Abstract
Background
Adoption of TRI in the United States is low and may be related to challenges learning the technique. We examined the relationships between operator transradial percutaneous coronary intervention (TRI) volume and procedural metrics and outcomes.
Methods and Results
We used CathPCI Registry® data from 07/2009–12/2012 to identify new radial operators, defined by an exclusively femoral PCI approach for 6 months after their first PCI in the database and ≥15 total TRIs thereafter. Primary outcomes of fluoroscopy time, contrast volume, and procedure success were chosen as markers of technical proficiency. Secondary outcomes included in-hospital mortality, bleeding, and vascular complications. Adjusted outcomes were analyzed using operator TRI experience as a continuous variable with generalized linear mixed models. Among 54,561 TRI procedures performed at 704 sites, 942 operators performed 1–10 cases; 942 operators performed 11–50 cases; 375 operators performed 51–100 cases; and 148 operators performed 101–200 cases. As radial caseload increased, more TRIs were performed in women, in STEMI patients, and for emergency indications. Decreased fluoroscopy time and contrast use were non-linearly associated with greater operator TRI experience, with faster reductions observed for newer (<30–50 cases) compared with more experienced (>30–50 cases) operators. Procedure success was high, while mortality, bleeding, and vascular complications remained low across TRI volumes.
Conclusions
As operator TRI volume increases, higher risk patients are chosen for TRI. Despite this, operator proficiency improves with greater TRI experience, and safety is maintained. The threshold to overcome the learning curve appears to be approximately 30–50 cases.
Keywords: transradial percutaneous coronary intervention, learning curve
Radial artery access for percutaneous coronary intervention (PCI) has potential advantages over the femoral approach. Compared with femoral PCI (TFI), transradial PCI (TRI) has been associated with reduced access site bleeding, fewer vascular complications, shorter hospital length of stay, and a trend towards reduced mortality in patients with ST-segment elevation myocardial infarction (STEMI).1–6 The transradial approach is also preferred by patients over the femoral route.5 Despite these potential benefits, TRI has been associated with increased radiation exposure, greater contrast use, and higher rates of procedural failure.2, 5, 7, 8 Overall rates of TRI use in this country remain low at 10–16% of PCI procedures.9 Furthermore, TRI use in the U.S. has been associated with a risk-treatment paradox whereby high-risk patients who may benefit the most from the radial approach are least likely to undergo TRI.10, 11
Challenges associated with TRI and low uptake of this technology may be partly related to technical proficiency for new operators. The concept of a learning curve, in which operator skill improves with greater experience, has been observed for many procedures, including TRI. Clinical trials and observational studies have reported no difference between TRI and TFI except at centers with proficient radial operators.2, 5, 12–17 To date, attempts to quantify the association between TRI experience and operator proficiency have been predominantly performed in small, single-center studies conducted outside of the U.S.2, 5, 13–18 Determining the minimum threshold to overcome the learning curve could be important for facilitating TRI adoption in the U.S., improving patient outcomes, and informing training guidelines and clinical trials of TRI. Therefore, we sought to: 1) characterize changes in patient selection for TRI procedures according to radial experience; 2) examine the relationship between TRI volume and procedural outcomes, including fluoroscopy time, contrast use, and procedural success; and 3) assess the relationship between TRI volume and in-hospital outcomes among U.S. operators using data from the National Cardiovascular Data Registry® CathPCI Registry®.
Methods
Data Source
Details about the CathPCI Registry have been previously described.19 Briefly, CathPCI Registry is an initiative of the American College of Cardiology and The Society for Cardiovascular Angiography and Interventions. As the nation’s largest PCI registry, the CathPCI Registry provides clinical and procedural data on patients undergoing cardiac catheterization and PCI from more than 1,400 U.S. hospitals. Trained data abstractors collect patient and procedural information at each participating institution via chart review using a standardized set of data elements and definitions (http://www.ncdr.com/WebNCDR/elements.aspx). Data quality is assured via automatic system validation and reporting of data completeness, random on-site audits, and site data manager training.
Study Population
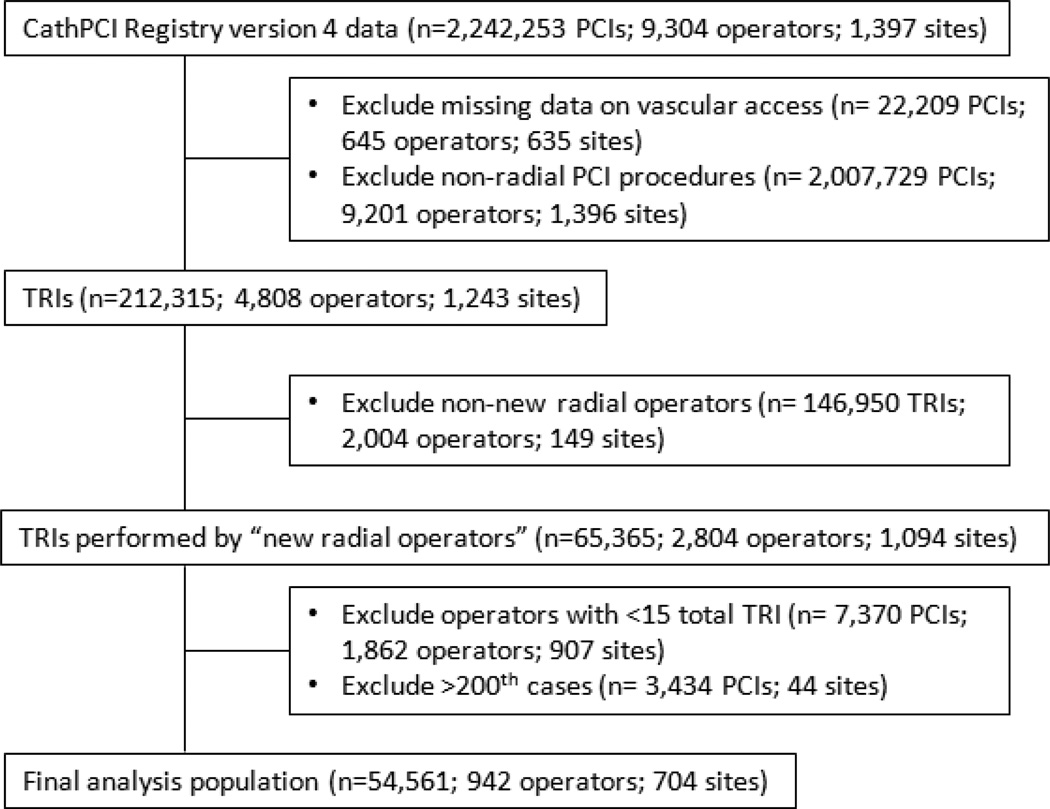
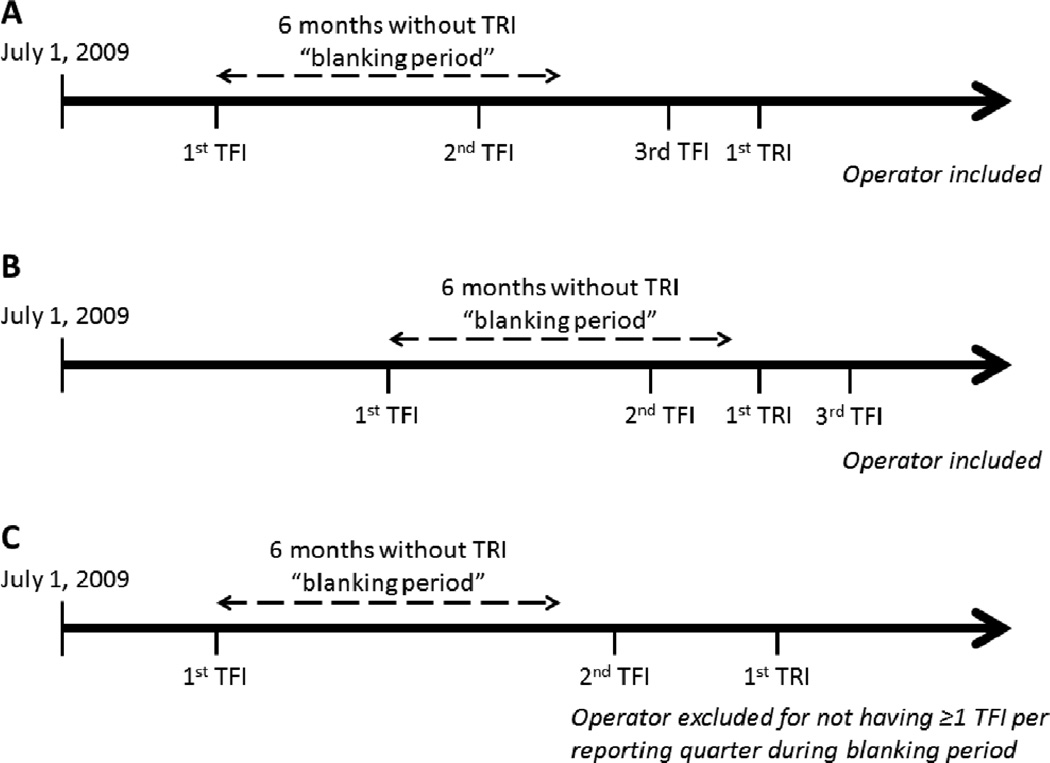
We examined 2,242,253 PCI procedures performed by 9,304 operators at 1,397 sites between July 2009 and December 2012 with data reported using version 4 of the CathPCI Registry collection form (Figure 1). PCIs with missing data regarding route of vascular access (n=22,209) and femoral PCIs (n=2,007,729) were excluded. We included “new” transradial operators (n=2,804), defined as those with a period of no TRI procedures but at least 1 femoral PCI per calendar reporting quarter for 6 months (“blanking period”) after the operator’s first femoral PCI in CathPCI Registry version 4 data (Figure 2). A TRI case volume of <15 cases proved insufficient to accurately estimate a learning curve. As a result, new radial operators performing <15 total TRIs (n=7,370 TRIs; 1,862 operators) were excluded, and modeling of the early learning curve (1st–14th cases) was performed using data from operators included in our analysis (≥15 total TRIs). Data from the >200th case TRI procedures for high-volume operators (n=3,434 TRIs) were also excluded due to insufficient operators to accurately model the learning curve in this range. However, these high-volume operators were not excluded from our study, as they contributed data from their first 200 TRI cases to our analyses. Our final analysis population consisted of 54,561 TRI procedures performed by 942 radial operators at 704 sites.
Figure 1.
Selection of TRI Procedures and Radial Operators. Shown is the flow diagram of TRI procedure and operator selection resulting in the final analysis population. PCI indicates percutaneous coronary intervention; TRI, transradial percutaneous coronary intervention.
Figure 2.
Definition of New Radial Operator. Depicted is the definition of a new radial operator used for inclusion in the analysis. Starting with the first TFI in version 4 CathPCI Registry, operators are required to have a “blanking period” of 6 months without a TRI, but at least 1 TFI per reporting quarter. Operators A and B have different start dates for their blanking period but both meet inclusion criteria. Operator C is excluded, as there is no TFI in the second reporting quarter. TFI indicates transfemoral percutaneous coronary intervention; TRI, transradial percutaneous coronary intervention.
Definitions and Outcomes
The following primary outcomes for our study were chosen as markers of operator proficiency: fluoroscopy time, contrast volume used, and procedure success. Procedure success is defined in the registry as residual stenosis ≤50% with a Thrombolysis in Myocardial Infarction flow grade ≥2 and at least 20% decrease in stenosis severity. For multi-vessel PCI procedures, procedure success was considered present if the number of lesions attempted and successfully dilated were equal. Since CathPCI Registry does not capture access site cross-over, we were unable to assess procedure success via the initial access site without cross-over. Secondary outcomes included in-hospital mortality, vascular complications, access site bleeding or hematoma, and any bleeding event within 72 hours post-PCI. Bleeding was defined as arterial access site bleeding—either overt, external bleeding, or a forearm hematoma >2 cm; retroperitoneal, gastrointestinal, or genitourinary bleeding; intracranial hemorrhage; cardiac tamponade; decrease of ≥3 g/dl in hemoglobin post-PCI in patients with pre-procedure hemoglobin ≤16 g/dl; or post-procedure non-bypass surgery related blood transfusion in patients with a pre-PCI hemoglobin of ≥8 g/dl.
Statistical Analysis
Radial volume was analyzed as a continuous variable for modeling, but to characterize patient and procedural features for descriptive purposes, the following categories of operator TRI volume were used: 1st–10th, 11th–50th, 51st–100th, and 101th–200th cases. These groupings represent “running tallies” of TRI experience such that operators can contribute to more than one group and serve as their own controls; this approach allows for assessments of average changes in patient selection and procedural metrics over individual learning curves. Categorical variables were presented as frequencies and percentages, and continuous variables were summarized as medians with interquartile ranges (IQRs). Comparisons among categorical and continuous variables were performed using Pearson Chi-Square and Kruskal-Wallis tests, respectively. Observed rates of the primary and secondary outcomes were reported according to operator TRI experience. We also compared observed rates with predicted bleeding rates using the CathPCI Registry PCI bleeding risk model applied to patients in categories of operator TRI volume.20
To examine the relationship between radial case volume and outcomes, generalized linear mixed models (linear regression for the continuous outcomes of fluoroscopy time and contrast volume; logistic regression for the categorical outcome of procedure success) were developed using radial case volume as a continuous variable. Relationships were plotted as curves for outcome versus TRI case volume such that any given slope along the curve represents the rate of change in outcome with increasing TRI experience. Restricted cubic splines were employed to explore potential non-linear relationships between case volume and outcomes, with random effects placed on the intercept and slopes for each component of the restricted cubic spline for case volume, assuming an unstructured covariance matrix. Intra-operator clustering of radial case volume was accounted for in the mixed models through use of an auto-regressive (order 1) covariance matrix that assumes that radial procedures performed closer in time to each other are likely more similar with respect to operator-level effects than those performed further apart. This mixed model also accounts for operator-specific effects such as case-mix selection. Analyses were repeated after adjustment for the following variables: age, female sex, body mass index, diabetes, cerebrovascular disease, peripheral vascular disease, chronic lung disease, glomerular filtration rate, prior PCI, New York Heart Association class, ejection fraction, cardiogenic shock, presentation with ST-segment-elevation myocardial infarction (STEMI), cardiac arrest within 24 hours, PCI status (elective, urgent, emergent, salvage), chronic total occlusion, bifurcation lesion, multi-vessel PCI, ad-hoc versus scheduled PCI, and hospital academic status.
To determine the threshold for overcoming the learning curve, the presence of potential inflection points along the relationship curves between case volume (per case increase) and outcomes by fitting linear splines using a single knot point was tested. Candidate knot points were chosen for testing based on visual inspection of curves. Given that linear splines with a single knot are less flexible than the restricted cubic spline methodology used for the primary analysis, similar Akaike Information Criterion (AIC) values ensured that the fit of data using linear versus cubic splines was comparable.21 The slopes of the curve before versus after the knot point were compared using score tests. In this approach, the slope of the curve indicates the rate and magnitude of change of the outcome of interest as TRI volume increases, and a significant difference between the pre- versus post-knot slopes indicates that the knot point can be considered an inflection point along the curve. Finally, pre-specified sensitivity analyses were performed after additionally adjusting for previous femoral PCI experience, defined as the cumulative number of femoral PCI procedures performed during the study period prior to the TRI procedure being modeled, and in high-risk subgroups including females and patients ≥75 years old. Analyses could not be repeated in patients undergoing TRI for STEMI (n=3,798) due to the small sample size and the lack of convergence of our statistical models. For all analyses, p-values <0.05 were considered statistically significant without correction for multiple comparisons. All analyses were performed at the Duke Clinical Research Institute using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
Operator TRI Volume
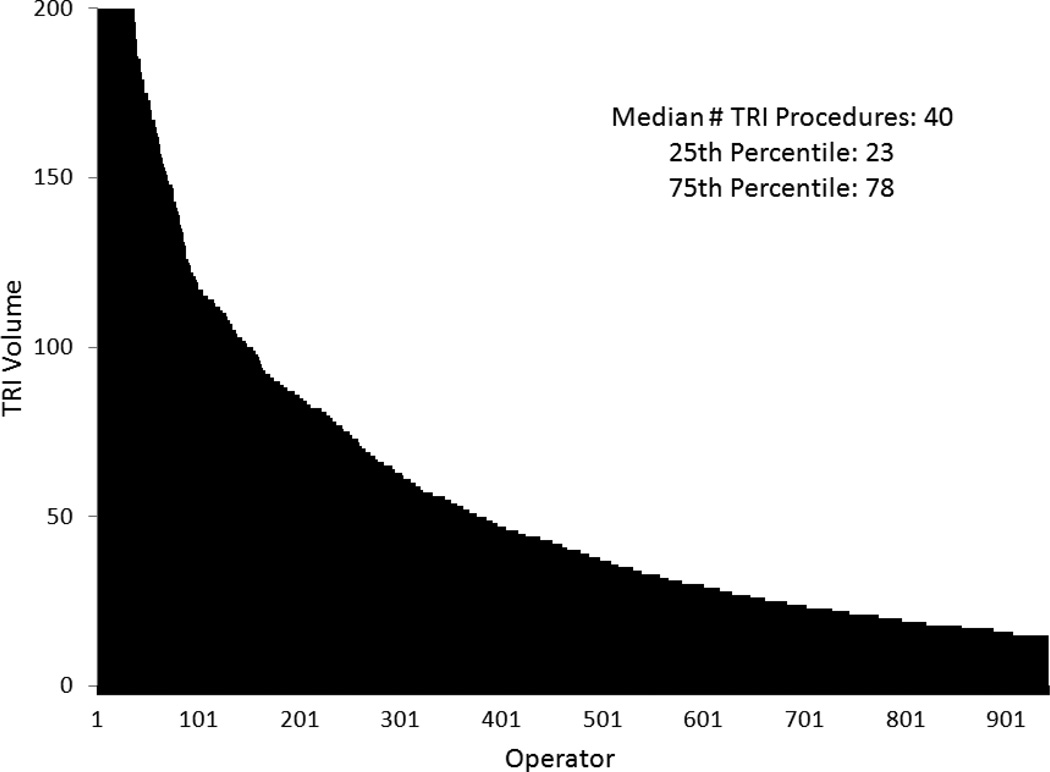
After exclusions (Figure 1), 54,561 TRI procedures performed by 942 new radial operators at 704 sites were included in our analysis. The distribution of TRI volume among operators is shown in Figure 3. The median total number of TRI procedures performed by individual operators during our study period was 40, and the 25th and 75th percentiles for TRI experience were 23 and 78 procedures, respectively.
Figure 3.
Distribution of TRI Volume among Operators. Shown is the distribution of total TRI procedures performed by operators included in our analysis. TRI indicates transradial percutaneous coronary intervention.
Patient and Procedure Characteristics
Overall, the median age of patients undergoing TRI was 63 years (IQR 55, 71), 29.7% (n=16,182) of patients were female, and 7.0% (n=3,798) of patients presented with STEMI. Among the total study cohort, 89.7% (n=48,920) of TRIs were performed at the same time as diagnostic angiography (ad hoc). TRIs were performed for emergency indications in 7.9% (n=4,315), in multiple vessels in 11.3% (n=6,159), and for high complexity lesions in 52.2% (n=28,442). Patient and procedure characteristics were examined according to categories of operator TRI volume (Tables 1 and 2). As TRI volume increased, more females, patients with NYHA class IV heart failure, patients presenting with STEMI, and patients with higher bleeding risk, were selected to undergo PCI using a radial versus femoral approach. Operators also performed more emergent PCIs, multi-vessel PCIs, and technically complex PCIs as they gained experience with TRI.
Table 1.
Patient Characteristics According to TRI Case Volume
| 1–10 (N=9,420 TRIs; 942 operators) |
11–50 (N=25,253 TRIs; 942 operators) |
51–100 (N=12,432 TRIs; 375 operators) |
101–200 (N=7,456 TRIs; 148 operators) |
|
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age (median, IQR), yrs | 63 (55,71) | 63 (55,71) | 64 (55,71) | 64 (55,72) |
| Female, % | 28.9 | 29.1 | 30.0 | 32.0 |
| Race/ethnicity, % | ||||
| Caucasian | 88.8 | 88.6 | 89.5 | 91.1 |
| African American | 8.2 | 8.4 | 7.8 | 7.0 |
| Hispanic | 4.1 | 4.4 | 4.0 | 3.4 |
| Other | 2.7 | 2.7 | 2.4 | 1.9 |
| Weight (median, IQR), kg | 91.8 (78.3,108.9) | 90.0 (77.3,104.5) | 89.0 (76.7,103.7) | 88.0 (75.8,102.4) |
| Height (median, IQR), cm | 173 (165,180) | 173 (165,180) | 173 (165,180) | 173 (165,180) |
| BMI (median, IQR), kg/m2 | 30.4 (26.6,36.1) | 29.8 (26.4,34.6) | 29.6 (26.1,34.1) | 29.5 (26.0,34.0) |
| Current/recent smoker, % | 27.3 | 27.9 | 28.8 | 29.5 |
| Hypertension, % | 83.5 | 82.0 | 81.2 | 81.3 |
| Dyslipidemia, % | 81.9 | 80.5 | 79.6 | 78.3 |
| Cerebrovascular disease, % | 11.0 | 10.0 | 10.0 | 11.0 |
| Peripheral artery disease, % | 13.7 | 11.0 | 10.7 | 11.7 |
| Chronic lung disease, % | 15.5 | 14.3 | 14.8 | 15.4 |
| Diabetes mellitus, % | 39.6 | 36.3 | 35.2 | 36.2 |
| Prior MI, % | 27.7 | 27.5 | 28.2 | 30.1 |
| Prior PCI,* % | 38.6 | 36.8 | 38.5 | 40.0 |
| Prior CABG, % | 7.3 | 6.7 | 7.4 | 9.5 |
| Prior CHF, % | 10.4 | 9.4 | 9.9 | 10.9 |
| GFR (median, IQR)† | 78.3 (63.3,93.5) | 78.7 (63.7,93.8) | 79.2 (64.0,94.9) | 78.5 (63.1,94.2) |
| Admission symptoms, % | ||||
| No angina | 9.2 | 7.1 | 5.7 | 4.5 |
| Atypical chest pain | 3.3 | 2.4 | 2.4 | 1.9 |
| Stable angina | 21.6 | 18.8 | 17.3 | 19.6 |
| Unstable angina | 42.8 | 43.5 | 43.1 | 41.2 |
| NSTEMI | 19.3 | 22.2 | 22.8 | 21.8 |
| STEMI | 3.8 | 6.0 | 8.8 | 11.1 |
| CHF/NYHA class w/in 2 wks, % | ||||
| No CHF | 90.7 | 91.1 | 91.3 | 90.8 |
| I | 1.4 | 1.6 | 1.3 | 2.0 |
| II | 2.8 | 2.6 | 2.4 | 2.5 |
| III | 3.1 | 3.0 | 3.0 | 3.2 |
| IV | 2.0 | 1.8 | 2.0 | 1.5 |
| Median predicted bleeding % (IQR) | 2.5 (1.7, 4.1) | 2.6 (1.7, 4.4) | 2.8 (1.8, 4.8) | 3.0 (1.9, 5.5) |
BMI indicates body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; GFR, glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TRI, transradial percutaneous coronary intervention
All p-values are <0.05 unless marked by an asterisk.
Table 2.
Procedure Characteristics According to TRI Case Volume
| 1–10 (N=9,420 TRIs; 942 operators) |
11–50 (N=25,253 TRIs; 942 operators) |
51–100 (N=12,432 TRIs; 375 operators) |
101–200 (N=7,456 TRIs; 148 operators) |
|
|---|---|---|---|---|
| PCI status, % | ||||
| Elective | 52.5 | 47.3 | 44.0 | 43.3 |
| Urgent | 43.1 | 45.7 | 46.0 | 44.6 |
| Emergent/salvage | 4.3 | 7.0 | 9.9 | 12.2 |
| Number of diseased vessels, % | ||||
| 1 | 53.9 | 54.1 | 51.5 | 50.9 |
| 2 | 32.3 | 32.3 | 33.6 | 33.0 |
| 3 | 13.7 | 13.6 | 15.0 | 16.0 |
| Number of treated lesions, % | ||||
| 1 | 76.8 | 75.9 | 74.7 | 74.7 |
| 2 | 18.7 | 19.8 | 20.6 | 20.9 |
| 3+ | 4.5 | 4.3 | 4.7 | 4.5 |
| Multi-vessel PCI, % | 10.7 | 11.1 | 11.5 | 12.5 |
| Previously treated lesion, % | 10.3 | 10.8 | 11.4 | 11.4 |
| High lesion complexity (high/C), % | 49.4 | 51.2 | 53.9 | 56.4 |
| Bifurcation lesion, % | 11.4 | 12.3 | 13.7 | 12.4 |
| Thrombus present, % | 7.5 | 10.4 | 12.9 | 13.4 |
| Saphenous vein graft PCI, % | 2.5 | 2.2 | 2.6 | 3.4 |
| Minimum stent diameter ≥3mm, % | 30.3 | 28.7 | 29.9 | 31.9 |
| Total stent length, median (IQR) | 36 (24,50) | 37 (26,52) | 38 (27,53) | 38 (26,53) |
BMI indicates body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; GFR, glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TRI, transradial percutaneous coronary intervention
All p-values are <0.05.
Outcomes According to TRI Volume
Overall, the median observed procedure fluoroscopy time was 14.3 minutes (IQR 9.6, 21.3), and the median observed contrast volume used was 180.0 mL (IQR 131.0, 230.0) per TRI. The rate of overall procedure success was high (96.0%, n=51,636). Ad hoc TRI procedures had greater median fluoroscopy times (14.6 vs. 11.4 minutes; p<0.001) and median contrast use (180 vs. 130 mL; p<0.001), but similar procedure success rates (95.9% vs. 96.5%; p=0.05) compared with scheduled TRI procedures. As shown in Table 3, observed median fluoroscopy times and contrast use for all procedures decreased significantly with increasing operator TRI volume (p<0.001). Despite more complex patient case-mix with greater TRI experience, procedure success remained consistently high across categories of TRI experience (p=0.44). Among patients with multi-vessel disease and without prior CABG, there was no substantial difference in the proportion of successfully treated vessels per number of diseased vessels as operator TRI volume increased (Supplemental Table 1). Overall rates of secondary outcomes were low for in-hospital mortality (0.5%, n=246), vascular complications (0.1%. n=79), access site bleeding (0.1%, n=45), access site hematoma (0.1%, n=81), or any observed bleeding event (2.3%, n=1,221). Although patients at progressively higher risk for bleeding were selected for TRI as operator experience increased, the rate of observed bleeding remained low and trended toward less bleeding across the spectrum of operator TRI volume (Table 3).
Table 3.
Outcomes According to TRI Case Volume
| 1–10 (N=9,420 TRIs; 942 operators) |
11–50 (N=25,253 TRIs; 942 operators) |
51–100 (N=12,432 TRIs; 375 operators) |
101–200 (N=7,456 TRIs; 148 operators) |
p- value |
|
|---|---|---|---|---|---|
| Primary | |||||
| Median fluoroscopy time, min (IQR) | 16.0 (10.8,23.6) | 14.6 (10.0,21.5) | 13.5 (9.2,20.2) | 12.6 (8.4,19.3) | <.001 |
| Median contrast volume, mL (IQR) | 185.0 (140.0,245.0) | 180.0 (135.0,231.0) | 175.0 (130.0,230.0) | 175.0 (130.0,225.0) | <.001 |
| Procedure success, % | 96.2 | 96.0 | 95.8 | 96.1 | 0.44 |
| Secondary | |||||
| In-hospital mortality, % | 0.5 | 0.4 | 0.5 | 0.5 | 0.79 |
| Vascular complication, % | 0.1 | 0.1 | 0.2 | 0.2 | 0.12 |
| Access site bleeding, % | 0.1 | 0.1 | 0.1 | 0.1 | 0.57 |
| Access site hematoma, % | 0.2 | 0.2 | 0.1 | 0.1 | 0.40 |
| Any bleeding event, % | 2.7 | 2.2 | 2.4 | 2.0 | 0.03 |
BMI indicates body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; GFR, glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation myocardial infarction; TRI, transradial percutaneous coronary intervention
Relationship Between TRI Volume and Outcomes
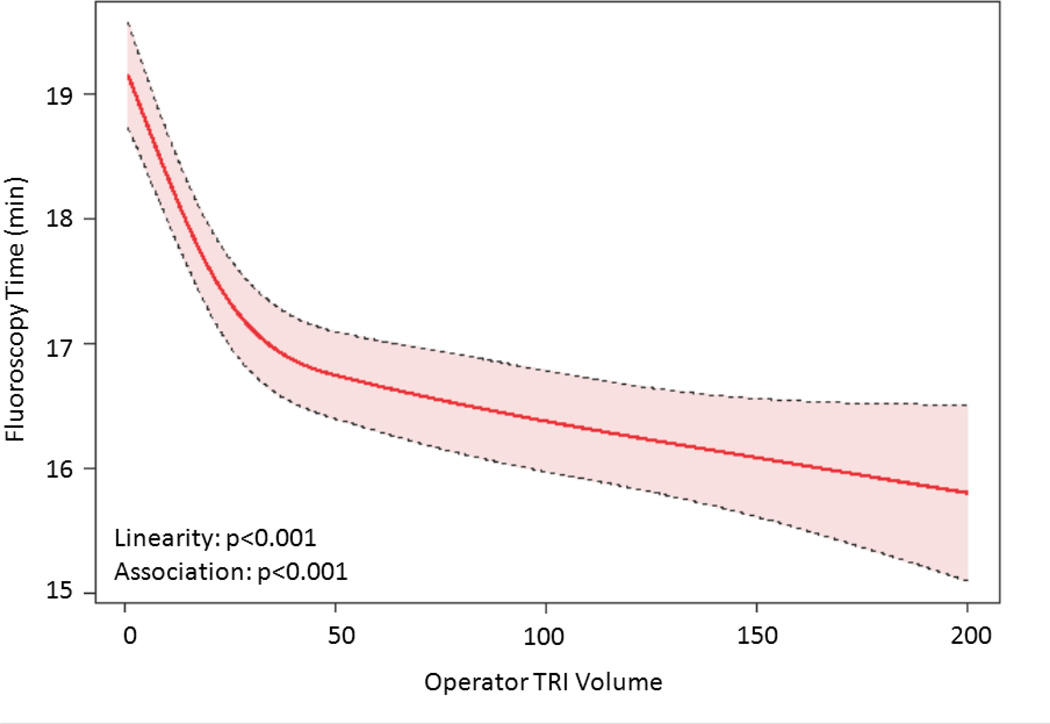
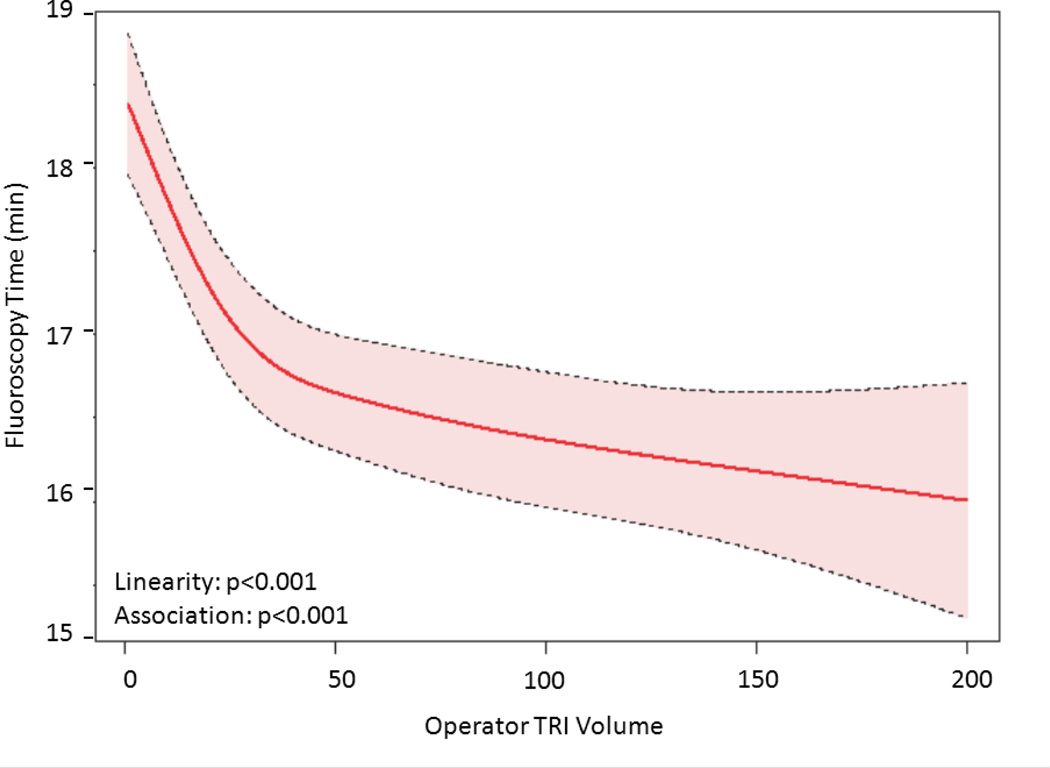
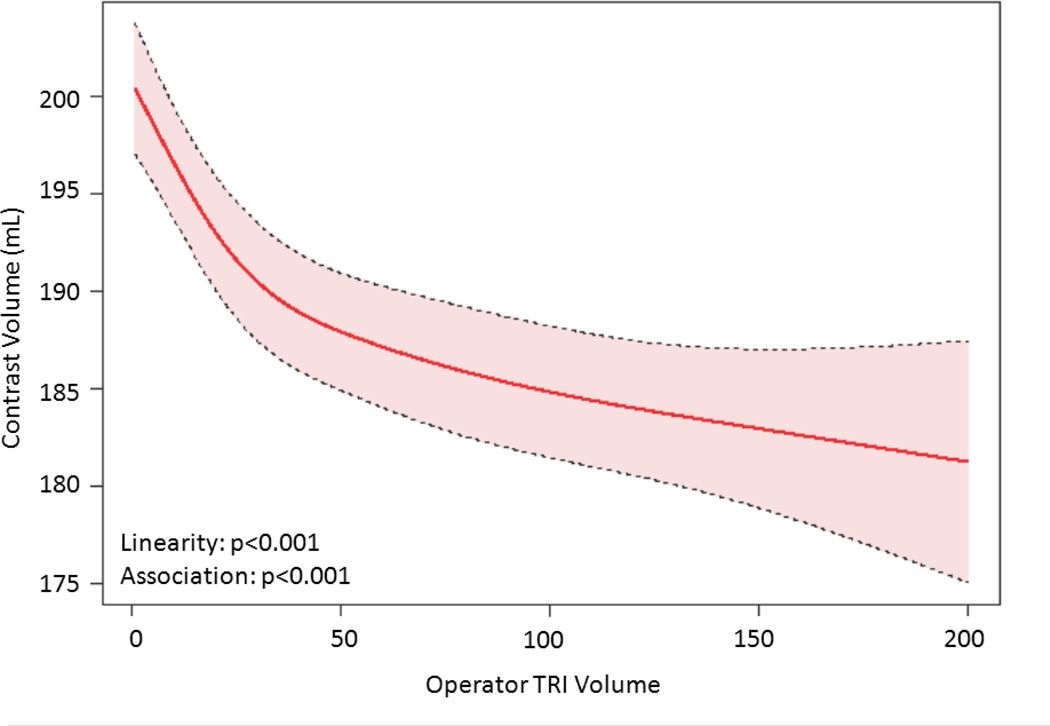
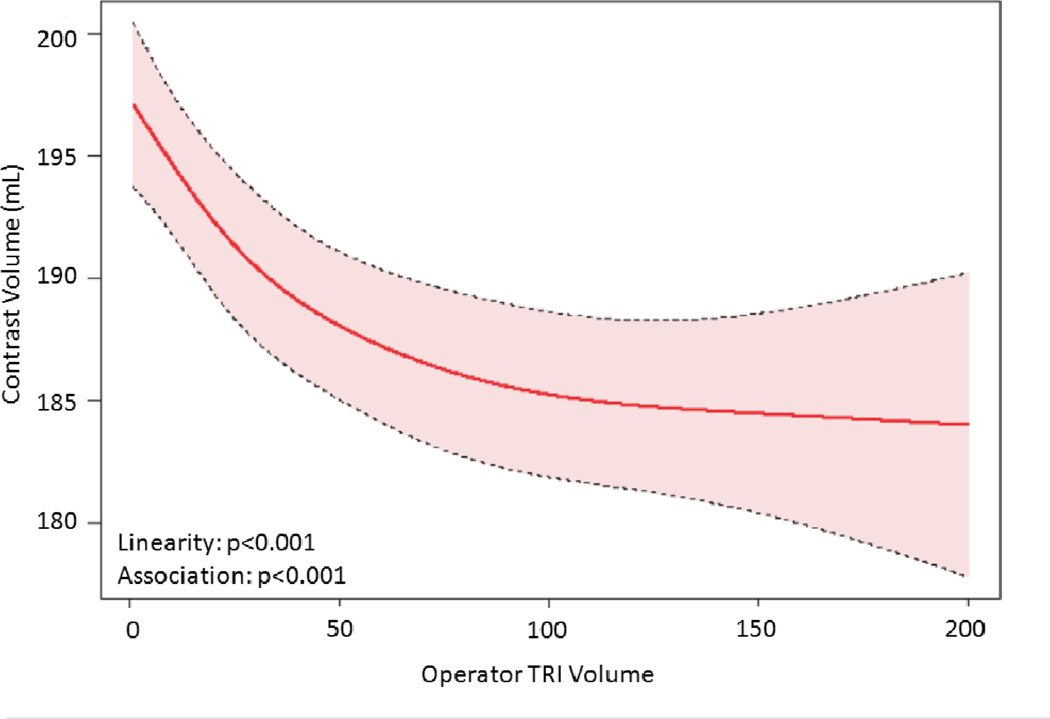
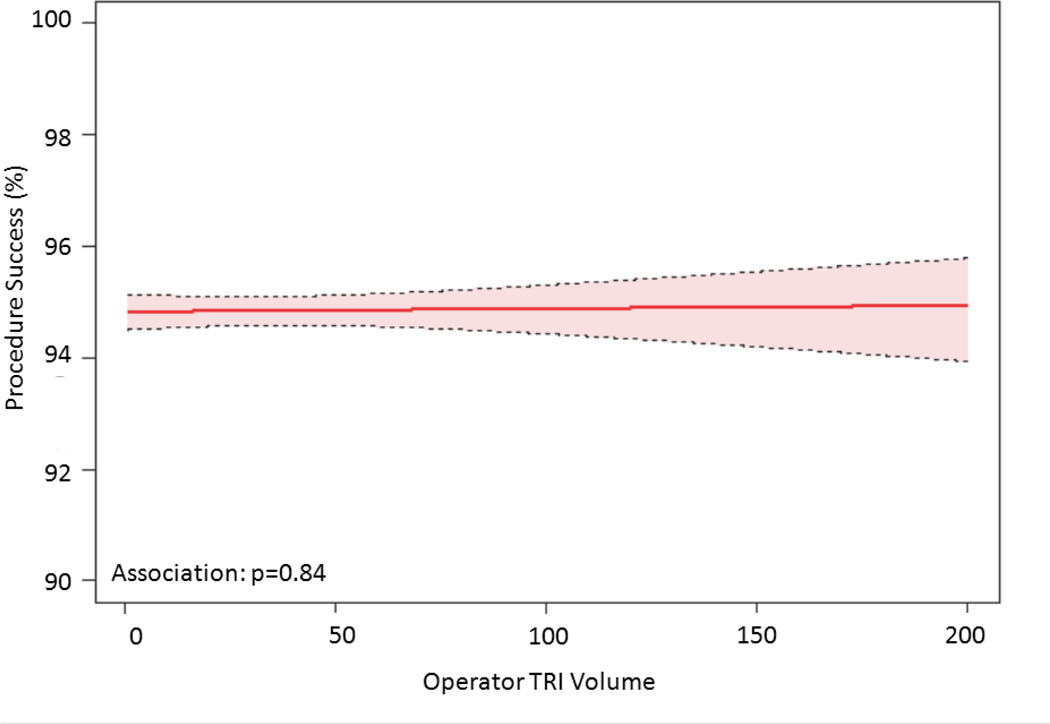
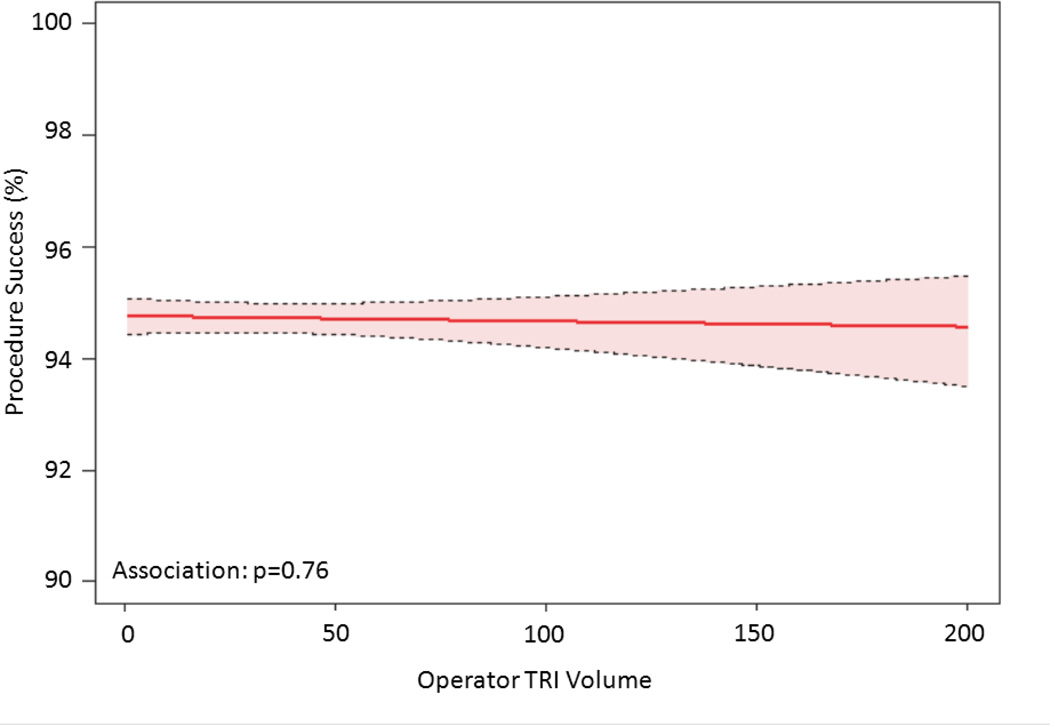
The relationship between TRI volume and procedural outcomes was assessed. After modeling, a significant non-linear association between increasing radial volume and decreasing fluoroscopy time (p<0.001 for non-linearity and association; Figure 4a) was observed. As shown in Figure 4b, TRI volume and fluoroscopy time remained significantly associated and demonstrated a non-linear relationship after adjustment for patient and procedural characteristics. A similarly significant non-linear association between increasing TRI volume and decreasing contrast use was observed (Figure 4c); this relationship also persisted after adjustment for patient and procedural characteristics (Figure 4d). In contrast, procedure success remained consistently high across the spectrum of TRI experience, and no significant association between TRI volume and procedural success using unadjusted or adjusted models (Figures 4e and 4f, respectively) was found.
Figure 4.
Relationship between Operator TRI Volume and Procedural Outcomes. Shown are curves depicting the unadjusted and adjusted relationships between TRI volume and fluoroscopy times (A and B, respectively); unadjusted relationships between TRI volume and contrast volume (C and D, respectively), and unadjusted and adjusted relationships between TRI volume and procedure success (E and F, respectively). Dotted lines represent 95% confidence intervals. TRI indicates transradial percutaneous coronary intervention.
The presence of inflection points along the curves for fluoroscopy time and contrast volume was investigated (Supplemental Table 2). Knot points between 30 and 50 TRI cases were chosen for evaluation based on visual inspection of the plots. Similar AIC values indicated that each pair of linear splines using these various knot points fit the data as well as the main model. For both fluoroscopy time and contrast volume, there were statistically significant differences between pre- versus post-knot slopes at each knot point tested, indicating that each could be considered an inflection point and that there is a range of case volumes beyond which improvements in procedural metrics begin to flatten. Furthermore, for both outcomes and across all knot points evaluated, the slopes before the knots were substantially steeper and more negative than slopes thereafter, indicating greater and faster reductions in fluoroscopy time and contrast volume use associated with increasing TRI volume along the earlier learning curve for inexperienced operators (<30–50 TRI cases) compared with the ongoing learning curve for more experienced operators (>50 TRI cases).
Sensitivity Analyses
Several pre-specified sensitivity analyses were performed. To assess for an effect of overall PCI experience on outcomes, analyses were repeated after including an adjustment variable for prior femoral PCI volume, but did not change the primary results. Next, a potential effect modification of the relationship between TRI volume and outcomes in high-risk subgroups of females and patients ≥75 years old was examined. In our cohort, 29.7% (n=16,182) of TRI procedures were performed in females, and 17.4% (n=9,502) of TRIs were performed in patients ≥75 years of age. Results of analyses repeated in both of these subgroups were consistent with our primary findings (Supplemental Figures 1 and 2).
Discussion
Despite important potential benefits of TRI over TFI, technical challenges associated with TRI may discourage new operators from adopting the radial approach. In this study, almost 55,000 TRI procedures performed by over 900 U.S. operators using data from the nation’s largest PCI registry were examined. We found that higher-risk patients and more complicated cases are selected for TRI as operator experience increases. In spite of this greater complexity in case selection, rates of bleeding and vascular complications and in-hospital mortality remain low, while procedural success rates remains high. We further demonstrate the existence of an operator learning curve whereby procedural metrics that are markers of operator proficiency significantly improve with increasing TRI volume. Based on our data, the threshold for overcoming this learning curve is ~30–50 cases, though this number will likely vary for each individual operator. These findings suggest that the learning curve for TRI is relatively shallow with the availability of modern interventional equipment and should inform TRI training guidelines in the U.S. Importantly, procedural metrics will likely continue to improve as an operator’s TRI experience increases.
The concept of a learning curve for TRI has previously been recognized,1, 2, 13, 22, 23 though few studies have attempted to quantify this relationship. Spaulding et al. found that an annual procedural volume of >80 transradial cases correlated with significantly lower rates of access failure and overall procedure time when using the left radial artery approach for coronary angiography.16 A more recent study examined metrics for radial diagnostic angiograms over 1 year for inexperienced operators and found significant improvements in procedure time, fluoroscopy time, and contrast use when comparing results from operators’ last 6 months experience (n=236 procedures) to their first 6 months (n=82 procedures).17 The only learning curve analysis to specifically assess TRI procedures is based on single-center data from Canada.15 In this study, authors showed that a case volume threshold of at least 50 TRI procedures was required for new radial operators to achieve similar procedural outcomes as experienced radial performers (>300 TRIs).
The results of our study add to and extend existing data in several ways. First, to our knowledge, this is the only U.S.-based multi-center study examining the TRI learning curve. Second, this work represents the largest radial learning curve analysis to date, incorporating data from ~55,000 TRI procedures performed by more than 900 operators at over 700 different centers. Consequently, these results characterize a national learning curve based on community practice data and are generalizable to the majority of U.S. operators interested in learning to perform TRI. Third, we used detailed clinical and procedural CathPCI Registry data that allowed for multivariable adjustment in examinations of TRI volume and operator proficiency. The strength of these findings is supported by sensitivity analyses among patient subgroups known to be at higher risk for bleeding and vascular complications and to have more challenging anatomy for the radial approach. Finally, the large sample size permitted use of rigorous statistical methodology to characterize the association between procedural outcomes and TRI volume and to test for the presence of a threshold along the learning curve.
There were several key differences in the findings from this analysis compared with those from previous studies. The previously reported threshold of 80 procedures was based on an older analysis of diagnostic angiograms, not TRIs, and this study predated wide availability of dedicated radial access equipment and radial training courses. Although our threshold of 30–50 cases is consistent with the minimum of 50 cases reported by Ball et al., in contrast to their findings, we did not observe that procedure success increased with greater TRI volume.15 This discrepancy is likely explained by fact that operators in our study selected increasingly more complex patients for TRI with additional TRI experience, whereas no similar trend was observed in the study by Ball et al. Therefore, we hypothesize that high procedure success rates early in the learning curve were likely related to the selection of lower-risk cases, while consistently high procedural success despite more complex patient selection later on reflected improved operator skill. Importantly, these CathPCI Registry data support a range of potential thresholds from 30–50 cases, not a single threshold. This range is consistent with the idea that decisions regarding an operator’s level of proficiency and comfort with TRI and, consequently, selection of patients for TRI during the learning phase, should be made on an individual basis. As recently demonstrated, successful adoption of TRI may ultimately require an operator to approach cases with a “radial first” mentality.24, 25 Additionally, non-operator-dependent factors, such as catheterization laboratory and ward staff training for preparing and caring for patients undergoing TRI or the availability of experienced mentors, need to be considered. Finally, we observed continued improvements in technical proficiency beyond the initial learning curve. This observation, which has been noted in a study of vascular complications and procedure success among experienced TRI operators,18 suggests that the volume-outcome relationship previously described for femoral PCIs also exists for TRI procedures.26
These data have important implications for adoption of TRI in the U.S. and for TRI training. Data from a recent international survey show that radial use in the U.S. lags behind many countries in the rest of the world.27 This may be partially related to lack of TRI training during fellowship for U.S. trainees, whereas early TRI exposure outside the U.S. is commonplace. Additionally, no formal society guidelines defining thresholds for TRI competency currently exist. A recent European consensus statement suggests an annual minimum of 80 diagnostic and interventional radial procedures to maintain proficiency after the initial learning curve is overcome, but the threshold for what constitutes initial competency in radial technique is not specified.28 Recommendations from the Society for Cardiovascular Angiography and Interventions also recognize the existence of the radial learning curve and outline suggested levels of scaled competency but do not specify minimum numbers of procedures meeting these standards.29 The absence of threshold for initial competency in TRI is likely due to the lack of large-scale systematic examinations of this topic. Such a paucity may be related to limited availability of data regarding factors influencing the learning curve and difficulty identifying and tracking operators using the radial approach for coronary angiography and PCI. Our data, which describe a national TRI learning curve, show that despite overall lower TRI volumes and predominance of post-fellowship TRI training, the learning curve is similar for U.S. versus non-U.S. operators. Therefore, these data may facilitate the development and adoption of formal TRI training guidelines in the U.S. These data also provide a national benchmark against which individual operators can track their progress along their own learning curves.
Limitations
This study has important limitations. First, despite multivariable adjustment, residual confounding may be present, and we assessed operator TRI proficiency using surrogates, which may also be markers of other unmeasured factors. Second, data regarding access site crossover (an important indicator of procedure success and operator proficiency) were unavailable, and outcomes were site-reported (not adjudicated). Nevertheless, the CathPCI Registry offers an extensive database that can provide insight into contemporary cardiology practice trends. Third, our “new operator” definition excluded a large number of experienced operators and TRI procedures. However, we were interested in assessing changes in new operator proficiency (i.e., those to whom the early learning curve for a novel technology is applicable). Additionally, our definition may have misclassified some operators as “new” (e.g., those practicing primarily at non-NDCR participating hospitals, including the Veteran’s Affairs health system, or at hospitals with intermittent NCDR participation). The likelihood of including such operators was reduced by imposing a 6-month blanking period, and the effect of including experienced operators would have been to mask the association between TRI volume and outcomes and bias our results toward the null. Furthermore, we focused on PCI procedures, and operators might begin performing radial procedures with diagnostic angiography; as a result, the true learning curve may be steeper than what we observed. Fourth, due to statistical limitations, low-volume operators with <15 total TRIs were excluded, and analyses were restricted to data accumulated through the 200th procedure for high-volume operators. However, we used data for the 1st through 15th TRI procedures from almost 1000 operators, and the inflection points along the learning curve in our study were well-below the 200th case, making it unlikely that these restrictions significantly impacted our results. Fifth, our results may reflect that some operators with initial TRI challenges or inadequate mentorship could have “dropped out” and given up on the technique rather than continue to improve their TRI proficiency. Finally, these data were limited to U.S. operators, the majority of whom learned to perform TRIs after completion of femoral PCI-based interventional cardiology fellowships. Therefore, the shape of the learning curve could be different for operators with early simultaneous exposure to both vascular approaches, yet the consistency of our results with data from Canada, where TRI training is typically acquired during fellowship, does not support this hypothesis.
Conclusions
In conclusion, adoption of TRI may be related to issues of operator proficiency and the need to overcome an assumed learning curve. Using data from the largest ongoing PCI registry in the U.S., we demonstrate that a TRI learning curve exists, and the threshold to overcome this learning curve in current U.S. practice is between 30 and 50 cases. In addition, we found that with growing TRI experience, operators are selecting more complex patients for TRI without sacrificing procedure success or increasing radiation exposure and contrast use. Despite overall lower TRI volumes, our data suggest that the learning curve for the U.S. compared with previously published data from outside of the U.S. is similar. These findings may allay the concerns of U.S. operators over the feasibility of incorporating TRI procedures into standard practice. These data should also inform the development of formal TRI training guidelines.
Supplementary Material
Acknowledgements
We thank Erin Hanley for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted. We also thank the participants of the CathPCI Registry for providing these data.
Funding Sources: Connie N. Hess received support from the National Institutes of Health (5T32HL069749-09). Funding sources had no role in the design, conduct, or reporting of the study.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg) Heart. 2008;94:1019–1025. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- 5.Louvard Y, Lefèvre T, Allain A, Morice M. Coronary angiography through the radial or the femoral approach: The CARAFE study. Catheter Cardiovasc Interv. 2001;52:181–187. doi: 10.1002/1522-726x(200102)52:2<181::aid-ccd1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (rival): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 7.Benit E, Missault L, Eeman T, Carlier M, Muyldermans L, Materne P, Lafontaine P, De Keyser J, Decoster O, Pourbaix S, Castadot M, Boland J. Brachial, radial, or femoral approach for elective Palmaz-Schatz stent implantation: a randomized comparison. Cathet Cardiovasc Diagn. 1997;41:124–130. doi: 10.1002/(sici)1097-0304(199706)41:2<124::aid-ccd3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Dehghani P, Mohammad A, Bajaj R, Hong T, Suen CM, Sharieff W, Chisholm RJ, Kutryk MJ, Fam NP, Cheema AN. Mechanism and predictors of failed transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. 2009;2:1057–1064. doi: 10.1016/j.jcin.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, Piana RN, Hillegass WB, Cohen MG, Gilchrist IC, Rao SV. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007–2012) Circulation. 2013;127:2295–2306. doi: 10.1161/CIRCULATIONAHA.112.000536. [DOI] [PubMed] [Google Scholar]
- 10.Wimmer NJ, Resnic FS, Mauri L, Matheny ME, Piemonte TC, Pomerantsev E, Ho KK, Robbins SL, Waldman HM, Yeh RW. Risk-treatment paradox in the selection of transradial access for percutaneous coronary intervention. J Am Heart Assoc. 2013;2:e000174. doi: 10.1161/JAHA.113.000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 12.Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187–2195. doi: 10.1016/j.jacc.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997;29:1269–1275. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SL, Renslo R, Sinow R, French WJ. Learning curve in the use of the radial artery as vascular access in the performance of percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn. 1998;44:147–152. doi: 10.1002/(sici)1097-0304(199806)44:2<147::aid-ccd5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Ball WT, Sharieff W, Jolly SS, Hong T, Kutryk MJ, Graham JJ, Fam NP, Chisholm RJ, Cheema AN. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011;4:336–341. doi: 10.1161/CIRCINTERVENTIONS.110.960864. [DOI] [PubMed] [Google Scholar]
- 16.Spaulding C, Lefevre T, Funck F, Thebault B, Chauveau M, Ben Hamda K, Chalet Y, Monsegu H, Tsocanakis O, Py A, Guillard N, Weber S. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39:365–370. doi: 10.1002/(SICI)1097-0304(199612)39:4<365::AID-CCD8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Looi JL, Cave A, El-Jack S. Learning curve in transradial coronary angiography. Am J Cardiol. 2011;108:1092–1095. doi: 10.1016/j.amjcard.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Burzotta F, Trani C, Mazzari MA, Tommasino A, Niccoli G, Porto I, Leone AM, Tinelli G, Coluccia V, De Vita M, Brancati M, Mongiardo R, Schiavoni G, Crea F. Vascular complications and access crossover in 10,676 transradial percutaneous coronary procedures. Am Heart J. 2012;163:230–238. doi: 10.1016/j.ahj.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. doi: 10.1136/heartjnl-2012-303379. [DOI] [PubMed] [Google Scholar]
- 20.Rao SV, McCoy LA, Spertus JA, Krone RJ, Singh M, Fitzgerald S, Peterson ED. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry® CathPCI Registry®. JACC Interv. 2013;6:897–904. doi: 10.1016/j.jcin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 22.Jolly SS, Cairns J, Niemela K, Steg PG, Natarajan MK, Cheema AN, Rao SV, Cantor WJ, Dzavik V, Budaj A, Sheth T, Valentin V, Fung A, Widimsky P, Ferrari E, Gao P, Jedrzejowski B, Mehta SR, Investigators R. Effect of radial versus femoral access on radiation dose and the importance of procedural volume: a substudy of the multicenter randomized RIVAL trial. JACC Cardiovasc Interv. 2013;6:258–266. doi: 10.1016/j.jcin.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Lo TS, Ratib K, Chong AY, Bhatia G, Gunning M, Nolan J. Impact of access site selection and operator expertise on radiation exposure; a controlled prospective study. Am Heart J. 2012;164:455–461. doi: 10.1016/j.ahj.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Turner S, Sacrinty M, Manogue M, Little W, Gandhi S, Kutcher M, Santos R, Applegate R. Transitioning to the radial artery as the preferred access site for cardiac catheterization: an academic medical center experience. Catheter Cardiovasc Interv. 2012;80:247–257. doi: 10.1002/ccd.23387. [DOI] [PubMed] [Google Scholar]
- 25.Rao SV, Krucoff MW. Radial first: paradox+proficiency=opportunity. J Am Heart Assoc. 2013;2:e000281. doi: 10.1161/JAHA.113.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post PN, Kuijpers M, Ebels T, Zijlstra F. The relation between volume and outcome of coronary interventions: a systematic review and meta-analysis. Eur Heart J. 2010;31:1985–1992. doi: 10.1093/eurheartj/ehq151. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodes-Cabau J, Larose E, Costerousse O, Hamon M, Mann T. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3:1022–1031. doi: 10.1016/j.jcin.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, Sabate M, Mauri-Ferre J, Huber K, Niemela K, Haude M, Wijns W, Dudek D, Fajadet J, Kiemeneij F. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013;8:1242–1251. doi: 10.4244/EIJV8I11A192. [DOI] [PubMed] [Google Scholar]
- 29.Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011;78:823–839. doi: 10.1002/ccd.23052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.