Abstract
Background
Healthcare associated mold infections (HAEMI) increase morbidity and mortality in children with leukemia. Excavation adjacent to Children’s Medical Center Dallas (CMCD) April 2006–February 2007 provided an opportunity to determine if excavation adjacent to a hospital building is associated with increased risk of developing HAEMI in children receiving intensive chemotherapy for acute leukemia.
Methods
Children who began receiving intensive chemotherapy for acute leukemia at CMCD from 2004–2008 were identified (N=275). Exposures to the CMCD campus during intensive chemotherapy and duration of neutropenia per exposure were recorded. Proven, probable or possible invasive fungal disease (IFD) was classified using EORTC/MSG guidelines. Institutional guidelines categorized mold infections as definite or possible HAEMI. A bivariate time-to-event model compared the association of excavation with HAEMI and yeast infections, controlling for neutropenia.
Results
There were 7454 CMCD exposures, 1007(13.5%) during excavation. Of 50 cases of IFD, 31 were HAEMI. By time-to-event analysis exposure to the CMCD campus during the excavation period was significantly associated with HAEMI (HR=2.8, P=0.01) but not yeast infections (HR=0.75, P=0.75). Neutropenia was significantly associated with both HAEMI and yeast infections (P<0.001). Voriconazole prophylaxis did not prevent HAEMI in 42% of the 14 patients with AML who had been receiving this agent.
Conclusion
This study is the first to demonstrate an association between exposure to hospital construction that includes excavation and HAEMI in pediatric oncology patients. Since neutropenic patients need protection from aerosolized fungal spores during visits to expanding medical centers, preventive strategies with adherence monitoring need additional study.
Keywords: Hospital construction, Mold infections, Acute Leukemia, Pediatric Oncology
INTRODUCTION
Aggressive chemotherapeutic regimens have improved long-term survival for pediatric cancer patients to nearly 80% [1], but one consequence has been an increase in the incidence of invasive fungal disease (IFD) [2, 3]. Patients receiving therapy for acute leukemia have several risk factors for developing IFD: 1) presence of long term central venous catheters; 2) frequent episodes of prolonged, severe neutropenia; 3) frequent and prolonged exposure to broad-spectrum antibiotics; 4) therapy with high-dose corticosteroids; 5) hyperglycemia; 6) mucositis; and 7) frequent, prolonged exposure to the healthcare environment [4–9]. To date, the largest study of pediatric patients with cancer reported that 67% of IFD occurred during therapy for acute leukemia with 11-year cumulative incidence rates of 9 and 10% for patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia/lymphoma (ALL), respectively [10]. Yeast infections result from intrinsic organisms present on host mucosa, most frequently Candida spp., that invade the bloodstream. In contrast, mold infections result from exposure to spores of extrinsic environmental pathogens, most frequently by inhalation. Historically, Aspergillus spp. have been the most frequently identified molds. Recently, molds isolated from cancer patients have been more varied and include Fusarium, Scedosporium, Zygomycetes, Bipolaris, Paecilomyces, Exserohilum species, etc.[11, 12].
The increased risk of IFD in immunocompromised patients exposed to aerosolized mold spores in hospital environments during periods of construction at adult health care facilities is well documented [13–17]. Children’s Medical Center Dallas (CMCD) underwent major renovation from 2006–2008, including an excavation of the grounds (April 1, 2006–January 31, 2007) for construction of a new tower connected to the existing buildings. This study was designed to ascertain whether the risk of developing healthcare associated environmental mold infections (HAEMI) in children receiving intensive chemotherapy for acute leukemia who were exposed to CMCD, either as inpatients or ambulatory patients, was increased during the excavation period, despite recommendations issued by the Infection Prevention and Control Department, intended to reduce the exposure of these high risk patients to mold spores. Our study evaluated the association between exposure to hospital construction and the risk of developing HAEMI in pediatric oncology patients by comparing the risk of developing HAEMI with that of invasive yeast infections that are also increased by prolonged neutropenia, but not by exposure to spores associated with excavation.
PATIENTS and METHODS
Patients
We identified a retrospective cohort of all patients who received therapy for ALL or AML at CMCD from January 1, 2004 to December 31, 2008. Intensive therapy was defined as the period from the initiation of Induction to the beginning of Maintenance therapy for patients with ALL. National Cancer Institute (NCI) standard risk ALL patients included those who were 1–9 years old at diagnosis and had initial white blood cell count <50,000 cell/mm3. NCI high-risk ALL patients included those who were <1 year or >10 years old at diagnosis or those who had initial white blood cell count >50,000 cell/mm3. For patients with AML, intensive therapy began with Induction and continued until recovery of an absolute neutrophil count (ANC) of 500 cell/mm3 after their final course of chemotherapy. The Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas approved the study.
Infection Prevention and Control (IPC) Precautions
To minimize mold exposure during excavation, the following precautions were recommended by the IPC Department [18–21]: 1) Windows of the existing adjacent building were resealed before excavation began; 2) Construction crews were instructed to maintain the excavation site in a moist state to reduce the quantity of aerosolized mold spores; 3) Air handling systems were optimized as follows: a) The inpatient oncology/hematopoietic stem cell transplant (HSCT) and intensive care units had central high-efficiency particulate air (HEPA) filtered air handling units with redundant units programmed to turn on in the case of equipment failure; b) The outpatient clinic was equipped with portable HEPA filters in rooms and hallways until December 2007 when the move to a different building provided central HEPA filtration; and c) Portable HEPA filters were placed in the rooms of neutropenic oncology patients admitted to hospital floors without central HEPA filtration and patients were confined to their rooms; 4)When travelling out of HEPA-filtered areas for diagnostic or therapeutic procedures, an N-95 respirator was placed on high risk patients; 5) Oncology and HSCT unit staff were encouraged to enter and exit the hospital via enclosed sky-bridges to avoid contamination of clothing and shoes with spores emanating from the construction site; 6) Families were given N-95 respirators and instructed to place them on the patient upon arrival at the CMCD campus before exiting the vehicle and until arriving to a HEPA-filtered area; and 7) An “Infection Control Valet” parking service was provided for all oncology patients, immediately adjacent to the hospital entrance, to reduce environmental exposure during walks from the parking garage. Audits of adherence to the recommended use of N-95 respirators and “Infection Control Valet” use were not performed.
Antimicrobial prophylaxis
The only prophylactic anti-bacterial agents received by the patients included in this study were oral trimethoprim-sulfamethoxazole, inhaled or intravenous pentamadine for prevention of Pneumocystis jiroveci. Infants with ALL received protocol directed fluconazole prophylaxis. Voriconazole prophylaxis was initiated for all patients with AML, beginning in September 2007 [22]. Previously, patients with AML received fluconazole prophylaxis per physician preference. Granulocyte colony stimulating factor was used as directed by protocol.
Data Collection
Baseline data collected on all patients included gender, ethnicity, age at diagnosis of malignancy, the type of malignancy and treatment plan. We reviewed patient records for all exposures to CMCD that occurred during intensive chemotherapy. Exposures included every outpatient visit and hospitalization. The duration of each exposure and the duration of severe neutropenia was determined for each exposure. Severe neutropenia was defined as an ANC below 500cell/mm3 or if the patient had not achieved a complete remission regardless of ANC. During each exposure, the presence of other risk factors for developing IFD was recorded: number of days receiving intravenous broad-spectrum antibiotics; mucositis; hyperglycemia (serum glucose ≥200mg/dL); and the administration of corticosteroids. Data collection stopped when patients developed a mold infection, transferred to another facility, received a hematopoietic stem cell transplant, relapsed or died before completing intensive therapy or completed intensive therapy in first remission. Additional data were collected on patients who developed IFD: the treatment phase, the pathogen and extent of disease, additional risk factors, antifungal medication(s) used and length of therapy, outcome of the IFD, any delays in treatment of the underlying malignancy, and the patient’s status at the time of analysis (alive, death from IFD or complications of IFD therapy, death from other causes).
All diagnostic studies for IFD were reviewed and days of antifungal therapy were recorded for each patient. Our standard practice is to initiate antifungal coverage and evaluate for IFD when: 1) A severely neutropenic patient remains febrile on the 5th day of an admission for fever and neutropenia; 2) A severely neutropenic patient defervesces and then develops new fever [23] or develops clinical signs suggestive of IFD. Evaluations include a computerized tomography scan (CT) of the chest without intravenous (IV) contrast, an abdominal ultrasound, and biopsy and/or cultures of suspicious lesions or drainage. A fiber-optic nasal evaluation at the bedside by an attending staff otolaryngologist was done sporadically until November 2006 when it became standard practice for evaluation of prolonged fever and neutropenia. Additional imaging, bronchoalveolar lavage specimens, lung and other tissue biopsies were obtained when clinically indicated and considered safe.
Classification of IFD
We identified patients who developed proven, probable, or possible IFD using the most recent guidelines of the European Organization for Research and Treatment of Cancer and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) [24]. Since no consensus guidelines existed for classifying mold infections as healthcare associated (HAEMI) or community acquired, in 2003, the CMCD Infection Prevention and Control Department developed institutional guidelines to classify mold infections as definite or possible HAEMI. Patients with definite HAEMI had a proven invasive mold infection with initial signs and symptoms appearing for the first time during a hospitalization > seven days after admission or within seven days of a previous hospitalization. Mold infections were classified as a possible HAEMI 1) if they were possible or probable invasive infections with initial symptoms that first appeared after day seven of a hospitalization or if there were ≥ 2 clinic visits in the past 2 weeks or 2) Proven mold infections when initial signs and symptoms may have been present at admission or developed within 7 days of hospitalization and/or with a history of 2 clinic visits in the previous 2 weeks, but no diagnostic testing was performed and no other exposure was identified. All IFD cases were jointly reviewed and classified by Oncology and Infectious Disease physicians and members of the IPC staff.
We used a bivariate time-to-event model to assess the evidence that development of a HAEMI during intensive therapy was associated with excavation at CMCD[25]. This model simultaneously estimated the excavation hazard rates for HAEMI and invasive yeast infections by fitting a proportional hazards model to the observed times to development of either type of infection. Indicators for excavation and neutropenia were used as time-dependent covariates. We used the robust covariance estimate aggregated over patient ID, to account for observations of the same patient in both strata of the bivariate time-to-event model [26] To visualize the incidence of HAEMI and yeast infections over time, we fit a discrete proportional hazards model to the weekly event data, expanding calendar week in a quadratic spline basis with 8 knots placed uniformly across percentiles of the data. SAS/STAT® software, version 9.2, was used for all analyses.
RESULTS
We identified 275 patients who received intensive chemotherapy at CMCD for acute leukemia from 2004–2008 (Table 1). Ninety-three percent (257) of patients received all of their intensive chemotherapy at CMCD. The study population included an almost equal gender distribution and several racial and ethnic groups were represented (Table 2). Of 7454 CMCD exposures, 1007 (13.5%) occurred during excavation.
TABLE I.
Malignancies in Study Population in Years 2004–2008 (N=275)
| Malignancy | 2004 | 2005 | 2006 | 2007 | 2008 | Totals |
|---|---|---|---|---|---|---|
| Standard risk ALL | 22 | 19 | 16 | 27 | 28 | 112 |
| High risk ALL | 15 | 29 | 21 | 20 | 29 | 102 |
| Lymphoblastic Lymphoma | 3 | 4 | 3 | 1 | 1 | 12 |
| AML | 9 | 3 | 12 | 12 | 12 | 49 |
|
| ||||||
| Totals | 46 | 51 | 50 | 59 | 69 | 275 |
ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia
TABLE II.
Baseline and Clinical Characteristics of Patients (N=275)
| Characteristics | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 142 (51.6) |
| Female | 133 (48.4) |
| Race | |
| Caucasian | 120 (43.6) |
| Hispanic | 114 (41.4) |
| African American | 28 (10.2) |
| Asian | 8 (2.9) |
| Other | 5 (1.8) |
| Diagnosis | |
| Standard Risk ALL | 112 (40.7) |
| High Risk ALL | 102 (37.1) |
| Lymphoblastic Lymphoma | 12 (4.4) |
| AML | 49 (17.8) |
| Antifungal Prophylaxis | |
| None | 239 (86.9) |
| Fluconazole | 22 (8.0) |
| Voriconazole | 14 (5.1) |
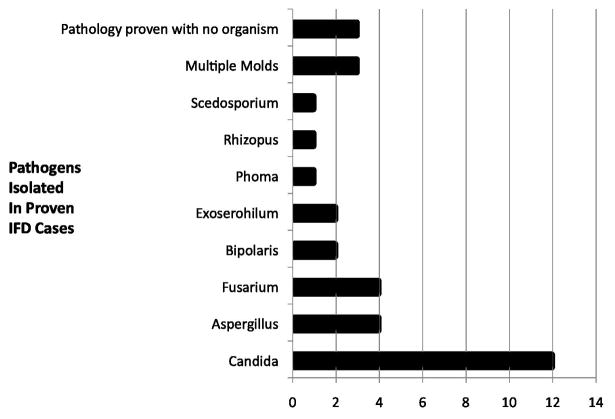
There were 50 cases of proven (n=34) or possible (n=16) IFD in 275 patients (18%) (Table 3). IFD was more common in patients with AML and NCI high-risk ALL (cumulative incidence 41% and 21% respectively). Thirteen (26%) cases of IFD were yeast infections: proven (n=12) or possible (n=1), with all proven infections caused by Candida spp. (Figure 1). Thirty-seven (74 %) cases of IFD were caused by molds: 22 proven and 15 possible. Among patients with mold infections, eight had multiple sites of involvement, 17 lung only, seven sinus only and five another single site of disease. Thirteen cases of mold infections were initially diagnosed by nasal fiber-optic endoscopy. In addition to Aspergillus spp, several other pathogenic molds were identified (Figure 1). Thirty-one mold infections were classified as being possible (n=23) or definite (n=8) HAEMI. The six community-acquired mold infections were not included in the time to event analysis, but were included in the descriptive summaries.
TABLE III.
Invasive Fungal Disease in Study Population
| Invasive Fungal Disease (N=50) | |
| Proven Yeast | 12 |
| Possible Yeast | 1 |
| Proven Mold | 22 |
| Possible Mold | 15 |
| HAEMI (N=31) | |
| Definite HAEMI | 8 |
| Possible HAEMI | 23 |
HAEMI, Healthcare Associated Environmental Mold Infections
Fig. 1.
Proven cases of IFD in study population. All proven yeast infections were due to Candida spp while a variety of mold pathogens were identified.
Time-to-event analysis, controlling for neutropenia, found that excavation exposure was significantly associated with HAEMI (HR=2.8, P=0.01) but not with yeast infections (HR=0.75, P=0.78). Each day of neutropenia was significantly associated with the development of both yeast and HAEMI (HR=1.1, P<0.001). When hyperglycemia, mucositis, antifungal prophylaxis and steroid treatment were added to the model, exposure to excavation continued to be significantly associated with developing HAEMI (HR=2.6, P=0.03) but not yeast infections (HR=0.69, P=0.73) and neutropenia continued to be significantly associated with both HAEMI and yeast infections (HR=1.1, P <0.001), but none of the other variables in the model were significantly associated with developing HAEMI or yeast infections (all P>0.4).
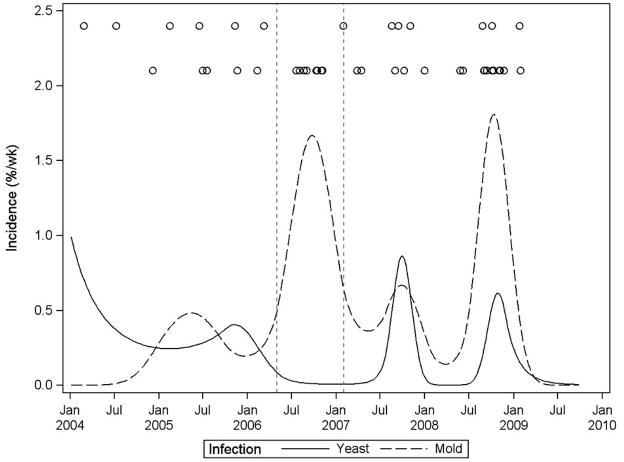
Figure 2 illustrates the incidence of HAEMI and yeast infections in neutropenic patients throughout the study period. During the excavation period, there is a prominent peak in HAEMI incidence with no concurrent peak in incidence of yeast infections. Following the excavation period, HAEMI and yeast infections rise and fall concurrently.
Fig. 2.
Incidence of HAEMI (dashed curve) and yeast infections (solid curve) in neutropenic patients throughout the study period with superimposed HAEMI (bottom circles) and yeast infection (top circles) events. The excavation period is indicated by vertical dashed lines.
The median length of severe neutropenia at the time of IFD diagnosis was 16 days (range 0–35 days). The median duration of antifungal therapy in IFD cases was 162 days (range 8–990 days). Sixteen of the 50 (32%) patients with IFD died; eleven deaths (69%) occurred in patients with mold infections. Ten of the 16 deaths (62.5%) were attributed to progressive IFD, complications of IFD therapy or relapse attributed to chemotherapy modifications (Table 4). Six deaths were not related to IFD (Table 4). The diagnosis of IFD required major changes to planned chemotherapy in 36% of cases (18/50). Five patients received less intensive chemotherapy and 13 patients received no further intensive chemotherapy. Four of these 18 patients developed recurrent leukemia, and died.
TABLE IV.
Deaths in Patients Diagnosed with IFD
| Cause of Death | Deaths (N=16) |
|---|---|
Complications of mold infection
|
9 |
| Progressive yeast infection | 1 |
| Relapse unrelated to IFD | 2 |
| Secondary AML | 1 |
| Liver failure due to Asparaginase toxicity | 2 |
| Liver failure due to Adenovirus | 1 |
IFD, Invasive Fungal Disease; AML, Acute Myeloid Leukemia
Twenty-two patients (5 infants with ALL and 17 with AML) had been receiving fluconazole prophylaxis. Four (18%) patients, all with AML, developed IFD: two cases of proven invasive Candidiasis, one case of proven disseminated Fusariosis, and one case of proven infection with Aspergillus and Scedosporium spp. Voriconazole prophylaxis was given to 14 patients with AML and six (42.9%) of these patients developed a mold infection. In this group, there were three possible mold infections (CT scans with pulmonary nodules consistent with mold etiology), and one case each of proven Aspergillosis, Bipolaris and Zygomycetes infections. No yeast infections occurred in patients receiving voriconazole prophylaxis.
DISCUSSION
This study linked hospital excavation to incident HAEMI in pediatric oncology patients. Despite the prospective recommendations for measures designed to reduce the risk of mold exposure while on the CMCD campus, excavation was found to be significantly associated with increased risk of developing HAEMI. Our data support the findings of previous studies in adults that demonstrate a temporal relationship between hospital construction and an increased incidence of HAEMI and those of previous studies in children demonstrating the association of prolonged, severe neutropenia with the development of IFD caused by both yeasts and molds [4–6].
We cannot be certain if the recommended environmental safeguards were ineffective because we did not monitor adherence. Lehrnbecher et al have demonstrated that pediatric oncology patient adherence to anti-infection precautions is highly variable [27]. Improving family education and monitoring adherence will likely enhance the effectiveness of known preventive measures in the future. Recent publications have demonstrated the effectiveness of infection prevention and control measures to reduce the risk of HAEMI during construction when there is collaboration among infection prevention and control, construction, and oncology staff members [28, 29].
It is important to note that even after the excavation was completed, there were other construction-related activities in progress until the new tower was completed. In September 2008, an invited external environmental consultant observed changes in building pressurization that could have potentially allowed airflow from the new tower during construction into the existing adjacent tower that housed oncology patients. This could potentially explain the spike in HAEMI noted in late 2008. Additionally, mold species similar to those that caused disease in patients were recovered from air sampling of the physical therapy gym and selected patient rooms. Finally, Figure 2 shows that HAEMI and yeast infections rise and fall concurrently following the excavation period, suggesting that variation in host susceptibility was a contributor.
Six of 14 (42.9%) patients with AML who received voriconazole prophylaxis developed mold infections. Three cases did not have an etiologic agent confirmed and one was caused by Zygomycetes, a mold known to emerge following the use of voriconazole prophylaxis[30–33]. Voriconazole levels were not routinely evaluated until September of 2011. Thus, explanations for failure of voriconazole prophylaxis include poor patient adherence, inadequate dosing resulting in sub therapeutic serum concentrations, or selective pressure leading to emergence of molds that are resistant to voriconazole. Continued surveillance is required in order to further define the risk of emergence of resistant molds in patients receiving voriconazole prophylaxis when therapeutic serum levels are achieved.
The limitations of this study include 1) the retrospective nature of the study; 2) inclusion of possible and probable as well as proven mold infections, as defined by EORTC/MSG guidelines; 3) absence of biopsy material in many patients; and 4) the infrequency of obtaining autopsies, since some IFD may be diagnosed only at autopsy[34]. In addition, defining the exact time of onset of symptoms, prior to diagnosis of a mold infection, was often challenging due to the nonspecific nature of clinical presentation and the uncertainty of incubation period; therefore, some infections may have been misclassified as HAEMI. Spore concentrations in the air outside of CMCD were not measured before, during and after the excavation. However, it is well established that excavation is associated with increased spore concentrations in the surrounding air [35, 36]. In addition, there may have been unprotected environmental exposures that were not recorded in the medical record, (e.g., trips to radiology or other hospital locations lacking HEPA-filtered air without wearing N–95 respirators). Another limiting factor is that there were dust-producing construction and renovation projects and occasional episodes of water leaks throughout our hospital before the initiation and after the completion of the excavation phase of construction and specific patient exposures were impossible to ascertain. Although hospital policies required the construction of appropriate barriers for dust – producing activities and prompt remediation of water leaks that did not dry within 72 hours, undetected breaches may have occurred. Lastly, the routine incorporation of direct nasal endoscopy during the last three months of the initial excavation period may have improved our ability to identify cases of proven invasive mold infections and the routine administration of voriconazole to patients with AML, beginning after the completion of the initial excavation phase may have reduced the incidence of yeast infections.
Despite these limitations, our study has several strengths. To reduce selection bias we identified a large, well-characterized cohort and all eligible patients were analyzed. Established diagnostic criteria were used to identify and describe IFD. All IFD cases were reviewed and classified jointly by staff from Oncology, Infectious Disease and IPC. The validity of our results is strengthened by the fact that our findings are consistent with previous studies that demonstrate that neutropenia is significantly associated with any type of IFD [4–6] and findings in adult oncology populations.
In conclusion, pediatric oncology patients receiving intensive chemotherapy for acute leukemia are at increased risk for developing IFD during periods of severe neutropenia. Exposure to hospital construction that includes excavation is associated with an increased risk of developing HAEMI but not yeast infections. Since construction is inevitable at most U.S. medical centers, future studies are needed to determine how to achieve increased adherence to recommended preventive measures, to develop and validate new environmental preventive strategies, to improve diagnostic testing for mold infections, to increase rates of obtaining biopsy and autopsy specimens, and to assess the effectiveness of prophylaxis with newer antifungal agents. Prevention of exposure to mold spores is the optimal strategy for prevention of HAEMI.
Acknowledgments
We thank Anju Varghese for her assistance in the categorization of fungal infections. This work was supported in part by grants by the NIH KL-2.
References
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26(4):781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Marinus A, Cohen J, et al. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. J Infect. 1998;37(2):173–80. doi: 10.1016/s0163-4453(98)80173-4. [DOI] [PubMed] [Google Scholar]
- 4.Empiric antifungal therapy in febrile granulocytopenic patients. EORTC International Antimicrobial Therapy Cooperative Group. Am J Med. 1989;(86):668–72. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 5.Walsh TJ, Rubin M, Hathorn J, et al. Amphotericin B vs high-dose ketoconazole for empirical antifungal therapy among febrile, granulocytopenic cancer patients. A prospective, randomized study. Arch Intern Med. 1991;151:765–770. [PubMed] [Google Scholar]
- 6.Pizzo PA, Robichaud KJ, Wesley R, Commers JR. Fever in the pediatric and young adult patient with cancer. A prospective study of 1001 episodes. Medicine. 1982;61(3):153–65. doi: 10.1097/00005792-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–38. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 8.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–75. [PubMed] [Google Scholar]
- 9.Hamad M. Antifungal immunotherapy and immunomodulation: a double-hitter approach to deal with invasive fungal infections. Scand J Immunol. 2008;67(6):533–43. doi: 10.1111/j.1365-3083.2008.02101.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosen GP, Nielsen K, Glenn S, et al. Invasive fungal infections in pediatric oncology patients: 11-year experience at a single institution. J Pediatr Hematol Oncol. 2005;27(3):135–40. doi: 10.1097/01.mph.0000155861.38641.ca. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TJ, Groll A, Hiemenz J, et al. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10 (Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 12.Malani AN, Kauffman CA. Changing epidemiology of rare mould infections: implications for therapy. Drugs. 2007;67(13):1803–12. doi: 10.2165/00003495-200767130-00001. [DOI] [PubMed] [Google Scholar]
- 13.Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol. 2001;66(4):257–62. doi: 10.1002/ajh.1054. [DOI] [PubMed] [Google Scholar]
- 14.Streifel AJ, Lauer JL, Vesley D, et al. Aspergillus fumigatus and other thermotolerant fungi generated by hospital building demolition. Appl Environ Microbiol. 1983;46(2):375–78. doi: 10.1128/aem.46.2.375-378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pini G, Faggi E, Donato R, et al. Invasive pulmonary aspergillosis in neutropenic patients and the influence of hospital renovation. Mycoses. 2008;51(2):117–22. doi: 10.1111/j.1439-0507.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang CC, Cheng AC, Devitt B, et al. Successful control of an outbreak of invasive aspergillosis in a regional haematology unit during hospital construction works. J Hosp Infect. 2008;69(1):33–38. doi: 10.1016/j.jhin.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Sarubbi FA, Jr, Kopf HB, Wilson MB, et al. Increased recovery of Aspergillus flavus from respiratory specimens during hospital construction. Am Rev Respir Dis. 1982;125(1):33–38. doi: 10.1164/arrd.1982.125.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SM, Streifel AJ. Infection control considerations during construction activities: land excavation and demolition. Am J Infect Control. 2001;29(5):321–28. doi: 10.1067/mic.2001.118410. [DOI] [PubMed] [Google Scholar]
- 19.Weber DJ, Peppercorn A, Miller MB, et al. Preventing healthcare-associated Aspergillus infections: review of recent CDC/HICPAC recommendations. Med Mycol. 2009;47 (Suppl 1):S199–209. doi: 10.1080/13693780802709073. [DOI] [PubMed] [Google Scholar]
- 20.Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 21.Siegel JD, Rhinehart E, Jackson M, et al. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vehreschild JJ, Böhme A, Buchheidt D, et al. A double-blind trial on prophylactic voriconazole (VRC) or placebo during induction chemotherapy for acute myelogenous leukaemia (AML) J Infect. 2007 Nov;55(5):445–9. doi: 10.1016/j.jinf.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34(6):730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 24.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei LJ, Lin DY, Weissfeld L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 26.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 27.Lehrnbecher T, Laws HJ, Boehm A, et al. Compliance with anti-infective preventive measures: A multicentre survey among paediatric oncology patients. Eur J Cancer. 2008;44(13):1861–5. doi: 10.1016/j.ejca.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Kidd F, Buttner C, Kressel AB. Construction: A model program for infection control compliance. Am J Infection Control. 2007;35:347–50. doi: 10.1016/j.ajic.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Berger J, Willinger B, Diab-Elschahawi M, et al. Effectiveness of preventive measures for hemato-oncologic patients undergoing stem cell transplantation during a period of hospital construction. Am J Infect Control. 2011;39:746–51. doi: 10.1016/j.ajic.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary care cancer center in the era of Aspergillosis-active antifungal therapy: a case control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 31.Oren I. Breakthrough zygomycosis during empirical voriconazole therapy in febrile patients with neutropenia. Clin Infect Dis. 2005;40(5):770–71. doi: 10.1086/427759. [DOI] [PubMed] [Google Scholar]
- 32.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoetic stem-cell transplants. N Engl J Med. 2004;350(9):950–52. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 33.Chamilos G, Kontoyiannis DP. Voriconazole-resistant disseminated Paecilomyces variotii infection in a neutropenic patient with leukemia on voriconazole prophylaxis. J of Infect. 2005;51(4):225–28. doi: 10.1016/j.jinf.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Seftel MD, Ho M, Pruthi D, et al. High rate of discordance between clinical and autopsy diagnoses in blood and marrow transplantation. Bone Marrow Transplant. 2007;40(11):1049–1053. doi: 10.1038/sj.bmt.1705855. [DOI] [PubMed] [Google Scholar]
- 35.Partridge-Hinckley K, Liddell GM, Almyroudis NG, Segal BH. Infection control measures to prevent invasive mould diseases in hematopoietic stem cell transplant recipients. Mycopathologia. 2009;168(6):329–37. doi: 10.1007/s11046-009-9247-z. [DOI] [PubMed] [Google Scholar]
- 36.Haiduven D. Nosocomial aspergillosis and building construction. Med Mycol. 2009;47 (Suppl 1):S210–16. doi: 10.1080/13693780802247694. [DOI] [PubMed] [Google Scholar]