MRI –guided interventions for the treatment of prostate cancer.
- 38.Partanen A, Yerram NK, Trivedi H, Dreher MR, Oila J, Hoang AN, Volkin D, Nix J, Turkbey B, Bernardo M, Haines DC, Benjamin CJ, Linehan WM, Choyke P, Wood BJ, Ehnholm GJ, Venkatesan AM, Pinto Pa. Magnetic resonance imaging (MRI)-guided transurethral ultrasound therapy of the prostate: a preclinical study with radiological and pathological correlation using customised MRI-based moulds. BJU Int. 2013;112:508–516. doi: 10.1111/bju.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuru TH, Roethke MC, Seidenader J, Simpfendörfer T, Boxler S, Alammar K, Rieker P, Popeneciu VI, Roth W, Pahernik S, Schlemmer H-P, Hohenfellner M, Hadaschik Ba. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J. Urol. 2013;190:1380–1386. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Ukimura O, Desai MM, Palmer S, Valencerina S, Gross M, Abreu AL, Aron M, Gill IS. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J. Urol. 2012;187:1080–1086. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 41.Betrouni N, Colin P, Puech P, Villers A, Mordon S. An image guided treatment platform for prostate cancer photodynamic therapy. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013;2013:370–373. doi: 10.1109/EMBC.2013.6609514. [DOI] [PubMed] [Google Scholar]
- 42.Punwani S, Emberton M, Walkden M, Sohaib A, Freeman A, Ahmed H, Allen C, Kirkham A. Prostatic cancer surveillance following whole-gland high-intensity focused ultrasound: comparison of MRI and prostate-specific antigen for detection of residual or recurrent disease. Br. J. Radiol. 2012;85:720–728. doi: 10.1259/bjr/61380797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, Jung AJ, Carroll PR, Coakley FV. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:e787–e793. doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Del Vescovo R, Pisanti F, Russo V, Battisti S, Cazzato RL, D’Agostino F, Giurazza F, Quattrocchi CC, Faiella E, Setola R, Giulianelli R, Grasso RF, Beomonte Zobel B. Dynamic contrast-enhanced MR evaluation of prostate cancer before and after endorectal high-intensity focused ultrasound. Radiol. Med. 2013;118:851–862. doi: 10.1007/s11547-012-0876-9. Study regarding follow-up with DCE after focal treatment of the prostate.
- 45.Shah V, Turkbey B, Mani H, Pang Y, Pohida T, Merino MJ, Pinto Pa, Choyke PL, Bernardo M. Decision support system for localizing prostate cancer based on multiparametric magnetic resonance imaging. Med. Phys. 2012;39:4093–4103. doi: 10.1118/1.4722753. [DOI] [PMC free article] [PubMed] [Google Scholar]


