Abstract
Background
The incidence of esophageal adenocarcinoma (EAC) has increased five-fold in the United States since 1975. The aim of our study was to estimate future U.S. EAC incidence and mortality and to shed light on the potential drivers in the disease process that are conduits for the dramatic increase in EAC incidence.
Methods
A consortium of three research groups calibrated independent mathematical models to clinical and epidemiologic data including EAC incidence from the Surveillance, Epidemiology, and End Results (SEER 9) registry from 1975–2010. We then used a comparative modeling approach to project EAC incidence and mortality to year 2030.
Results
Importantly, all three models identified birth cohort trends affecting cancer progression as a major driver of the observed increases in EAC incidence and mortality. All models predict that incidence and mortality rates will continue to increase until 2030 but with a plateauing trend for recent male cohorts. The predicted ranges of incidence and mortality rates (cases per 100,000 person years) in 2030 are 8.4–10.1 and 5.4–7.4 respectively for males, and 1.3–1.8 and 0.9–1.2 for females. Estimates of cumulative cause-specific EAC deaths among both sexes for years 2011–2030 range between 142,300 and 186,298, almost double the number of deaths in the past 20 years.
Conclusions
Through comparative modeling, the projected increases in EAC cases and deaths represent a critical public health concern that warrants attention from cancer control planners to prepare potential interventions.
Impact
Quantifying this burden of disease will aid health policy makers to plan appropriate cancer control measures.
Keywords: esophageal neoplasm, adenocarcinoma, incidence, mortality, projections
Introduction
The vast majority of esophageal cancers are either squamous cell carcinoma (SCC) or adenocarcinoma (EAC). While esophageal SCC incidence has been declining in the U.S. and other parts of the western world, EAC incidence has experienced an alarming five-fold increase over the past four decades (1). There is no consensus regarding the causes of this increase in EAC incidence, although an increasing prevalence of gastroesophageal reflux disease (GERD) related to increases in abdominal obesity (2) and wider eradication of Helicobacter pylori infection (3, 4) have been suggested, among others. GERD is a risk factor for Barrett’s esophagus (BE), a pre-dysplastic condition associated with progression to EAC (5). Current EAC prevention efforts have focused on endoscopic screening for BE, although the benefit and effectiveness are uncertain (6, 7). Recent analyses of historical trends in EAC incidence and mortality in the U.S. suggest that EAC incidence continues to rise, although the EAC incidence rate may be beginning to plateau in recent years (8, 9). Projections of future EAC incidence and mortality would provide important data for health policy makers as they track cancer trends and plan appropriate cancer control policy. In this analysis, we utilized independent mathematical models in a comparative modeling approach to make future EAC incidence and mortality projections.
Mathematical modeling is a powerful methodology which can be used to make projections by systematically integrating available data. Despite its potential, one common criticism of modeling is that independent modeling efforts often yield disparate results. These differences can primarily be attributed to differing model inputs and model structures, and often a lack of transparency in model assumptions, all impeding wider acceptance of simulation modeling results. The Cancer Intervention and Surveillance Modeling Network (CISNET) is a National Cancer Institute (NCI) funded consortium of mathematical and computational modelers who work closely together to address many of these limitations using a comparative modeling approach. Comparative modeling is a powerful method to improve each of the participating models by providing an environment where experts in modeling methodology, cancer control and clinical management can easily and openly collaborate. This context allows for an iterative process where common calibration targets are shared but other aspects of the individual models are free to differ. After each model is calibrated and applied independently, the prediction targets are shared and analyzed cooperatively, providing a transparent setting for iterative improvement of the models, and enhancing our understanding of the natural history of a particular type of cancer. After model development and refinement, CISNET researchers can then use their models to evaluate the impact of potential interventions on trends in cancer incidence and mortality, helping to optimize cancer control strategies. CISNET models have been utilized by the U.S. Preventive Services Task Force (USPSTF) for breast (10) and colorectal cancer screening guidelines (11), in formulating draft recommendations for lung cancer screening (12), and by the Centers for Medicare and Medicaid Services (CMS) to compare the effectiveness of CRC screening strategies (13, 14).
The CISNET consortium focuses on five cancer sites: breast, colorectal, lung, prostate, and esophagus. The esophagus group is comprised of four academic institutions that have developed three independent models of EAC. Our three independent models were calibrated to historic EAC incidence and mortality rates between the years 1975–2010 for all males and all females from the Surveillance, Epidemiology and End Results (SEER 9) database (15). After calibration, we compared the calibration results from the three models to reinforce each model’s validity against the available data. Afterward, the models were used to generate independent projections of EAC incidence and mortality to the year 2030. We also outputted the BE prevalence, progression rates to detected cancer in patients with BE, and average EAC sojourn time (time interval between preclinical EAC and clinically detected EAC); all are important underlying factors that may be subject to secular trends related to period and/or birth cohort. In modeling these trends we had developed a better understanding of the observed increases in EAC incidence and mortality.
Materials and Methods
The Models
We analyzed EAC incidence and mortality rates by using three mathematical models that were developed independently by participants in the NCI’s CISNET consortium: the Multiscale Esophageal AdenoCarcinoma (MSEAC) Model from the Fred Hutchinson Cancer Research Center (Seattle, WA) - FHCRC model, the Esophageal AdenoCarcinoma Model (EACMo) from the Massachusetts General Hospital (Boston, MA) - MGH model, and University of Washington (Seattle, WA) and the Microsimulation Screening Analysis model from Erasmus University Medical Center (Rotterdam, The Netherlands) - UW-MISCAN-Esophagus model. The major features of the models are also summarized in the Appendix. Each model computes the life histories of a population of hypothetical individuals from birth (UW-MISCAN and FHCRC) or age 20 (MGH) to death and has a natural history component that tracks the progression of esophageal disease or precursor states preceding adenocarcinoma. All three models include the following health states: healthy, GERD symptoms, BE without dysplasia, BE with dysplasia, preclinical cancer, clinically diagnosed cancer, and death. The UW-MISCAN and MGH models further categorize dysplasia in BE into low-grade dysplasia (LGD) and high-grade dysplasia (HGD).
The primary differences between these models are in the modeling methodology. The FHCRC model is a biological model based on the paradigm of initiation, promotion, and progression where carcinogenesis arises from the accumulation of mutations and clonal expansion of partially altered cells on the pathway to malignancy (Appendix Figure A1). The FHCRC model also combines likelihood and multiscale spatial simulation methods to represent health states as observation or detection processes built into a detailed tissue- and cell-level model of carcinogenesis (16, 17). It also includes random transitions from normal esophageal tissue to BE that occur with a rate which reflects the prevalence of GERD in the general population (18, 19). The mathematical construct of the model yields a numerical hazard function, which predicts the age-specific incidence of EAC and therefore was used for parameter estimation. In contrast, the MGH and UW-MISCAN models are empirically-based simulations of natural histories. The MGH model is a Markov state transition simulation model which simulates a cohort of hypothetical individuals and does not allow for disease regression (Appendix Figure A4) (2, 20, 21). The UW-MISCAN model is a microsimulation model using a discrete event formalism which simulates individuals one at a time and also allows for disease regression in the health states prior to cancer (Appendix Figure A7).
Although the three models differ substantially in their modeling structure, they all generate predictions of incidence and mortality as a function of age and stage of EAC diagnosis.
Modeling of EAC Trends
Mathematical models can be utilized to investigate the impact of shifting risk factor patterns on the population burden of cancer. Other CISNET cancer groups have modeled known risk factors, such as the Lung group that used historical smoking data to investigate the total number of lives saved between 1975 and 2000 as a result of the implementation of tobacco control policies (22). Since EAC incidence has experienced a dramatic increase, it is imperative that our modeling incorporates the mechanism(s) that effectively shed light on the potential causes of this increase. The three CISNET groups opted to capture this increase using a generalization of the traditional age-period-cohort (APC) formalism (23–25) in which age, period and cohort trends are applied to rates within the natural history model. The FHCRC group used likelihood methods to evaluate period and cohort trends affecting specific cell kinetic rates, including trends on BE incidence. The MGH and UW-MISCAN groups varied the transition rates between health states in the models which depend on age, calendar year and birth year of the cohort. The traditional Age-Period-Cohort (APC) models are log-linear models which simultaneously analyze the age, period, and cohort effects in data from registries such as the SEER 9 Program. By replacing the age effects in the traditional APC approach with functionally constrained parametric models, we finesse a well-known ‘linear-trend’ non-identifiability problem (26–28) allowing us to explore both period and cohort effects jointly. These effects were estimated together with the age dependent parameters (UW-MISCAN) or after the age dependent parameters were established (MGH). For FHCRC, the period and cohort effects were calibrated jointly with estimation of the cell kinetic parameters and mutation rates in the EAC hazard function. For the details of modeling EAC trends in each model, see the Appendix and corresponding Figures A2,3,5,6, and 8.
Common Calibration Targets
All three CISNET models were calibrated to EAC incidence rates from the Surveillance, Epidemiology, and End Results (SEER 9) program data for all men and all women aged 20–84 years in the United States from 1975–2010. Following the method in one of our previous analyses (8), the cancer incidence rates are comprised of cancers defined/identified by the International Classification of Diseases for Oncology, third edition (ICD-O-3) histology codes 8140– 8141, 8143– 8145, 8190– 8231, 8260–8263, 8310, 8401, 8480–8490, 8550–8551, 8570–8574, and 8576. Standard mortality statistics are not available for EAC because death certificates do not include the histology of the cancer. However, incidence-based mortality (IB mortality) data in SEER utilize cancer registry information to link characteristics of the incident cancer (e.g. stage, histology) to individual death certificates (29). The incidence and mortality rates generated by the models are also stratified by the SEER historic stages: localized (confined to primary site), regional (spread to regional lymph nodes), distant (cancer metastasized), and unknown (unstaged) (30). The MGH group opted to calibrate to IB mortality rates along with the EAC incidence from SEER. The UW-MISCAN group used SEER stage-specific EAC survival data stratified by calendar groups, while the FHCRC group used SEER specific survival rates stratified by gender and age groups, as model inputs to calculate the mortality rates. For the UW-MISCAN and FHCRC groups, the IB mortality rates from SEER are used to validate their mortality outputs. All three models also use U.S. census data and projections for past (from 1975 onwards) and future (up to 2030) population size (31).
Model Outputs
The primary endpoints or model outputs for this study were the projections of overall EAC incidence and mortality rates up to calendar year 2030. SEER incidence and mortality rates corresponding to the years 1975 to 2010 were organized by birth cohorts and used as model calibration targets. We also calculated the cumulative EAC deaths from 2011 to 2030, using national population projections from the United States Census Bureau (31), to quantify the burden of disease on society. Other predictions included the average time between developing preclinical cancer and cancer diagnosis (EAC sojourn time) and the annual rate of patients diagnosed with BE (without dysplasia or with LGD) progressing to clinically diagnosed EAC, to be compared with rates in the literature. These progression rates were calculated for age-at-diagnosis with BE at age 60 and with five years of follow-up time. The cancer progression rate measures the percentage of BE patients who advance to EAC. While the MGH and FHCRC models calculated the cancer progression rate as a prediction mainly based on fitting EAC incidence patterns, the UW-MISCAN model included calibration to progression rates of BE patients with age-at-diagnosis at age 65 and average 5 year follow-up, averaged between calendar years 1990–2005 (0.18% for males, 0.08% for females). However, the estimated cancer progression rates from UW-MISCAN for the other values of age-at-diagnosis with BE are model predictions. Additional intermediate outputs included the prevalence of GERD and BE adjusted to the 2000 U.S. population. GERD and BE are the two important precursor states of EAC. The existing literature reports a wide range of estimates on the prevalence of these two precursor states. As part of the comparative modeling method, we independently integrated the available evidence to produce estimates. The approaches to estimating these prevalence values among the three models are described in the Appendix.
Results
Calibration and Projections of Incidence and Mortality Rates
All three CISNET models were calibrated to individual birth-cohort incidence data from SEER. The EAC incidence and mortality rates for each cohort were aggregated to generate age-adjusted rates for the general population (age-adjusted to the 2000 U.S. Standard Population). The EAC incidence rates by calendar year until year 2010 for each birth cohort from the three independent models appeared to fit SEER 9 data well and appeared reasonably consistent with each other (refer to Figure A9 in the Appendix).
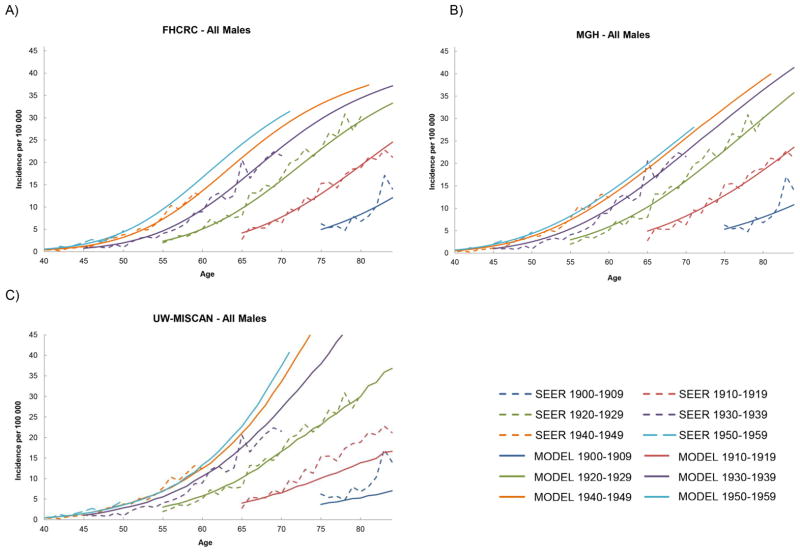
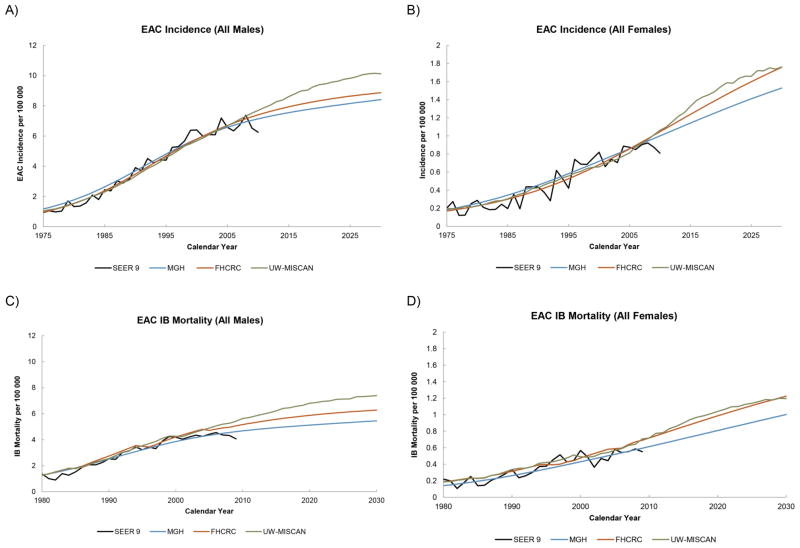
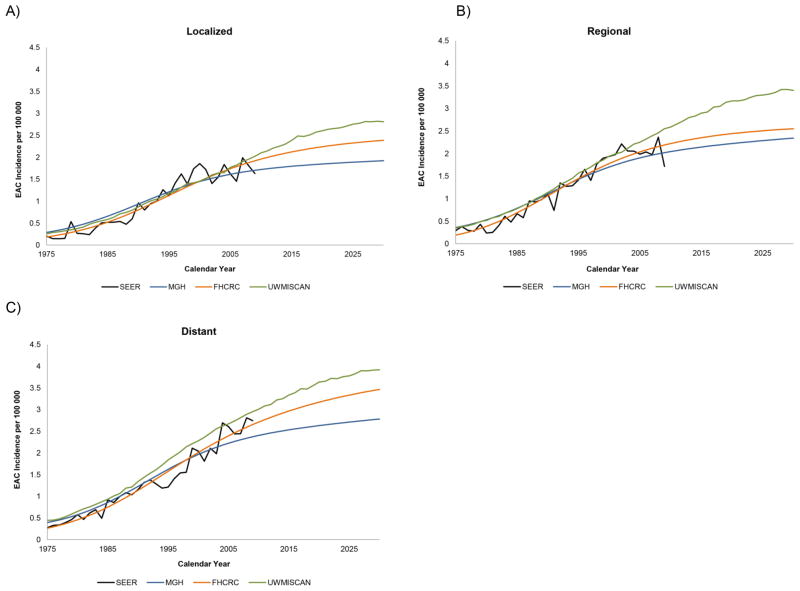
The male incidence rates by age until year 2030 for each birth cohort with projections from each model are shown in Figures 1a–1c. All three models project that the EAC incidence will continue to increase. For later birth cohorts, incidence results show that the incremental differences between birth cohorts has decreased, indicating a deceleration of the birth cohort effect. For females, the projected incidence rates from all models also showed increasing incidence rates by advancing birth cohorts (Appendix Figure A10). However, the trend in the incremental differences between birth cohorts was not as clear, likely due to the relatively low incidence rates for females with greater statistical variance of the estimated parameters resulting in model predictions with greater uncertainty. After aggregating the projections for all cohorts, the total EAC incidence and mortality rates between ages 20–84 (age-adjusted to the 2000 U.S. population) were calculated and are shown in Figures 2a–2d. Despite the differences in approach and mathematical formalism between the three models, all three models yielded small variations in their model fits to SEER and projections to 2030 for total EAC incidence and mortality rates. The three models all projected an increase in EAC incidence and mortality until 2030. The ranges of incidence and mortality rates for all males in 2030 predicted by three models are 8.4–10.1 and 5.4–7.4 cases per 100,000 person years, respectively. These translate to a 7–10 and 7–8 fold increase in the EAC incidence and mortality rates, respectively, from 1975 to 2030. For all females, the future incidence and mortality rates in 2030 are estimated to be 1.3–1.8 and 0.9–1.2 cases per 100,000 person years, respectively. From 1975 to 2030, the increases in incidence and mortality rates for females will increase by 8–9 and 9–10 times. A closer look at the incidence rate stratified by stage-at-diagnosis revealed that the localized EAC incidence rates exhibited the slowest increases, followed by regional and finally, distant (Figures 3a–3c).
Figure 1.
The EAC incidence rates by 10 year birth cohorts for all males. The cohort born in 1959 would be 71 years old in calendar year 2030. (A) FHCRC – All Males (B) MGH – All Males (C) UW-MISCAN – All Males.
Figure 2.
The SEER and projected EAC incidence rates for both males and females are shown in (A) and (B). The black line is the SEER data and the colored lines are predictions from the three simulation models. The incidence based mortality rates are shown in (C) and (D).
Figure 3.
The SEER and projected EAC incidence rates for all males stratified by stage at diagnosis are shown below. (A) Localized (B) Regional (C) Distant.
To quantify the impact on public health, we calculated the number of cumulative EAC cancer deaths estimated using our predicted mortality rates and population projections from the U.S. Census Bureau, summarized in Table 1. The estimated cumulative cause-specific EAC deaths from males and females in the years 2011–2030 will be between 142,300 and 186,298 cases. Compared to the EAC deaths in the years 1991–2010, the predicted number of cumulative cases will approximately double. In order to separate out the effect of changes in demographic composition, we repeated the calculation using the 2000 U.S. Standard Population. Our results indicate that the cumulative EAC deaths in the years 2011–2030 will be 1.6 times more than those in the previous two decades.
Table 1.
Cumulative EAC Deaths over Time
| Future Cumulative EAC Deaths: | Group | 1991–2010 | 2011–2030 |
|---|---|---|---|
|
| |||
| Males | MGH | 72,884 | 122,525 |
| FHCRC | 81,069 | 140,000 | |
| UW-MISCAN | 83,118 | 160,750 | |
|
| |||
| Females | MGH | 9,110 | 19,775 |
| FHCRC | 10,397 | 24,736 | |
| UW-MISCAN | 10,489 | 25,548 | |
|
| |||
| Total | MGH | 81,994 | 142,300 |
| FHCRC | 91,466 | 164,736 | |
| UW-MISCAN | 93,607 | 186,298 | |
The top two rows of the table display the predicted numbers of cumulative EAC deaths; the 1st and 2nd rows are numbers for males and females respectively. The bottom row of the table shows the male and female numbers combined.
Abbreviations: EAC = esophageal adenocarcinoma
Progression Rate and Prevalence of Precancerous States
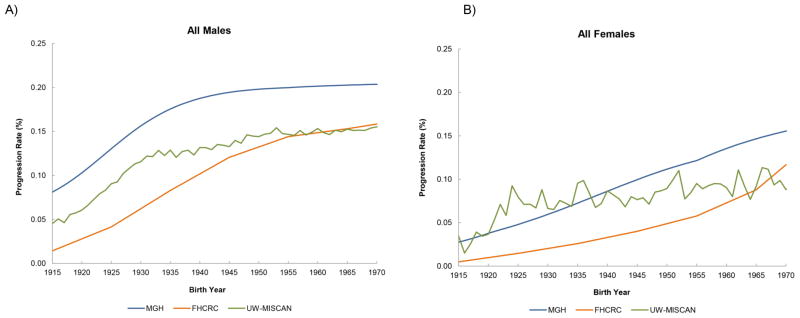
The rates of BE patients progressing to clinical EAC are shown in Figures 4a–4b. The results are shown for patients born between year 1915 to 1970 (these patients will be at age 60 between year 1975 and 2030) who were diagnosed with non-dysplastic BE or LGD at age 60 with five years of follow-up time. All three models suggested a strong birth cohort effect on the progression rates with increasing progression rates in younger birth cohorts until the cohorts born in 1940, followed by a leveling off in the cohorts born after 1940. For males born after 1940, the ranges of progression rates are 0.10–0.20% per person year. However, the three models predict that in contrast to males, progression rates for females have not yet leveled off.
Figure 4.
Progression rates by birth year are shown below. The progression rates were calculated for age-at-diagnosis with BE at age 60 with five years of follow-up time. (A) All Males (B) All Females.
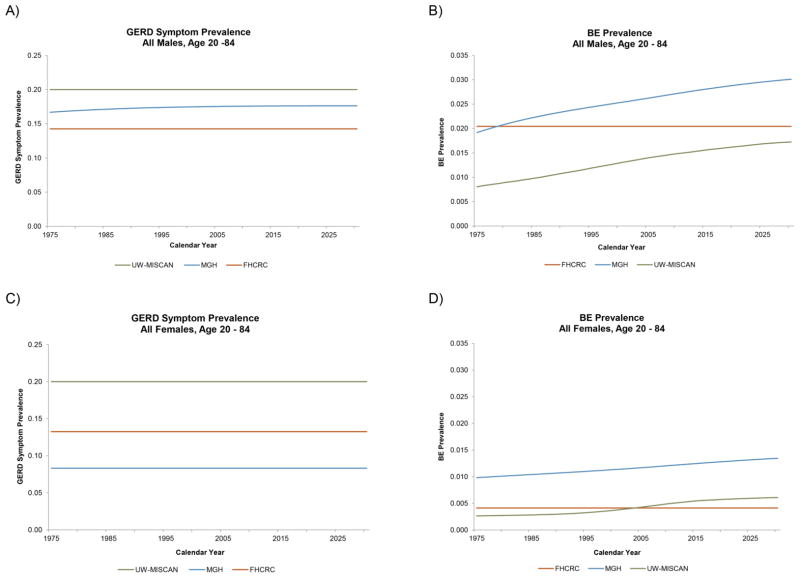
Figures 5a–5d show the prevalence of GERD symptoms and BE for males and females generated by the three CISNET modeling groups. The three groups have varying estimates for GERD prevalence, but overall showed similar trends. The outputs of GERD prevalence from three models remain relatively constant over time. For BE prevalence, the FHCRC model predicted no change with calendar year, while BE prevalence predictions from MGH and UW-MISCAN have increased over the past years.
Figure 5.
The estimated GERD symptom and BE prevalence of all males (top row) and all females (bottom row) in the US. (A) GERD Symptom Prevalence, All Males (B) BE Prevalence, All Males (C) GERD Symptom Prevalence, All Females (D) BE Prevalence, All Females.
EAC Sojourn Time
The EAC sojourn time is a useful concept to understand the nature of disease progression and detection. However, it is difficult to measure in clinical settings since it depends on unobservable events in the disease process (for example the appearance of preclinical cancer). The capacity of the models to predict this important time scale can be a strength and will help us to examine the impact of screening and specific interventions. Figures 6a–6b show the EAC sojourn time as estimated from the three models; these estimates show considerable differences in spite of model calibration using the same SEER 9 data. The EAC sojourn time estimate from the UW-MISCAN group is relatively constant over birth cohorts. Meanwhile, the EAC sojourn time predicted by the MGH model shows an increase with advancing birth years. The FHCRC model predicted that the EAC sojourn time decreases with birth year. The average male sojourn times are 10.2, 7.1, and 4.9 years for FHCRC, MGH, and UW-MISCAN, respectively. For females, the average sojourn times are 13.3, 9.0, and 4.5 years for FHCRC, MGH, and UW-MISCAN, respectively. The variation between this secondary prediction from the three groups can thus be attributed to fundamental differences in model structure and model-specific constraints used to facilitate model calibration.
Figure 6.
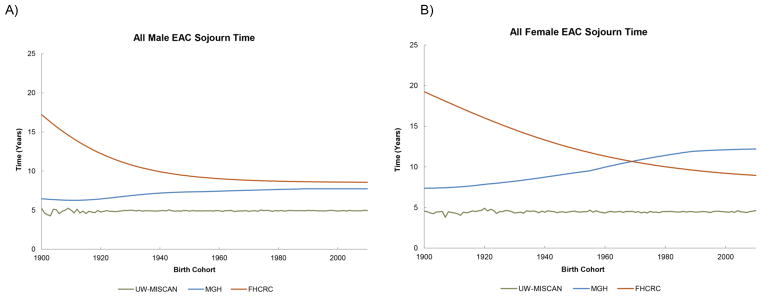
The EAC sojourn times (time between preclinical cancer and cancer diagnosis) for males and females are shown below. (A) All Male EAC Sojourn Time (B) All Female EAC Sojourn Time.
Discussion
Three independent mathematical models were calibrated to U.S. SEER 9 data, specifically the EAC incidence and IB mortality rates from 1975 to 2010. The models were then used to generate incidence and mortality projections until the year 2030. Although the models differ considerably in structure and design, from biologically-based modeling at the cellular level to empirically-based simulations of natural histories, the models’ projections (excluding sojourn time estimates) are consistent with one another. Furthermore, all three models identify modification of cancer progression rates (modeled as a birth cohort effect) as an important driver of the observed temporal trends for EAC incidence and mortality. We believe that comparative modeling greatly benefits from an interdisciplinary, collaborative approach to cancer risk prediction, increasing the credibility of model projections and deepening our understanding of EAC epidemiology and natural history. We conclude that EAC incidence and mortality rates will likely continue to increase until 2030 although the rate of the increase appears to weaken with advancing birth cohorts. For the period from 2011 to the year 2030 we estimate that there will be between 142,300 and 186,298 cumulative EAC deaths in the U.S. which is about double the number of EAC deaths that occurred between 1991 and 2010.
Several studies have reported the progression rates of patients with non-dysplastic BE to EAC. Hvid-Jensen et al. reported one of the lower rates of progression, 0.12% per person year to EAC among patients with non-dysplastic BE and LGD (32). A meta-analysis by Desai et al. reported a progression rate of 0.33% per person year from BE to EAC when only higher quality, more recent studies were included (33). A frequently cited progression rate of 0.5% per person year was reported from a meta-analysis that attempted to adjust, using a funnel plot, for publication bias (34). These reported progression rates range between 0.1% and 0.5% per person year and are consistent with our model estimates for younger cohorts.
The reporting of EAC sojourn time is rare in the literature. Previously, the MGH group had estimated that the range of sojourn times was between 4 to 9 years (35), which is in agreement with current results from both the MGH and UW-MISCAN groups. These sojourn time estimates are also consistent with a study which suggested the time from endoscopically detectable esophageal cancer to clinical symptoms as on average 4–5 years (36). The variability of EAC sojourn time estimates from three models can be attributed to the differences in the modeling approaches between research groups and inclusion or exclusion of additional calibration targets in addition to SEER 9 data. The three groups used different mathematical frameworks to model the disease progression of EAC. The UW-MISCAN model is a microsimulation model using discrete event formalism, the MGH model is a Markov transition state model, and the FHCRC group has developed a multiscale model that includes multistage clonal expansion processes and detailed spatio-temporal simulations of BE screening outcomes. Additional differences included disease pathways that were unique to each group’s model. For example, the UW-MISCAN model allows disease regressions prior to patients developing preclinical EAC while the MGH model does not allow for such regressions. In contrast, the FHCRC model explicitly incorporates cell death and differentiation and thus allows for clonal extinction of precursor lesions including preclinical cancer. The longer FHCRC estimates are likely a consequence of defining preclinical cancers as clonal lesions of any size, including smaller and earlier malignancies such as intramucosal carcinoma that may be difficult to detect endoscopically.
Our study has several limitations. First, although there are some known risk factors associated with EAC, none seem dominant. We opted to model the trend in EAC incidence and mortality by varying the transition rates between health states (MGH and UW-MISCAN) and the biological parameters (FHCRC) using a generalization of the age-period-cohort (APC) formalism. Thus, varying the transition rates as a proxy for changing risk factor trends did not allow us to investigate the etiology of EAC or to develop a cancer prevention and control policy to reduce the risk of developing EAC. However, the identification of cancer progression as an important driver of EAC trends may provide a focus for future investigation and possible interventions. We have developed a computational framework which allows us to update the analysis as additional clinical evidence on the key risk factor(s) and their impact on EAC emerges. Second, all of our models depict the biological progression following a specific sequence: healthy, absence or presence of GERD symptoms, BE without dysplasia, BE with dysplasia, preclinical cancer, and detected cancer. Although this is the commonly accepted paradigm for EAC carcinogenesis, all EACs may not follow this prescribed sequence or alternative pathways may exist within this paradigm adding heterogeneity which the models do not capture (37, 38).
Despite the limitations, our study has several strengths. We present incidence and mortality projections to 2030 using comparative modeling to investigate EAC trends in multiple birth cohorts. By modeling multiple cohorts separately, our models comprehensively capture incidence and mortality rates with changing age structures as different birth cohorts age. Our comparative modeling approach compares and contrasts the results from independently developed simulation and likelihood-based models using common calibration targets. The approach to resolve differences in model outputs is one of the benefits of comparative modeling, which has been used in other CISNET comparative modeling analyses. When differences are found, it provides the opportunity to pinpoint the source, which could be a result of error(s) in the model(s), or a consequence of fundamental lapses in knowledge surrounding the natural history of the disease. This approach provides a check for model validity but also the opportunity to identify and discuss gaps in knowledge. This iterative and in many aspects integrative process is perhaps the major strength of comparative modeling and the CISNET consortium, where preliminary model results can be discussed in an open and non-threatening environment conducive to model enhancements that improve risk predictions and therefore credibility.
A statistical analysis on EAC incidence between years 1973–2006 reported that the overall incidence may be plateauing in recent years (9). A subsequent analysis which included three additional years of incidence data found that the EAC incidence rate continues to increase, although at a decelerating rate (8). Our projections using three comprehensive computational cancer models also suggest that the incidence and mortality rates of EAC will continue to increase; however, the rate of increase appears to be slowing down for the younger male cohorts. In summary, we used a comparative modeling approach to examine U.S. population trends in EAC. The specific causes of the historical increase in EAC incidence and mortality remain unclear. However, our joint modeling of potential drivers behind the increasing incidence and mortality trends implicates an enhanced BE-to-EAC progression as significant factor (Figures 4a–4b), in addition to trends that may be driving up the prevalence of BE in the U.S. population as predicted by the MGH and UW-MISCAN models (Figures 5a–5d). The future projected increase in cumulative EAC deaths and incidence reflect a significant concern and burden to society. Our findings highlight the importance of public health and cancer control planning with potential interventions to curtail the projected EAC morbidity and mortality.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Institutes of Health (grant U01CA152926 to the CISNET Esophagus group and grant K25CA133141 to Dr. Kong)
Footnotes
Disclosures: None
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Kong CY, Nattinger KJ, Hayeck TJ, Omer ZB, Wang YC, Spechler SJ, et al. The impact of obesity on the rise in esophageal adenocarcinoma incidence: estimates from a disease simulation model. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2011;20:2450–6. doi: 10.1158/1055-9965.EPI-11-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrift AP, Pandeya N, Smith KJ, Green AC, Hayward NK, Webb PM, et al. Helicobacter pylori infection and the risks of Barrett’s oesophagus: a population-based case-control study. Int J Cancer. 2012;130:2407–16. doi: 10.1002/ijc.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, et al. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. Journal of the National Cancer Institute. 2004;96:388–96. doi: 10.1093/jnci/djh057. [DOI] [PubMed] [Google Scholar]
- 5.Thrift AP, Kramer JR, Qureshi Z, Richardson PA, El-Serag HB. Age at onset of GERD symptoms predicts risk of Barrett’s esophagus. The American journal of gastroenterology. 2013;108:915–22. doi: 10.1038/ajg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of Endoscopic Surveillance on Mortality From Barrett’s Esophagus-Associated Esophageal Adenocarcinomas. Gastroenterology. 2013;145:312–9. e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Annals of internal medicine. 2003;138:176–86. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2010;19:1468–70. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 10.Screening for breast cancer: U S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 11.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 12.USPSTF. US Preventive Services Task Force Draft Recommendation Statement. 2013. Screening for Lung Cancer. AHRQ Publication No. 13-05196-EF-3. [Google Scholar]
- 13.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, van Ballegooijen M, Kuntz KM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. Journal of the National Cancer Institute. 2010;102:1238–52. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Wilschut JA, Zauber AG, van Ballegooijen M. Stool DNA testing to screen for colorectal cancer in the Medicare population: a cost-effectiveness analysis. Annals of internal medicine. 2010;153:368–77. doi: 10.1059/0003-4819-153-6-201009210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SEER. Surveillance, Epidemiology, and End Results (SEER) Program. 2010 ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973–2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 16.Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States) Cancer Causes Control. 2006;17:971–81. doi: 10.1007/s10552-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 17.Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013;73:1086–96. doi: 10.1158/0008-5472.CAN-12-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruigomez A, Wallander MA, Lundborg P, Johansson S, Rodriguez LAG. Gastroesophageal reflux disease in children and adolescents in primary care. Scandinavian journal of gastroenterology. 2010;45:139–46. doi: 10.3109/00365520903428606. [DOI] [PubMed] [Google Scholar]
- 19.Ruigomez A, Rodriguez LAG, Wallander MA, Johansson S, Graffner H, Dent J. Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharm Ther. 2004;20:751–60. doi: 10.1111/j.1365-2036.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 20.Hur C, Hayeck TJ, Yeh JM, Richards EB, Spechler SJ, Gazelle GS, et al. Development, Calibration, and Validation of a U.S. White Male Population-Based Simulation Model of Esophageal Adenocarcinoma. PloS one. 2010;5:e9483. doi: 10.1371/journal.pone.0009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–7. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moolgavkar SH, Holford TR, Levy DT, Kong CY, Foy M, Clarke L, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst. 2012;104:541–8. doi: 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Statistics in medicine. 1987;6:469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 24.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–24. [PubMed] [Google Scholar]
- 25.Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:15095–100. doi: 10.1073/pnas.222118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupper LL, Janis JM, Karmous A, Greenberg BG. Statistical age-period-cohort analysis: a review and critique. J Chronic Dis. 1985;38:811–30. doi: 10.1016/0021-9681(85)90105-5. [DOI] [PubMed] [Google Scholar]
- 27.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 28.Zheng T, Holford TR, Boyle P, Chen Y, Ward BA, Flannery J, et al. Time trend and the age-period-cohort effect on the incidence of histologic types of lung cancer in Connecticut, 1960–1989. Cancer. 1994;74:1556–67. doi: 10.1002/1097-0142(19940901)74:5<1556::aid-cncr2820740511>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: An application to female breat cancer. Journal of clinical epidemiology. 1994;47:1451–61. doi: 10.1016/0895-4356(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 30.Stout NK, Knudsen AB, Kong CY, McMahon PM, Gazelle GS. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27:533–45. doi: 10.2165/11314830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bureau UC. US Census Bureau. [last accessed May 1, 2013];US population projections. 2013 http://www.census.gov/population/www/projections.
- 32.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of Adenocarcinoma among Patients with Barrett’s Esophagus. New England Journal of Medicine. 2011;365:1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 33.Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–6. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and Cancer in a Large Multicenter Cohort of Patients With Barrett’s Esophagus. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2006;4:566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Hur C, Hayeck TJ, Yeh JM, Richards EM, Spechler SJ, Gazelle GS, et al. Development, calibration, and validation of a U.S. white male population-based simulation model of esophageal adenocarcinoma. PloS one. 2010;5:e9483. doi: 10.1371/journal.pone.0009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guanrei Y, Songliang Q, He H, Guizen F. Natural history of early esophageal squamous carcinoma and early adenocarcinoma of the gastric cardia in the People’s Republic of China. Endoscopy. 1988;20:95–8. doi: 10.1055/s-2007-1018145. [DOI] [PubMed] [Google Scholar]
- 37.Cook MB. Optimization and expansion of predictive models for Barrett’s esophagus and esophageal adenocarcinoma: could a life-course exposure history be beneficial? Am J Gastroenterol. 2013;108:923–5. doi: 10.1038/ajg.2013.83. [DOI] [PubMed] [Google Scholar]
- 38.Shaheen NJ. Editorial: Predicting the risk of Barrett’s esophagus: does the BERET fit? Am J Gastroenterol. 2013;108:363–5. doi: 10.1038/ajg.2012.455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.