Abstract
The biologic characteristics of mesenchymal stem cells (MSCs) isolated from two distinct tissues, bone marrow and adipose tissue were evaluated in these studies. MSCs derived from human and non-human primate (rhesus monkey) tissue sources were compared. The data indicate that MSCs isolated from rhesus bone marrow (rBMSCs) and human adipose tissue (hASCs) had more similar biologic properties than MSCs of rhesus adipose tissue (rASCs) and human bone marrow MSCs (hBMSCs). Analyses of in vitro growth kinetics revealed shorter doubling time for rBMSCs and hASCs. rBMSCs and hASCs underwent significantly more population doublings than the other MSCs. MSCs from all sources showed a marked decrease in telomerase activity over extended culture; however, they maintained their mean telomere length. All of the MSCs expressed embryonic stem cell markers, Oct-4, Rex-1, and Sox-2 for at least 10 passages. Early populations of MSCs types showed similar multilineage differentiation capability. However, only the rBMSCs and hASCs retain greater differentiation efficiency at higher passages. Overall in vitro characterization of MSCs from these two species and tissue sources revealed a high level of common biologic properties. However, the results demonstrate clear biologic distinctions, as well.
Keywords: mesenchymal stem cells, bone marrow, adipose tissue, differentiation, telomerase, transcription factors
Mesenchymal stem cells (MSCs) can be derived from specific organs, such as gut, lung, liver, and bone marrow [Wei et al., 2000]. Adult MSCs have been successfully isolated from both bone marrow and adipose tissue. Bone marrow traditionally has been viewed as the home of hematopoietic stem cells. It is also known to contain MSCs as part of the stromal fraction. The non-hematopoietic connective tissue of the bone marrow microenvironment is derived from a heterogeneous population of stromal precursor cells, whose progeny support and regulate hematopoiesis [Weiss, 1976; Lichtman, 1981; Tavassoli and Friedenstein, 1983; Bianco and Gehron Robey, 2000]. MSCs represent a small, non-hematopoietic subpopulation of cells that reside in the bone marrow, and were initially described by Friedenstein et al. [1974] in the 1970s.
Adipose tissue, like bone marrow, is derived from the mesenchyme and contains a supportive stroma that is easily isolated. Based on this, adipose tissue may represent a source of stem cells that could have far-reaching effects on several fields. These cells can be isolated from adipose tissue in significant numbers and exhibit stable growth and proliferation kinetics in culture [Aust et al., 2004]. Isolation of a population of stem cells with similar biologic potential from human adipose tissue has been described earlier [Zuk et al., 2002; Aust et al., 2004; Estes et al., 2004; Dubois et al., 2005; Mitchell et al., 2005]. It was originally thought that tissue-specific adult stem cells were only capable of differentiation along cell lineages of their tissue of origin; however, recent studies revealed the ability of these MSCs from bone marrow and adipose tissue to differentiate into lineages of other tissues [Arnhold et al., 2004; Dawn and Bolli, 2005; Tang et al., 2005]. Adipose tissue stem cells (ASCs), like bone marrow MSCs (BMSCs), differentiate in vitro toward the osteogenic, adipogenic, neurogenic, myogenic, and chondrogenic lineage when treated with established lineage specific factors [Zuk et al., 2001, 2002; Mizuno et al., 2002]. The selective differentiation depends on the specific environmental cues, usually a combination of growth factors and cytokines supplied in vitro. The multipotentiality of BMSCs and ASCs makes them promising candidates for mesodermal defect repair and disease management.
A limited number of studies have been undertaken to compare the biologic properties and potential of these distinct MSC populations [Romanov et al., 2005]. Nevertheless, several aspects of MSC biology remain controversial. In this study, the biologic properties of adult MSCs isolated from two tissue sources in human and non-human primates were characterized and compared. The data clearly demonstrate significant levels of similarity between these two types of MSCs such as growth kinetics, differentiation ability, cell surface markers, telomerase activity, and gene expression. However, distinct biologic differences unique to each lineage are noted, as well.
MATERIALS AND METHODS
Isolation and Expansion of MSCs
Human bone marrow MSCs (hBMSCs) from normal donors were obtained from the Tulane Center for Distribution of Adult Stem Cells (wolfe@tulane.edu) and were prepared as described previously [Colter et al., 2001; Prockop et al., 2001]. Bone marrow specimens were obtained under a protocol approved by the Institutional Review Board (IRB) of the Tulane University Health Sciences Center.
Human adipose tissue stem cells (hASCs) were isolated from subcutaneous white adipose tissue based on standard protocols [Estes et al., 2004]. Tissue specimens were obtained under protocol approved by the IRB of the Pennington Biomedical Research Center. All human samples were obtained from healthy participants based on clinical examination and laboratory tests who were not receiving any medications.
Rhesus BMSCs were collected and cultured as previously described [Izadpanah et al., 2005]. All animal procedures in this study conformed to the requirements of the Animal Welfare Act and the Institutional Animal Care and Use Committee (IACUC) of the Tulane National Primate Research Center approved protocols before implementation. The animals were housed under conditions approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Healthy Rhesus monkeys (Macaca mulatta) aged 6 months to 12 years old of both sexes were used for these studies.
Adipose tissue was obtained from normal healthy rhesus macaques under local anesthesia. The raw adipose tissue was processed according to established methodologies to obtain a stromal vascular fraction [Zuk et al., 2002]. To isolate MSCs, samples were digested at 37°C for 30 min with 0.075% collagenase (Sigma, St. Louis, MO). The cells were resuspended in red cell lysis buffer. The MSCs were cultured in complete medium containing alpha-MEM (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS, Atlanta Biological, Atlanta, GA), 1% L-glutamine (Invitrogen), and 1% penicillin/streptomycin (Invitrogen) in a 37°C incubator with a 5% CO2 atmosphere.
Colony Forming Unit (CFU)
Following expansion, cells were plated at 5, 50, 100, 500, 1,000, 5,000, 10,000, 15,000, or 125,000 cells per 10-cm2 plate (Nalgene Nunc, Rochester, NY). The cultures were incubated for 2 weeks and the medium was changed every 2–3 days during culture. After 12–14 days of culture, the cells were fixed and stained with 1% crystal violet in 100% methanol (Sigma). The number of colonies greater than 3 mm in diameter were counted and recorded.
Flow Cytometry
Flow cytometric analysis of cell surface markers was performed on cultured MSCs at passages 1, 10, 20, and 30. Cells were incubated with fluorescent isothiocyante (FITC) and phycoerythrin (PE)-labeled antibodies for CD3, CD4, CD8, CD11b, CD13, CD31, CD59, CD90, CD105, CD106, CD146, CD161, CD164, Stro-1, and HLA-1 (Pharmingen, San Diego, CA), and CD8 and CD13 (Serotec, Raleigh, NC). Non-specific fluorescence was determined using cell preparations that were incubated with conjugated anti-mouse IgG-FITC and IgG-PE. Cells were then fixed with 1% paraformaldehyde and analyzed on a Becton Dickinson FACSCalibur (Becton Dickinson, San Jose, CA) using Cell Quest software.
Telomerase Assay
Telomerase activity was measured according to telomere repeat amplification protocol (TRAP) by using the TeloTAGGG telomerase PCR ELISA kit according to manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN). Briefly, telomerase adds telomeric repeats (T2AG3) to the end of biotin-labeled primers. The extension products of telomerase were amplified using polymerase chain reaction (PCR). The telomerase-mediated elongated product was detected by hybridization to digoxigenin-labeled probes. The level of enzyme activity was evaluated and determined by photometric enzyme immunoassay.
Telomere Length Assay
Telomere length was analyzed using the TeloTAGGG Telomere Length Assay kit according to manufacturer’s instructions (Roche Molecular Biochemical). Genomic DNA was extracted from MSCs using a DNA extraction kit (Qiagen, Valencia, CA). One microgram of genomic DNA was digested with the mixture of HinfI and RsaI restriction endonucleases and was electrophoresed through a 0.8% agarose gel and transferred to a positively charged nylon membrane. The membrane was then hybridized to a digoxigenin (DIG)-labeled telomeric oligonucleotide (TTAGGG)3. The DNA/oligonucleotide hybridization products were visualized, after reaction with a chemiluminescent substrate, using Versa Doc imaging system (Bio-Rad, Hercules, CA).
MSC Differentiation Assays
Adipogenic and osteogenic differentiation were induced according to our standard protocol [Izadpanah et al., 2005]. The acquisition of the adipogenic phenotype was determined by staining the monolayers with 0.5% Oil Red-O solution. MSC colonies that underwent adipogenic differentiation exhibited cells that contained numerous, variable-size lipid vesicles. Osteogenic mineralization was assessed by staining with 40 mM Alizarin red (pH 4.1, Sigma).
For chondrogenic differentiation, the pellet culture system described by Sekiya et al. [2001] was used. MSC cell pellets were cultured in chondrogenic differentiation media, which consisted of high glucose DMEM supplemented with 500 ng/ml BMP-6 (R&D system), 10 ng/ml TGF-β3, 10-7 M dexamethasone, 50 μg/ml ascorbate 2-phosphate, 40 μg/ml proline, 100 μg/ml pyruvate, and 50 mg/ml ITS + premix (Becton-Dickinson: 6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml bovine serum albumin, 5.35 mg/ml linoleic acid). The media was replaced every 2–3 days for 21 days. Pellets were then fixed in formalin, embedded in paraffin and sectioned. The sections were stained with Toluidine Blue.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was isolated from ~2 × 106 cells from each passage using an RNA extraction kit (Qiagen). Oligo (dT)-primed reverse transcription was performed on aliquots (1 μg) of total RNA as a template and used the resultant cDNA for PCR amplification with the following primers: Oct-4 primers 5′-CGC-ACC-ACT-GGC-ATT-GTC-AT-3′ and 5′-TTC-TCC-TTG-ATG-TCA-CGC-AC-3′ (approximately 200 bp). Sox2 primers 5′-GGC-AGC-TAC-AGC-ATG-ATG-CAG-GAG-C-3′ and 5′-CTG-GTC-ATG-GAG-TTG-TAC-TGC-AGG-3′ (approximately 200 bp). Rex-1 primers 5′-TGA-AAG-CCC-ACA-TCC-TAA-CG-3′ and 5′-CAA-GCT-ATC-CTC-CTG-CTT-TGG-3′ (approximately 200 bp). RT-PCR reactions were performed for the GAPDH gene as a control for efficiency of the amplification in the reactions (5′-ATG-GGG-AAG-GTG-AAG-GTC-GG-3′ and 5′-GGA-GTG-GGT-GTC-GCT-GTT-GAA-3′; approximately 500 bp). The PCR products were visualized and analyzed by 1.5% agarose gel electrophoresis.
Immunocytochemistry
MSCs were cultured on sterile glass cover slips and fixed by incubation in 1% paraformaldehyde/PBS for 3–5 min, permeabilized with 0.5% Triton X-100 in PBS for 15 min, and postfixed for 10 additional minutes in 4% paraformaldehyde in PBS. The intracellular staining patterns and distribution of Oct-4 and Sox-2 proteins were analyzed by immunostaining with an anti-Oct-4 monoclonal antibody (mAb), which recognizes an epitope located at amino acid 143–359 of 44 kDa Oct-4 protein (Chemicon, Temecula, CA, Cat#MAB4305,) and the rabbit anti-Sox-2 mAb, which recognizes the amino acid amino acids 265–283 of 34 kDa Sox2 protein (Chemicon, Cat#AB5603,). The FITC-conjugated anti-mouse IgG and Texas Red anti-rabbit IgG were used as the secondary antibodies (Molecular Probe, Carlsbad, CA).
Western Blot Analysis
MSC cultures were washed twice with ice-cold phosphate-buffered saline (PBS) and then lysed in 40 μl of lysis buffer (Promega, Madison, WI) and 1 μl of cocktail proteinase inhibitor (Sigma). Total protein concentration was measured using a Bradford assay containing Coomassie Plus protein reagent (Bio-Rad Laboratories) according to the manufacturer’s specifications. Equivalent amounts of total cell lysate were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) using 10% polyacrylamide gels. Proteins were electroblotted to PVDF membrane (Millipore, Billerica, MA). The membranes were then blocked and incubated in anti-Oct-4 (1:100), anti-Sox-2 (1:100), and anti-GAPDH antibody (rabbit polyclonal; 1:1,000; Abcam plc. Cambridge, UK, Cat#AB9485) overnight at 4°C. Alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgGs (1:1,000) were used as secondary antibodies (Bio-Rad Laboratories) for detection. The membranes were incubated with Western Blotting Detection Reagents (Bio-Rad Laboratories) according to the manufacturer’s instructions and detected using the Versa Doc imaging system (Bio-Rad Laboratories).
RESULTS
Isolation and Expansion of Rhesus BMSCs and ASCs
We sought to compare biologic properties of populations of both human and rhesus MSCs derived from bone marrow and adipose tissue. The human adipose and bone marrow MSCs were isolated using standard techniques [Colter et al., 2001; Prockop et al., 2001]. The volume of collected bone marrow from rhesus was ranged from 4 to 7 ml while the adipose tissue specimens from rhesus were between 5 and 20 g. The number of nucleated cells isolated per bone marrow sample was significantly higher than per adipose tissue sample (1–3 × 109 vs. 3–5 × 106), respectively.
Rhesus MSC cell cultures were established by plating all cells at a density of 1,000 cells/cm2. Rhesus bone marrow MSCs (rBMSCs)s grew to 80% confluence within 2 weeks; while under same culture conditions, rASCs took a markedly longer period of time to reach to 80% confluence, typically 3 weeks.
Culture Growth Kinetics
Under our culture conditions, human and rhesus BMSCs and ASCs were capable of proliferating for several passages (approximately 6–7 population doublings per passage). hASCs and rBMSCs expanded routinely beyond 30 passages (180–210 population doublings), whereas rASCs and hBMSCs became senescent by passage 20 (120–140 population doublings).
In rBMSC cultures, cell morphology (size and shape) persisted with only minimal alterations out to passage 30 (Fig. 1A, B). In contrast, marked alterations to cell morphology were observed in both rASCs and hBMSCs cultured up to 20 passages and beyond, as indicated by the appearance of large cells composed of an extensive cytoplasmic volume (Fig. 1F, H). The hASCs showed only minor morphologic alterations once passage 30 was exceeded (Fig. 1G, H).
Fig. 1.
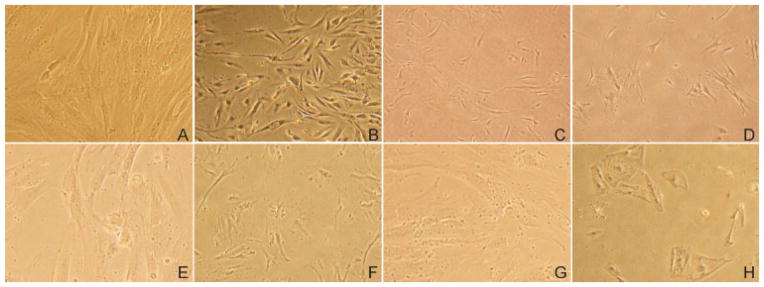
MSC morphology in low and high passages. Microscopic photographs of four types of MSCs cultures at low and high passages. rBMSCs and hASCs passage 1 (A and C) and passage 30 (B and D). Cultures of hBMSCs and rASCs at Passage 1 (E and G) and passage 20 (F and H), respectively.
The doubling time of each MSC culture was analyzed at multiple time points over extended culture periods. The time required for population doubling significantly increased in both rASCs and hBMSCs in cultures higher than passage 20 from 50 h to between 160 and 180 h. Passage 30 for these cultures could not be analyzed as the doubling time became so protracted that cells failed to reach that passage. In comparison, the doubling time of rBMSCs increased only modestly from 48 h at passage 1–55 h at passage 30. hASCs showed only a modest increase in doubling time over the same period from 48 h up to 60 h at passage 20 and 110 h at passage 30 (Fig. 2).
Fig. 2.
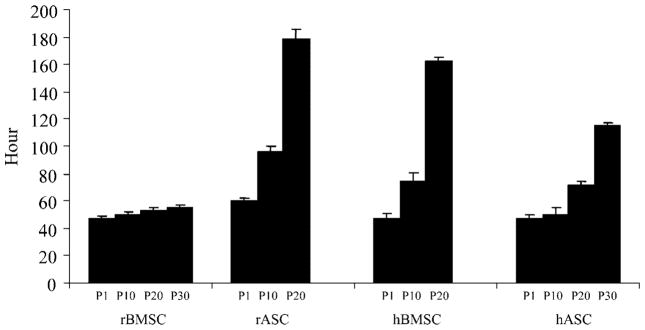
Population doubling time of MSCs. A known number of MSCs types from different passages were cultured. The total number of cells was determined at different time points to obtain the doubling time.
Estimation of MSC Frequency in Bone Marrow and Adipose Tissue
Each of the MSC lines (rBMSCs, rASCs, hBMSCs, and hASCs) was examined for the presence of CFU. MSC cultures at specific passages (1, 10, 20, for hBMSCs and rASCs; 1, 10, 20, 30 for hASCs and rBMSCs) were plated at densities of 1, 5, 10, and 25 cells/cm2. The efficiency of colony formation of the four cell types was comparable and ranged between 45% and 60% of the starting MSC population up to passage 10. The efficiency of colony formation decreased to 25%–35% in rASCs and hBMSCs at passage 10 and higher. Whereas, the CFU efficiency did not change in rBMSCs through 30 passages and the hASC cultures revealed a decrease in CFU efficiency to 35%–40% only at passage 30 (data not shown).
As previously reported, the estimated frequency of rhesus bone marrow MSCs ranged from 1 in 4.3 × 105 to 1 in 6.7 × 105, which is similar to the frequency in human bone marrow [Izadpanah et al., 2005]. The yield of adherent cells obtained from rhesus adipose tissue samples was 1 in 4.6 × 105 to 1 in 7.5 × 105 in processed tissue (5–20 g of adipose tissue). Aust et al. [2004] determined that the mean cell yield from hASC was 404 × 206 × 103 cells per milliliter of lipoaspirate. The morphologic homogeneity of MSC cultures at specific passages was apparent upon assessment of the cell surface antigen profile by flow cytometric analysis. The expression and levels of selected cell surface antigens was analyzed at multiple passages. Hematopoietic stem cell markers including CD3, CD4, CD8, CD11b, and CD13 were found to be negative in all MSCs at all passages. In addition, CD31 was also found to be negative. Each MSC cell line was strongly positive for CD59, a SCA-1 homolog, CD90 (Thy-1), and HLA-1, while they were negative for CD164. The four MSC populations were positive for MSC-specific surface markers such as CD105, CD106, CD146, CD161, and STRO-1. The surface marker profile of these cell types remained stable through passage 20–30.
Telomere Maintenance in MSCs
Telomerase enzyme activity was evaluated in each of the MSC cell lines using the amplified telomeric repeat (TRAP) method. Analysis of telomerase in MSC populations at different passages showed reduced activity at higher population doublings. The telomerase activity was stable in rBMSCs through passage 10. rBMSCs showed a 15% decrease at passage 20 and about 33% decrease at passage 30 (Fig. 3). Cultures of hASCs at passage 10, 20, and 30 showed 15%, 30%, and 60% decrease in telomerase activity, respectively. hBMSCs showed a marked decrease in enzyme activity through 20 passages, approximately an 80% decrease between passage 1 and 20. The level of telomerase activity in rASCs also decreased by 63% by passage 20.
Fig. 3.
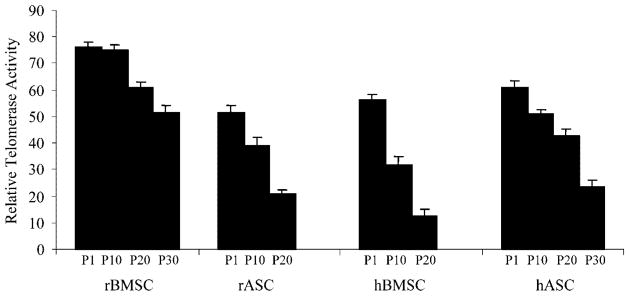
Telomerase activity in MSCs. Telomerase enzyme activity measured using a photometric enzyme immunoassay on samples isolated at different passages for each line of MSCs. The bars indicate the mean enzyme activity out of three replicates for every passage of each cell type.
Telomere length was determined as a mean terminal restriction fragment (TRF) length of genomic DNA based on Southern hybridization with a telomeric probe. Telomere length was analyzed in passages of rBMSCs, rASCs, hBMSCs, and hASCs. However, despite a significant decrease in telomerase activity in all MSC cultures, no significant change in the mean telomere length was observed for as long as 30 passages (Fig. 4).
Fig. 4.
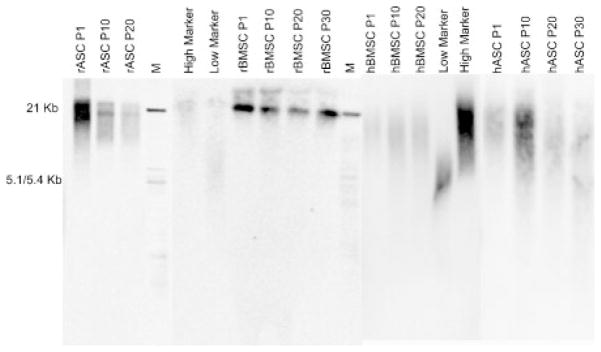
Telomere length at multiple passages of MSCs. Overall telomere length in passages of rASCs, rBMSCs, hASCs, and hBMSCs. Genomic DNA isolated from each indicated passage was digested with HinfI and RsaI and electrophoresed in a 0.8% agarose gel. Terminal restriction fragments were visualized, using a labeled (TTAGGG) probe.
Multilineage Differentiation Potential of MSCs
To study the multilineage capacity of each MSC line at selected passages, cells were differentiated toward the adipogenic, osteogenic, and chondrogenic lineages using lineage-specific induction factors. rBMSC and hASC cultures (passages of 1, 10, 20, and 30), and rASCs and hBMSCs (passages 1, 10, 20) were subjected to conditions promoting adipogenesis, osteogenesis, and chondrogenesis.
Adipogenesis
For the adipogenic differentiation, MSCs were cultured in adipogenic induction media for 12–14 days. The acquisition of the adipogenic phenotype was determined by staining the cell monolayers with Oil Red-O (Fig. 5A). The efficiency of adipogenesis was similar in low passage cultures for each type of MSC. Every MSC line showed multiple intra-cellular lipid filled droplets in 35%–50% of cells in adipogenic media. The cells containing lipid vesicles exhibited an expanded morphology with the majority of intracellular space occupied by droplets, which is consistent with the phenotype of mature adipocytes. MSCs continued to undergo adipogenesis, as indicated by the extensive accumulation of lipid vesicles, such that they readily resembled adipocytes in culture. The vesicle saturated cells survived for as long as 18–24 days in vitro. Adipogenesis was observed in rBMSCs and hASCs up to passage 20 at similar frequency. rBMSCs retained their adipogenic potential up to passage 30, whereas hASCs significantly lost this potential after passage 20. However, the frequency of adipogenesis was significantly reduced in rASCs and hBMSCs at cultures of passage 10 and higher. The ratio of the colonies that underwent adipogenic differentiation to the entire population of cells decreased as the passage number increased.
Fig. 5.
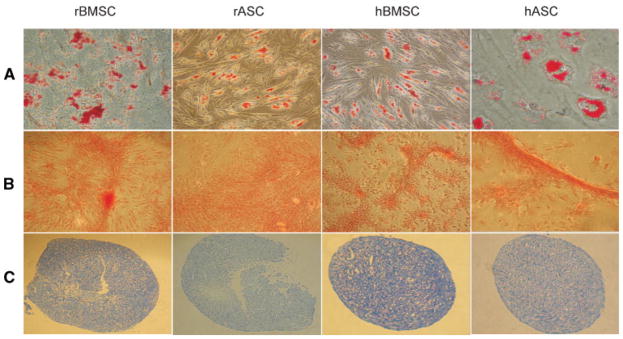
Differentiation of MSCs along mesodermal lineages. rBMSCs, rASCs, hBMSCs, and hASCs were differentiated along adipogenesis and formed lipid vesicles stained with Oil red-O (A). Mineralization revealed in osteogenic-differentiated MSCs using Alizarin red staining (B). MSCs under chondrogenesis condition promoted the formation of chondrocytes, sectioned paraffin-embedded micromass pellets were stained with Toluidine Blue (C).
The rate of adipogenic differentiation changed significantly as the passage number increased. In lower passages of rBMSCs the lipid containing droplets were detectable by light microscopy only after 6–8 days of differentiation, while the formation of lipid droplets was observed within 48 h at passage 30 (data not shown). No lipid droplets were observed in undifferentiated MSCs or in controls.
Osteogenesis
Differentiation of MSCs into osteocytes was induced by treating cells with low concentrations of ascorbic acid, beta-glycer-ophosphate, and dexamethasone [Cheng et al., 1994; Conget et al., 2001]. Human and rhesus MSCs that had undergone in vitro osteogenic differentiation proliferated rapidly and formed tightly packed colonies. In culture, dense, granular areas appeared within individual colonies, and as the cultures were incubated over 2 weeks, multiple layers of cells often formed. In some cases, these colonies gave rise to dense nodules from which radiated highly elongated spindle-shaped cells with large nuclei. Calcium deposition was demonstrated by staining monolayers with Alizarin Red (Fig. 5B). In early passage cultures, human and rhesus MSCs showed similar potential toward osteogenic differentiation. The percentage of colonies that underwent osteogenic differentiation ranged between 50% and 65% of the total colonies in all MSC types at low passage number. However, this frequency decreased to 30%–35% in hBMSCs and rBMSCs at passage 30, and 20%–25% in rASCs and hMSCs at passage 20.
Chondrogenesis
MSCs differentiated into chondrocytes using a micromass culture technique [Sekiya et al., 2001]. Micromass cultures changed to spheroids visible to the naked eye as early as 3 days after the initiation of chondrogenic induction and the nodules increased in size over the course of 3 weeks in culture. The nodules that formed in the micromass cultures were fixed and sections of the paraffin embedded cells stained positively for Toluidine Blue which is specific for the highly sulfated proteoglycans of cartilage matrices (Fig. 5C). All MSC types showed similar potentials to differentiate to chondrocytes at passage one. Both rASCs and hBMSCs cultures rapidly lost chondrogenic differentiation potential with this protocol at passage 5 and higher; whereas, rBMSCs and hASCs underwent differentiation for as long as 10 passages.
MSCs Transcription Factor Expression
A limited number of transcription factors appear to control the biology of stem cells. These factors have been suggested as candidates for the master regulator of initiation, maintenance, and differentiation of pluripotent cells [Ingra-ham et al., 1990; Li et al., 2005]. The expression of genes such as Oct-4, Sox-2, and Rex-1 was examined at multiple passages in culture and compared among the four stem cell populations. Oct-4 and Sox-2 mRNAs were expressed at consistent levels in all cell types, including the higher passages of cultured cells. Rex-1 mRNA expression did not show any significant change in levels out to passage 10. There was approximately a 10-fold decrease in Rex-1 mRNA levels in rASCs and hBMSCs at passage 20 and a fivefold decrease in rBMSCs Rex-1 expression at passage 30. However, hASCs did not down-regulate the transcription of Rex-1 mRNA at passage 30 (Fig. 6A). The results indicate that Oct-4 and Sox-2 mRNAs are constitutively expressed at higher passages, whereas Rex-1 expression is variable in vitro.
Fig. 6.
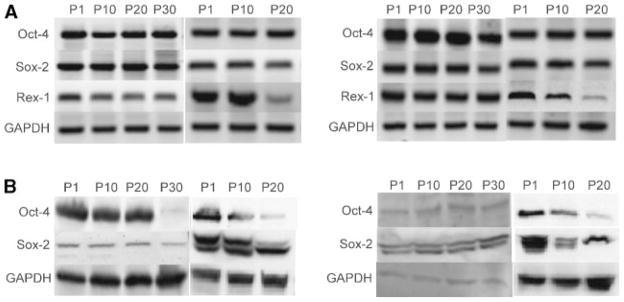
Transcription factor mRNA and expression in MSCs. Total cellular RNA analyzed by RT-PCR for Oct-4, Sox-2, and Rex-1 mRNA expression (A). The cell lysate from MSC types at different passages were subjected to Western blot analysis using Oct-4 and Sox-2 monoclonal antibodies to determine the level of expression of these two proteins (B).
Further assessment by Western blot analysis demonstrated that Oct-4 and Sox-2 protein expression levels in MSCs (Fig. 6B). Protein analysis revealed no alterations in Oct-4 expression in rBMSCs up to passage 20; however, the expression of Oct-4 was markedly decreased at passage 30. The rASCs revealed about fivefold decrease in Oct-4 at passage 10 when it was compared to passage 1, Oct-4 levels decreased significantly in these cells at passage 20. The Oct-4 expression pattern in hBMSCs was very similar to rASCs. Oct-4 expression showed about 5-fold decrease in passage 10 and more than 10-fold decrease at passage 20. The hASCs showed no detectable alterations in the level of Oct-4 expression through passage 30.
In general, the overall levels of Sox-2 were consistent among the cell lines, with the exception being rBMSCs, which showed the lowest levels of Sox-2. rBMSCs showed equivalent levels of Sox-2 expression up to passage 20 with only a modest decrease observed at passage 30. Sox-2 was consistently expressed in rASCs to passage 10, but fivefold diminution in expression at passage 20. hBMSCs showed 10-fold decrease in the level of expressed Sox-2 in passage 10 and 20 when compared to passage 1. The level of Sox-2 did not change in hASCs up to 30 passages (Fig. 6B).
Immunocytochemistry was employed to analyze the intracellular localization of Oct-4 and Sox-2 proteins at selected passages of MSCs (Fig. 7). As shown in Figure 7, there appeared to be much less Oct-4 signal in rASCs and hBMSCs in comparison to rBMSCs and hASCs. Both proteins were readily detectable at all passages analyzed; however marked alterations in the pattern of distribution of the staining were observed. Oct-4 was routinely detected in both the nuclear and cytoplasmic compartments of the MSCs; whereas Sox-2 showed more of nuclear and perinuclear localization. In low passage MSCs, Oct-4 and Sox-2 demonstrated a high level of co-localization in low passage MSCs. The levels of co-localization dropped significantly in higher passage MSCs.
Fig. 7.
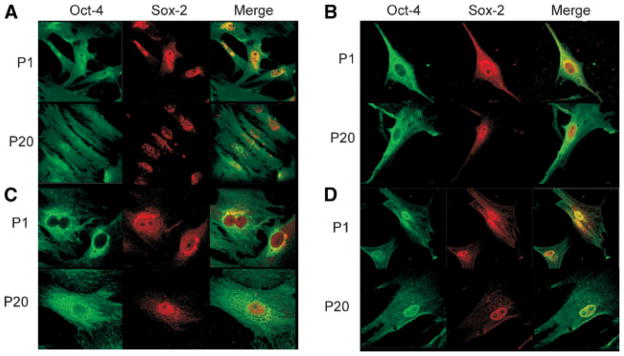
Oct-4 and Sox-2 localization in MSCs. Immunohistochemical staining of rBMSCs (A), hBMSCs (B), rASCs (C), and hASCs (D). Cells were fixed, permeabilized, and stained to visualize Oct-4 and Sox-2. Distribution and localization of these transcription factors exhibited at passage 1 and 20 each MSC types. Merge panel showed co-localization of these two proteins.
DISCUSSION
MSCs are multipotent stem cells that exist in marrow stroma, bone, tendon, ligament, and cartilage in fetal and postnatal organisms. This is the first comparison of the biologic properties of adult ASCs and BMSCs isolated from human and non-human primates. The goals of this study were twofold, to compare the biologic properties and differentiation potential of MSCs isolated from bone marrow and adipose tissue in adult humans and non-human primates. The results indicated the heterogeneity and growth kinetics of these cell populations and revealed the differences and similarities of MSCs isolated from the two tissue sources. There was no significant difference for growth kinetics and multi-lineage differentiation capacity between these MSC types of cells at low passages. The clonal efficiency of human and rhesus MSCs were similar at low passages. This ability is consistent in rBMSCs through passage 30, in hASCs through passage 20, and it decreased in rASCs and hBMSCs in passage 10 and higher. This would likely describe the more growth capability of rBMSCs and hASCs in higher passages in vitro. The estimated frequency of MSCs obtained from rhesus bone marrow was similar to the frequency of MSCs in human bone marrow. The difference observed in the frequency of the MSCs yield in human and rhesus adipose tissue could be related to the method of sampling. hASCs were obtained from lipoaspirate samples while rASCs were obtained from the adipose tissues using surgical procedure.
The human and non-human primate cells isolated from bone marrow and adipose tissue displayed a high degree of homogeneity with respect to cell surface markers. This is consistent with the others reports indicating the similarity of phenotype of adult MSCs in two distinct tissue [Pittenger et al., 1999; Conget et al., 2001; Aust et al., 2004]. The cell surface markers did not show any marked changes after extended passages for each of the cell types.
At each cell division, the alternative outcomes of self-renewal and differentiation are decided by the interplay between intrinsic factors and extrinsic instructive or selective signals. Human and other mammalian BMSCs and ASCs retain the ability to differentiate toward the adipogenic, osteogenic, and chondrogenic lineages with appropriate medium supplementation [Hauner et al., 1987; Pittenger et al., 1999; Zuk et al., 2001, 2002; Indrawattana et al., 2004]. As suggested in previous studies, rhesus adult MSCs also have the capacity to differentiate into multiple mesodermal lineages, including, bone, fat, and cells of ectodermal [Kang et al., 2004b; Izadpanah et al., 2005]. These observations have led to the comparison of these four types of MSCs. Adipogenesis occurred in all MSC types with very similar frequency through passage 5; in these cells lipid vesicles formed within 6–8 days of differentiation. The initial lipid formation was longer in passage 10 and higher for rASCs and hBMSCs while it did not change in rBMSCs and hASCs through passage 20. rBMSCs and hASCs showed modest alterations in differentiation capability through passage 20, whereas rASCs and hBMSCs dramatically lost their adipogenic ability after passage 10, and there was no adipogenic differentiation at passage 15.
Our data indicated that hASCs and rBMSCs differentiate to osteogenic lineage for extended durations in culture; whereas rASCs and hBMSCs lost this capability after passage 10. The doubling time for rBMSCs did not change during culture, but there was a moderate to marked increase in doubling time in all of the other cell types. All four cell types showed similar potential toward chondrogenesis in cells up to passage 5. However, only rBMSCs and hASCs retain this ability up to passage 10. These observations might indicate the existence of a relationship between the differentiation potential and doubling time of MSCs in vitro.
Analyses of the MSCs indicated no significant levels of telomere shortening in four types of MSCs in long time in vitro culture. The telomere length in human and rhesus monkey MSCs showed similar size [Allsopp et al., 1992; von Zglinicki et al., 1995; Weber et al., 2002; Van Ziffle et al., 2003]. Telomeres are protein DNA structures present at the ends of chromosomes and are essential for genetic stability and cell replication [Shiver et al., 2002]. The telomere length was found to decrease with aging in vivo [Hayflick, 1965; Harley et al., 1990]. The distribution pattern of telomerase activity among the MSC types showed that all cell types have the highest telomerase activity at passage one. Our data provide evidence that the level of telomerase activity varies between cell types. Quantitative differences determined that telomerase activity was significantly higher in early passage cells with rBMSCs containing the greatest telomerase activity relative to the other MSC types, which is consistent with earlier published data [Kakuo et al., 1999]. Telomerase activity decreased with passage number. The decrease was greatest in rASCs and hBMSCs. It was also observed that the decrease in telomerase activity in rASCs and hBMSCs between passages 1 and 10 was greater than the more modest decrease seen in rBMSCs and hASCs.
It was striking that the telomere length did not demonstrate detectable shortening in higher passages despite a considerable decrease in telomerase activity. Control of telomere length appears to depend on a number of factors in addition to changes in the levels of telomerase activity. There is evidence suggesting telomere length is not only under the control of telomerase, but there are phenomemena other than telomerase act to modulate telomere length [Bryan et al., 1998]. The extent of telomere shortening and the levels of telomerase activity often correlate with the stem cell characteristics [Greider and Blackburn, 1985; Morin, 1989; Prowse et al., 1993; Hirato et al., 1994; Bodnar et al., 1998; McEachern et al., 2000]. A causal relationship between telomere shortening and cellular senescence has been established by showing that transfection of the telomerase reverse transcriptase gene into various human mortal somatic cells results in telomere length elongation and extension of in vitro life span [Bodnar et al., 1998]. The data indicate that the cells with greatest reduction of telomerase activity are severely compromised in their remaining long-term proliferative and differentiation capacity. It has been reported that transfection of rhesus MSCs with the telomerase reverse transcriptase gene prolonged the duration of their multilineage differentiation ability [Kang et al., 2004a].
Transcription factors are responsible for regulating the biology of stem cells. Members of the transcription factor families POU and SOX exemplify this function cooperatively during early embryonic development [Villinger et al., 2001; Remenyi et al., 2003]. One of the important members of POU family is a transcription factor encoded by the Oct-4 gene. Oct-4, together with Sox-2, a member of SOX family, and Rex-1, is tightly regulated during embryonic development [Pesce et al., 1998a, b]. The expression profile of Oct-4 reflects a key role for this transcription factor in the specification and maintenance of pluripotent cells both in vivo and in vitro [Niwa, 2001]. Expression of stem-cell related transcription factors in rhesus MSCs has already been reported by our group [Izadpanah et al., 2005]. Our data suggested a dramatic decrease in expression levels of Oct-4 protein in higher passage MSCs. Only the hASC did not show marked declines in protein levels at higher passage. While the Sox-2 protein levels decreased in rASC and hASC passage 20, there was no change in expression of Oct-4 and Sox-2 mRNA in high-passage MSCs. This may lead us to speculate presence of a system in higher passage cells that inhibits the transcription of expressed genes to corresponding proteins. Additionally, the co-localization of Oct-4 and Sox-2 may play important role in differentiation potentials of MSCs (Fig. 7).
In summary, analysis of adult MSCs from two tissues has identified many similarities between the two populations in human and non-human primates, lending support to the theory that stem cells can be found within many tissues. An alternative explanation may be that pericytes associated with blood vessels may account for the presence of MSCs in tissues throughout the body. The data presented in this article extends the characterization of MSC populations within different adult tissue types and their potential for in vivo applications. Since there is no ethical limitation on using adult stem cells, the results of this study suggest that adipose tissue and bone marrow will prove to be important sources of pluripotent stem cells.
Acknowledgments
Grant sponsor: National Center for Research Resources; Grant sponsor: National Institutes of Health; Grant number: RR00164; Grant sponsor: State of Louisiana Millennium Health Excellence Fund; Grant sponsor: Louisiana Gene Therapy Research Consortium; Grant sponsor: Pennington Biomedical Research Foundation.
We thank Dr. Darwin Prockop for hBMSCs, Dr. Xavier Alvarez, staff of the confocal microscopy and the flow cytometry core laboratories for their help, and the staff of the Stem Cell Laboratory and Cell Biology Core Laboratory of the Pennington Biomedical Research Center for human adipose tissue preparation.
References
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Dunham MA, Reddel RR. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp Cell Res. 1998;239:370–378. doi: 10.1006/excr.1997.3907. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: Induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget PA, Allers C, Minguell JJ. Identification of a discrete population of human bone marrow-derived mesenchymal cells exhibiting properties of uncommitted progenitors. J Hematother Stem Cell Res. 2001;10:749–758. doi: 10.1089/152581601317210845. [DOI] [PubMed] [Google Scholar]
- Dawn B, Bolli R. Adult bone marrow-derived cells: Regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Halvorsen Y-DC, Ravussin E, Gimble JM. Primary stromal cell culture from adipose tissue: From liposuction to needle biopsy. Adipocytes. 2005;1:139–144. [Google Scholar]
- Estes BT, Gimble JM, Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91–126. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987;64:832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hirato J, Nakazato Y, Sasaki A, Hikima A, Shimizu S, Yamanouchi H. Krabbe’s disease with giant lamellar bodies in Purkinje cells. Acta Neuropathol (Berl) 1994;88:78–84. doi: 10.1007/BF00294363. [DOI] [PubMed] [Google Scholar]
- Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Albert VR, Chen RP, Crenshaw EB, 3rd, Elsholtz HP, He X, Kapiloff MS, Mangalam HJ, Swanson LW, Treacy MN, et al. A family of POU-domain and Pit-1 tissue-specific transcription factors in pituitary and neuroendocrine development. Annu Rev Physiol. 1990;52:773–791. doi: 10.1146/annurev.ph.52.030190.004013. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Joswig T, Tsien F, Dufour J, Kirijan JC, Bunnell BA. Characterization of multipotent mesenchymal stem cells from the bone marrow of rhesus macaques. Stem Cells Dev. 2005;14:440–451. doi: 10.1089/scd.2005.14.440. [DOI] [PubMed] [Google Scholar]
- Kakuo S, Asaoka K, Ide T. Human is a unique species among primates in terms of telomere length. Biochem Biophys Res Commun. 1999;263:308–314. doi: 10.1006/bbrc.1999.1385. [DOI] [PubMed] [Google Scholar]
- Kang SK, Putnam L, Dufour J, Ylostalo J, Jung JS, Bunnell BA. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells. Stem Cells. 2004a;22:1356–1372. doi: 10.1634/stemcells.2004-0023. [DOI] [PubMed] [Google Scholar]
- Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A, Bunnell BA. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004b;117:4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- Li X, Kato Y, Tsunoda Y. Comparative analysis of development-related gene expression in mouse preimplantation embryos with different developmental potential. Mol Reprod Dev. 2005;72:152–160. doi: 10.1002/mrd.20346. [DOI] [PubMed] [Google Scholar]
- Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: A review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen YD, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunophenotype of human adipose derived cells: Temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2005 doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. discussion 210–211. [DOI] [PubMed] [Google Scholar]
- Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001;26:137–148. doi: 10.1247/csf.26.137. [DOI] [PubMed] [Google Scholar]
- Pesce M, Gross MK, Scholer HR. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays. 1998a;20:722–732. doi: 10.1002/(SICI)1521-1878(199809)20:9<722::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998b;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–396. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- Prowse KR, Avilion AA, Greider CW. Identification of a nonprocessive telomerase activity from mouse cells. Proc Natl Acad Sci USA. 1993;90:1493–1497. doi: 10.1073/pnas.90.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi A, Lins K, Nissen LJ, Reinbold R, Scholer HR, Wilmanns M. Crystal structure of a POU/HMG/ DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 2003;17:2048–2059. doi: 10.1101/gad.269303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: Isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Tang LJ, Gao Y, Zhang Z, Li H, Shan YQ. Human bone marrow multipotent adult progenitor cells differentiate into hepatocyte-like cells with hepatocyte growth factor plus fibroblast growth factor-4 in vitro. Zhonghua Gan Zang Bing Za Zhi. 2005;13:652–655. [PubMed] [Google Scholar]
- Tavassoli M, Friedenstein A. Hemopoietic stromal microenvironment. Am J Hematol. 1983;15:195–203. doi: 10.1002/ajh.2830150211. [DOI] [PubMed] [Google Scholar]
- Van Ziffle JA, Baerlocher GM, Lansdorp PM. Telomere length in subpopulations of human hematopoietic cells. Stem Cells. 2003;21:654–660. doi: 10.1634/stemcells.21-6-654. [DOI] [PubMed] [Google Scholar]
- Villinger F, Rowe T, Parekh BS, Green TA, Mayne AE, Grimm B, McClure HM, Lackner AA, Dailey PJ, Ansari AA, Folks TM. Chronic immune stimulation accelerates SIV-induced disease progression. J Med Primatol. 2001;30:254–259. doi: 10.1034/j.1600-0684.2001.d01-57.x. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Weber M, Steinert A, Jork A, Dimmler A, Thurmer F, Schutze N, Hendrich C, Zimmerman U. Formation of cartilage matrix proteins by BMP-transfected murine mesenchymal stem cells encapsulated in a novel class of alginates. Biomaterials. 2002;23:2003–2013. doi: 10.1016/s0142-9612(01)00329-5. [DOI] [PubMed] [Google Scholar]
- Wei G, Schubiger G, Harder F, Muller AM. Stem cell plasticity in mammals and transdetermination in Drosophila: Common themes? Stem Cells. 2000;18:409–414. doi: 10.1634/stemcells.18-6-409. [DOI] [PubMed] [Google Scholar]
- Weiss L. The hematopoietic microenvironment of the bone marrow: An ultrastructural study of the stroma in rats. Anat Rec. 1976;186:161–184. doi: 10.1002/ar.1091860204. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]


