Abstract
Background and Purpose
Interleukin-6 (IL6) is a pro-inflammatory cytokine with known auto-regulatory feedback mechanisms. We hypothesized elevated high-sensitivity C-reactive protein (hsCRP) relative to IL6 confers an increased risk of ischemic stroke (IS), and low hsCRP relative to IL6 a decreased risk, for individuals in the prospective, multi-ethnic, population-based Northern Manhattan Study (NOMAS).
Methods
Serum hs-CRP and IL6 were measured in NOMAS participants at baseline. We created a trichotomized predictor based on the dominant biomarker in terms of quartiles: hsCRP-dominant; IL6-dominant; and co-dominant groups. Cox proportional hazards models were used to calculate hazard ratios and 95% confidence intervals (HR, 95%CI) for the association between inflammatory biomarker group status and risk of incident IS.
Results
Of 3298 participants, both hsCRP and IL6 were available in 1656 participants (mean follow-up 7.8 years, 113 incident IS). The hsCRP-dominant group had increased risk of IS (adjusted HR 2.62, 95%CI 1.56–4.41) and the IL6-dominant group had decreased risk (adjusted HR 0.38, 95%CI 0.18–0.82), compared to the referent group, after adjusting for potential confounders. Model fit was improved using the inflammation dominant construct, over either biomarker alone.
Conclusions
In this multi-ethnic cohort, when hsCRP quartile was higher than IL6 quartile, IS risk was increased, and conversely when IL6 quartiles were elevated relative to hsCRP, IS risk was decreased. Construct validity requires confirmation in other cohorts.
INTRODUCTION
Inflammatory biomarkers serve as immune system health indicators, and in vascular research, elevated levels support evidence of ongoing disease processes that can up-regulate atherosclerosis or induce pro-thrombotic states.(1) Cytokine profiles produce snapshots of dynamic and complex immune system responses to the milieu of acute and chronic stimuli. High sensitivity C-reactive protein (hsCRP) is one of the most investigated cytokines in cardiovascular research (2) and has been found to predict ischemic stroke in some, but not all populations.(3) Interleukin-6 (IL6) is a pro-inflammatory cytokine similarly associated with increased vascular risk,(4) but it is also paradoxically linked to anti-inflammatory moleculesthrough complex auto-inhibitory feedback mechanisms.
Low ratios of hsCRP to IL6 levels have been observed among statin users (5) and among morbidly obese patients after weight loss from gastric bypass surgery.(6) In both scenarios, a decrease in relative levels of serum hsCRP to IL6 may reflect improved immune system homeostasis and a reduction in the underlying inflammatory state. Previous analysis in the Northern Manhattan Study (NOMAS) failed to confirm an independent association between hsCRP and increased risk of stroke.(3) In this analysis, we a priori selected CRP and IL6 biomarkers based on existing literature, and then created a trichotomized variable for systemic inflammation, with categorization dependent on which component (hs-CRP or IL6) is dominant in terms of quartile levels: hs-CRP-dominant, IL6-dominant, and co-dominant, as a reference group. We hypothesized that stroke-free individuals with elevated hsCRP relative to IL6 would have an increased risk of ischemic stroke, and those with low hsCRP relative to IL6 a decreased risk, after adjusting for demographic and clinical risk factors.
METHODS
Study Design and Data Source
As previously reported, NOMAS is a population-based, urban, multi-ethnic prospective cohort study.(7) Participants were recruited using random-digit dialing from 1993 until 2003 and selected using the following eligibility criteria: (i) no prior diagnosis of stroke, (ii) over the age of 39 years; and (iii) resided in Northern Manhattan. The race-ethnic distribution of this cohort consists of 63% Hispanic, 20% non-Hispanic black, and 15% non-Hispanic white residents. All study participants provided informed consent and this study received approval from the Institutional Review Boards at Columbia University Medical Center and University of Miami.
Study Sample
An analytic sub-sample (50.2%) was selected from among the full NOMAS population (n=3,298) based on the availability of blood samples for assay for both hsCRP and IL6 (n=1656).
Follow-Up and Ascertainment of Outcomes
Annual telephone follow-up evaluation was conducted for all participants to assess vital status and intermittent hospitalization or illness, as previously described.(3) Primary stroke outcomes were defined by the first symptomatic occurrence of stroke according to World Health Organization criteria, as previously described.(8) Stroke subtype (ischemic or hemorrhagic) was decided by a consensus of two study neurologists, with a third neurologist adjudicating as needed. Myocardial infarction (MI) was defined according to criteria developed from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial.(9) MI was diagnosed by a study cardiologist. Death arising from stroke, MI, congestive heart failure, pulmonary embolism, arrhythmia/sudden death, aortic aneurysm, aortic or mitral stenosis and left ventricular hypertrophy was considered vascular death endpoints. All other causes of death were characterized as non-vascular. Causes of death were classified by a trained research associate for all participants and any uncertain cases were adjudicated by study physicians.
Primary Explanatory Variable: Relative HsCRP/IL6 Dominance
Serum samples were drawn from participants at baseline. Whole blood was collected in 5cc EDTA anti-coagulated tubes by a trained phlebotomist. Blood samples were centrifuged at 3000 rpm for 10 minutes, with serum samples immediately separated, aliquoted and stored in 1.2 mL cryule vials at −70°C until ready for batch testing. IL6 levels were measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) and hsCRP using the BNII nephelometry system (Dade Behring, Deerfield, IL).
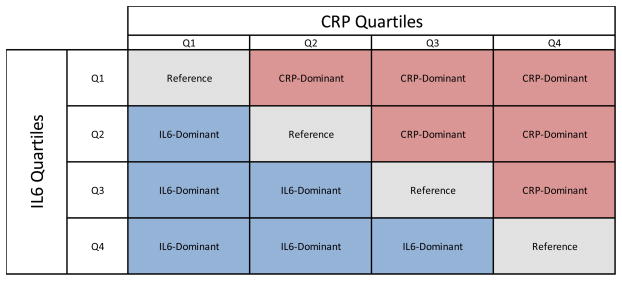
IL6 and hsCRP values were independently assigned quartile values (values between 1–4), based on the NOMAS cohort distribution. A continuous predictor of relative hsCRP quartile to IL6 quartiles was created and trichotomized, with categorization dependent on which component was dominant in terms of quartiles: hs-CRP-dominant, if hs-CRP-quartile>IL6-quartile (hs-CRP-quartile/IL6-quartile>1); IL6-dominant, if hs-CRP-quartile<IL6-quartile (hs-CRP-quartile/IL6-quartile<1); and reference group, if hs-CRP-quartile=IL6-quartile (hs-CRP-quartile/IL6-quartile=1) (Shown in Figure 1).
Figure 1. Operationalization of CRP-Dominant or IL6-Dominant Inflammation Construct.
captures the distribution of the NOMAS analytic subsample with regards to relative IL6 and CRP levels. This matrix representation identifies the various comparison groups in the analysis. The three inflammation categories are: hs-CRP-dominant, where hs-CRP-quartile>IL6-quartile (hs-CRP-quartile/IL6-quartile>1); IL6-dominant, where hs-CRP-quartile<IL6-quartile (hs-CRP-quartile/IL6-quartile<1); and the reference group or co-dominant group, where hs-CRP-quartile=IL6-quartile (hs-CRP-quartile/IL6-quartile=1). In the figure, the reference group is located in the diagonal portion of the matrix; the hs-CRP-dominant category is located in the upper-triangular portion of the matrix; and the IL6-dominant category is located in the lower-rectangular region of the matrix. Hs-CRP median (IQR) is 2.5 (1.1 – 5.8) and IL6 median (IQR) is 1.6 (0.8 – 2.9).
Covariates
Demographics and Behavioral Risk Factors
Demographic variables included: age (measured continuously); sex; education (less than high school (HS) or ≥HS educational attainment); race (self-reported and collapsed into: Hispanic, non-Hispanic black and non-Hispanic white, as reference); and insurance status collapsed into no-insurance or Medicaid, and private insurance or Medicare as reference. Behavioral risk factors included waist circumference, alcohol consumption, smoking, and physical activity. Waist circumference was measured by trained researchers and reported as a continuous variable. Alcohol intake was assessed using the National Cancer Institute food frequency questionnaire and collapsed into two categories: moderate consumption (≥1 drink per month and up to ≤2 drinks daily), and the remaining as reference group. Smoking status was assessed as current, past, and never smoker (reference). Leisure physical activity levels were measured via an adapted National Health Interview Survey and partitioned into 2 levels: moderate to heavy, and none to low activity.
Clinical Risk Factors
Clinical risk factors have been previously described(3) and are briefly discussed here. Hypertension was defined as systolic blood pressure (SBP)≥ 140 mm Hg or diastolic blood pressure of ≥ 90 mm Hg, prior history for diagnosis, patient self-report, or anti-hypertensive drug use. A continuous measure of SBP was used for modeling. Diabetes mellitus was defined as fasting glucose ≥ 126 mg/dl or a participant’s self-report of history of disease, or insulin or hypoglycemic drug use, and represented as a binary variable. Fasting lipid panels were measured to establish low density lipoprotein (LDL), high density lipoprotein (HDL), and modeled continuously. Coronary artery disease (CAD) was established by self-report or health provider diagnosis and reported as a binary variable.
Statistical Methods
The baseline characteristics and their relations to inflammatory categories (hsCRP-dominant, IL6 dominant and referent group) were calculated. We fitted Cox proportional hazard regression models to estimate hazard ratios and 95% CI (HR, 95% CI) for the trichotomized predictor, both unadjusted and after adjusting for demographics, medical and behavioral risk factors: age, sex, race-ethnicity, education, insurance status, history of coronary artery disease, diabetes status, systolic blood pressure, waist circumference, HDL, LDL, cigarette smoking, alcohol consumption, physical activity and cholesterol medication. The primary outcome was ischemic stroke. Secondary outcomes included total strokes, MI, vascular mortality and non-vascular mortality. Final models and interaction models of inflammation status with covariates were examined using the likelihood-ratio test (LRT) with specified degrees of freedoms (df) to ensure overall associations on outcomes. After examining the proportional hazards assumption, we censored follow-up at 10 years in our final models. Inverse probability weighting was conducted as a sensitivity analysis to correct a potential bias due to only using complete observations for hsCRP and IL6 values. (10) (11) All statistical analyses were conducted using SAS, version 9.2.3 (Cary, NC).
RESULTS
The characteristics of this cohort are presented in Table 1. Briefly, the mean age was 69.1 (SD=10.3) years, 21.5% white, 24.4% black, and 54.1% Hispanic. There were 517 (31.2%) in the hsCRP-dominant, 544 (32.9%) in IL6-dominant, and 595 (35.9%) in the no-dominance reference group. The median follow-up was 7.8 years, with 113 incident ischemic strokes, 135 all-cause strokes, 116 myocardial infarctions, 225 vascular mortality endpoints and 259 non-vascular mortality endpoints. The median biomarker levels were hsCRP=2.54 mg/L (IQR 1.06–5.84) and IL6=1.58 pg/mL (IQR 0.82–2.91) overall, with race/ethnic specific distributions reported in Table 1.
Table 1.
Baseline Characteristics and Risk Factors of the NOMAS sub-cohort with CRP and IL6 serum biomarkers available
| Baseline Descriptive Statistics (Overall and By Inflammation Category)
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analytic Subsample n= 1656 | HsCRP Dominant n=517 (31.2%) | IL6 Dominant n=544 (32.9%) | No Dominance n=595 (35.9%) | |||||||
|
|
|
|
|
|
|
|||||
| Parameter | n, % OR mean, SD |
n, % OR mean, SD |
n, % OR mean, SD |
n, % OR mean, SD |
K-W or χ2 (p-value) | |||||
|
|
|
|
|
|
|
|||||
| Demographics | Age, mean, SD, y | 69.1 | 10.3 | 68 | 9.6 | 70.5 | 10.9 | 68.7 | 10.1 | <0.01 |
| Sex, | ||||||||||
| Male, n, % | 583 | 35.2% | 170 | 32.9% | 214 | 39.3% | 199 | 33.5% | 0.05 | |
| Race / Ethnicity, n, % | ||||||||||
| Non-Hispanic White | 345 | 21.5% | 82 | 16.3% | 129 | 24.5% | 134 | 23.3% | ||
| Non-Hispanic Black | 391 | 24.4% | 123 | 24.5% | 130 | 24.7% | 137 | 23.8% | <0.01 | |
| Hispanic | 868 | 54.1% | 297 | 59.2% | 267 | 50.8% | 304 | 52.9% | ||
| Education Level, n, % | ||||||||||
| ≥ High school | 764 | 46.1% | 219 | 28.7% | 250 | 32.7% | 295 | 38.1% | 0.05 | |
| Insurance Status | ||||||||||
| No Insurance / Medicaid | 742 | 45.2% | 243 | 47.5% | 242 | 44.7% | 257 | 43.6% | 0.42 | |
| Private Insurance / Medicare | 901 | 54.8% | 269 | 52.5% | 299 | 55.3% | 333 | 56.4% | ||
|
|
|
|
|
|
|
|||||
| Behavioral Risk Factors | Smoking Status, n, % | |||||||||
| Non-Smoker | 794 | 48.0% | 253 | 48.8% | 258 | 47.6% | 283 | 47.6% | ||
| Past Smoker | 583 | 35.2% | 172 | 33.2% | 182 | 33.6% | 229 | 38.5% | 0.72 | |
| Current Smoker | 277 | 16.7% | 93 | 18.0% | 102 | 18.8% | 83 | 13.9% | ||
| Moderate Alcohol Consumption, n, % | ||||||||||
| Yes | 556 | 33.7% | 170 | 30.6% | 185 | 33.2% | 201 | 36.2% | 0.92 | |
| Physical Activity, n, % | ||||||||||
| Moderate or Heavy | 139 | 8.4% | 34 | 24.5% | 51 | 96.7% | 54 | 38.9% | 0.20 | |
| Waist, inches, mean, SD | 36.6 | 5.0 | 37.3 | 5.0 | 35.6 | 4.7 | 36.9 | 5.0 | < 0.001 | |
|
|
|
|
|
|
|
|||||
| Clinical Risk Factors | Hx of coronary artery disease, n, % | 353 | 21% | 115 | 32.6% | 115 | 32.6% | 123 | 34.8% | 0.81 |
| Hx of Diabetes Mellitus, n, % | 255 | 15.5% | 111 | 43.5% | 55 | 21.6% | 201 | 36.2% | < 0.001 | |
| Cholesterol Rx, n, % | 288 | 13.8% | 91 | 17.0% | 62 | 11.4% | 75 | 12.6% | <0.01 | |
| HDL, mg/dl, mean, SD | 47.4 | 14.7 | 47.7 | 13.5 | 47.8 | 15.2 | 47.6 | 15.2 | 0.66 | |
| LDL, mg/dl, mean, SD | 129 | 36.2 | 132.8 | 36.7 | 126.6 | 36.6 | 128.4 | 35.3 | 0.05 | |
| Systolic blood pressure, mm Hg mean + SD | 143 | 21.3 | 144.3 | 20.7 | 142.0 | 22.4 | 144.0 | 21.3 | 0.06 | |
| Hs-CRP mg/L, median (IQR) | 2.5 (1.1 – 5.8) | 4.7 | 1.1 | 3.1 | ||||||
| Non-Hispanic White | 2.1 (0.9 – 4.8) | --- | --- | --- | ||||||
| Non-Hispanic Black | 2.5 (1.0 – 6.6 | --- | --- | --- | ||||||
| Hispanic | 2.7 (1.2 – 6.0) | --- | --- | --- | ||||||
| Interleukin 6, pg/mL, median, (IQR) | 1.6 (0.8 – 2.9) | 0.8 | 2.3 | 1.9 | ||||||
| Non-Hispanic White | 1.5 (0.9 – 2.9 | --- | --- | --- | ||||||
| Non-Hispanic Black | 1.6 (0.7 – 3.2) | --- | --- | --- | ||||||
| Hispanic | 1.6 (0.8 – 2.8) | --- | --- | --- | ||||||
|
|
|
|
|
|
|
|||||
| Outcomes | Ischemic Stroke, n, % (Primary Outcome) | 113 | 6.8% | 65 | 13.0% | 16 | 2.9% | 32 | 5.4% | <0.001 |
| Time to ischemic stroke, year, SD | 7.8 | 2.5 | 7.6 | 2.5 | 7.9 | 2.4 | 7.9 | 2.4 | <0.001 | |
| All Stroke (Secondary Outcome) | 135 | 8.2% | 77 | 14.0% | 21 | 3.9% | 37 | 6.2% | <0.001 | |
| All MI (SecondaryOutcome) | 116 | 7% | 38 | 7.4% | 30 | 5.5% | 48 | 7.4% | 0.23 | |
| Vascular Mortality (Secondary Outcome) | 225 | 13.6% | 69 | 14% | 78 | 13.9% | 78 | 13.2% | 0.93 | |
| Non-Vascular Mortality (Secondary Outcome) | 259 | 15.6% | 62 | 12.4% | 107 | 19.1% | 90 | 15.2% | 0.01 | |
|
|
|
|
|
|
|
|||||
Abbreviations: Q, quartile; SD, Standard Error; K-W, Kurts-Wallace Test; χ2, Chi-Squared Test; Y, years
HsCRP Dominance and IL6 Dominance and Ischemic Stroke Risk
The categorical hsCRP-dominant group had an increased risk of ischemic stroke in the fully adjusted model (adjusted HR 2.62, 95%CI 1.56–4.41), compared to the referent group. The IL6-dominant group had a decreased risk of ischemic stroke in the adjusted models (HR 0.38, 95%CI 0.18–0.82), compared to the referent group (Table 2). The set of HsCRP-dominant and IL6-dominant variables improved model fit (LRT, p-value=0.0042) compared to the model including only trichotomized hsCRP and IL6 variables in the same model.
Table 2.
HsCRP-Dominant and IL6-Dominant Inflammation and Ischemic Stroke Risk
| Association of Inflammation Status and ischemic Stroke, by Missing Data Approach
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
Model 1. Ischemic Stroke (Primary Outcome, Events=113)
|
||||||||
| Missing Data Approach | Inflammation Category | HR | Unadjusted 95% CI | p-value* | HR | Adjusted** 95% CI | p-value* | ||
|
|
|
|
|
||||||
| Complete Case Analysis | Hs-CRP Dominant | 3.03 | 1.86 | 4.91 | <0.001 | 2.62 | 1.56 | 4.41 | <0.001 |
| IL6 Dominant | 0.46 | 0.22 | 0.97 | 0.38 | 0.18 | 0.82 | |||
| Reference | 1.00 | --- | --- | 1.00 | --- | --- | |||
|
|
|
|
|
||||||
| IPW Corrected Estimates | Hs-CRP Dominant | 2.14 | 1.47 | 3.15 | N/A | ||||
| IL6 Dominant | 0.38 | 0.19 | 0.77 | ||||||
| Reference | 1.00 | --- | --- | ||||||
| Association of inflammation Status and ischemic Stroke, stratified by Insurance Status & Race-Ethnicity
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
Model 2. Ischemic Stroke (Primary Outcome, Events=113)
|
||||||||
| Insurance Stratum | Inflammation Category | HR | Unadjusted 95% CI | p-valueŧ | HR | Adjusted** 95% CI | p-valueŧ | ||
|
|
|
|
|
||||||
| Private Insurance or Medicare | Hs-CRP Dominant | 3.08 | 1.61 | 5.89 | 0.02 | 2.94 | 1.51 | 5.73 | <0.001 |
| IL6 Dominant | 0.16 | 0.04 | 0.71 | <0.001 | 0.13 | 0.03 | 0.58 | <0.001 | |
| Reference | 1.00 | --- | --- | --- | 1.00 | --- | --- | --- | |
|
|
|
|
|
||||||
| No Insurance or Medicaid | Hs-CRP Dominant | 3.05 | 1.42 | 6.56 | 0.02 | 2.38 | 1.07 | 5.28 | <0.01 |
| IL6 Dominant | 0.95 | 0.60 | 1.51 | 0.40 | 0.88 | 0.34 | 2.30 | 0.46 | |
| Reference | 1.00 | --- | --- | --- | 1.00 | --- | --- | --- | |
|
|
Model 3. Ischemic Stroke (Primary Outcome, Events=113)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Race-Ethnic Stratum | Inflammation Category | HR | Unadjusted 95% CI | p-valueŧ | HR | Adjusted** 95% CI | p-valueŧ | ||
|
|
|
|
|
||||||
| Black | Hs-CRP Dominant | 1.66 | 0.79 | 3.47 | 0.09 | 1.18 | 0.52 | 2.68 | 0.35 |
| IL6 Dominant | 0.09 | 0.01 | 0.70 | 0.01 | 0.07 | 0.01 | 0.54 | 0.01 | |
| Reference | 1.00 | --- | --- | ------ | 1.00 | --- | --- | --- | |
|
|
|
|
|
||||||
| Hispanic | Hs-CRP Dominant | 3.01 | 1.35 | 6.74 | <0.01 | 2.46 | 1.08 | 5.63 | 0.02 |
| IL6 Dominant | 0.99 | 0.36 | 2.73 | 0.49 | 0.92 | 0.33 | 2.54 | 0.44 | |
| Reference | 1.00 | --- | --- | --- | 1.00 | --- | --- | --- | |
|
|
|
|
|
||||||
| White | Hs-CRP Dominant | 9.25 | 2.70 | 31.76 | <0.001 | 9.50 | 2.70 | 33.30 | <0.001 |
| IL6 Dominant | 0.70 | 0.12 | 4.17 | 0.69 | 0.62 | 0.10 | 3.70 | 0.60 | |
| Reference | 1.00 | --- | --- | --- | 1.00 | --- | --- | --- | |
Abbreviations: CI, confidence interval; HR, Hazard ratio; IPW, inverse probability weighted estimates
p value: based on likelihood ratio test
p value: based on wald chi square test of added interaction effect, after testing using likelihood ratio omnibus test
Models were adjusted for age, sex, race-ethnicity, education, insurance status, smoking, alcohol consumption, physical activity, waist size, diabetes mellitus, cholesterol treatment, high-density lipoprotein, low-density lipoprotein, systolic blood pressure
Missing Data Sensitivity analyses
We performed a sensitivity analysis to account for the fact that our analytic sample included 50% of the full NOMAS cohort. Inverse probability weight (IPW) corrected estimates for hsCRP dominant (adjusted HR=2.40, 95% CI, 1.73–3.34) and IL6 dominant (adjusted HR=0.38, 95% CI, 0.19–0.77) categories with ischemic stroke risk are presented in Table 2. These results were similar to those for the complete case analysis and possible selection biases are therefore deemed negligible.
Interactions
The effects of inflammation status on the risk of IS differed by insurance status (LRT with 2 df, p=0.04) and race-ethnicity (LRT with 4 df, p=0.01). Compared to the reference (co-dominant group), the IL6-dominant group was inversely associated with the risk of ischemic stroke among those with private insurance or Medicare (adjusted HR=0.13, 95%CI, 0.03–0.58), but not among those with no insurance or Medicaid (adjusted HR=0.88, 95%CI, 0.34–2.30). The association between hsCRP dominant status and ischemic stroke risk was not modified by insurance status (Table 2).
The associations between hsCRP-dominant status and risk of ischemic stroke were modified by race-ethnicity; compared to the reference, hsCRP-dominance was associated with risk of IS among whites (adjusted HR=9.50, 95% CI, 2.70–33.3) and Hispanics (adjusted HR=2.46, 95% CI, 1.08–5.63), but not among blacks (adjusted HR=1.18, 95%CI, 0.52–2.68). The association of the IL6 dominant group on the risk of IS was not modified by race-ethnic status. No further interactions were detected for the inflammation construct (Table 2).
HsCRP Dominance and IL6 Dominance and Secondary Outcomes
Overall, the association of the inflammation construct with total stroke risk (hemorrhagic and ischemic) was similar to those with ischemic stroke. The hsCRP-dominant group had an increased risk of total stroke in the fully adjusted model (adjusted HR 2.48, 95%CI 1.56–3.95), compared to the referent group. The IL6-dominant group had a decreased risk of total stroke in the adjusted models (HR 0.41, 95%CI 0.21–0.82), compared to the referent group (Table 3). The inflammation categories were not associated with MI, vascular mortality, or non-vascular mortality (Table 3).
Table 3.
HsCRP Dominant, IL6 Dominant Inflammation and Secondary Outcomes
| Association of Inflammation Status and Secondary Outcomes: Total Stroke, MI, Vasular Mortality & Non-Vascular Mortality
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
Total Stroke (Secondary Outcome, events=135)
|
|||||||
| Inflammation Category | HR | Unadjusted 95% CI | p-value* | HR | Adjusted** 95% CI | p-value* | ||
|
|
|
|
||||||
| Hs-CRP Dominant | 2.89 | 1.86 | 4.49 | 2.48 | 1.56 | 3.95 | ||
| IL6 Dominant | 0.51 | 0.27 | 0.99 | <0.001 | 0.41 | 0.21 | 0.82 | <0.001 |
| Reference | 1.00 | --- | --- | 1.00 | --- | --- | ||
|
|
|
|
||||||
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
|
Myocardial Infarction (Secondary Outcome, Events=116)
|
|||||||
| Inflammation Category | HR | Unadjusted 95% CI | p-value* | HR | Adjusted** 95% CI | p-value* | ||
|
|
|
|
||||||
| Hs-CRP Dominant | 0.97 | 0.63 | 1.49 | 0.79 | 0.49 | 1.26 | ||
| IL6 Dominant | 0.68 | 0.43 | 1.07 | 0.19 | 0.65 | 0.40 | 1.04 | 0.17 |
| Reference | 1.00 | --- | --- | 1.00 | --- | --- | ||
|
|
|
|
||||||
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
|
Vascular Mortality (Secondary Outcome, Events= 225)
|
|||||||
| Inflammation Category | HR | Unadjusted 95% CI | p-value* | HR | Adjusted** 95% CI | p-value* | ||
|
|
|
|
||||||
| Hs-CRP Dominant | 1.28 | 0.92 | 1.77 | 1.21 | 0.86 | 1.71 | ||
| IL6 Dominant | 1.02 | 0.74 | 1.40 | 0.27 | 0.88 | 0.63 | 1.24 | 0.23 |
| Reference | 1.00 | --- | --- | 1.00 | --- | --- | ||
|
|
|
|
||||||
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
|
Non-Vascular Mortality (Secondary Outcome, Events=259)
|
|||||||
| Inflammation Category | HR | Unadjusted 95% CI | p-value* | HR | Adjusted** 95% CI | p-value* | ||
|
|
|
|
||||||
| Hs-CRP Dominant | 0.92 | 0.67 | 1.27 | 0.93 | 0.66 | 1.31 | ||
| IL6 Dominant | 1.22 | 0.92 | 1.61 | 0.18 | 0.97 | 0.72 | 1.30 | 0.91 |
| Reference | 1.00 | --- | --- | 1.00 | --- | --- | ||
|
|
|
|
||||||
Abbreviations: CI, confidence interval; HR, Hazard ratio
p value: based on likelihood ratio test
p value: based on wald chi square test of added interaction effect, after testing using likelihood ratio omnibus test
Models were adjusted for age, sex, race-ethnicity, education, insurance status, smoking, alcohol consumption, physical activ size, diabetes mellitus, cholesterol treatment, high-density lipoprotein, low-density lipoprotein, systolic blood pressure
DISCUSSION
In this prospective cohort study of community-dwelling individuals followed for 10 years, we found that compared to the co-dominant group, (i) those identified to have hsCRP-dominant inflammation status at baseline had a 2.62 fold risk of ischemic stroke and (ii) those individuals categorized as having IL6-dominant inflammation had a 0.62-fold risk of ischemic stroke. The relationship between our inflammation construct and ischemic stroke risk remained consistent after sensitivity analyses for missing observations. These findings represent possible stroke risk stratification approaches and may identify individuals that can benefit from more aggressive preventative measures.
We found IL6-dominance to be protective against stroke outcomes, as compared to the referent no-dominant inflammation group. We hypothesize that individuals with IL6-dominance benefit from inflammation pathways with greater auto-regulatory and anti-inflammatory associations of IL6. The role of IL6 in cardiovascular research is predominantly that of a risk factor (4), and its feedback mechanisms as an anti-inflammatory cytokine are not well established among cardiovascular researchers. Causal thinking in cardiovascular research may benefit from incorporating the concepts of dynamic feedback and change over time as they relate to biomarker risk prediction.(12)
Several notable CVD studies have assessed relationships between hsCRP and IL6. The Cardiovascular Health Study (CHS) found associations between elevated >3mg/L hsCRP (HR ) stroke events (HR=1.26, 95%CI, 1.05–1.51), as well as vascular events and all-cause mortality, in contexts of the detectable atherosclerosis.(13) Increasing levels of IL6 SD units were also found to be associated with incident vascular events (adjusted RR=1.14, 95%CI, 1.08–1.21) among the CHS cohort.(14) Recent findings from CHS have shown that doubling of IL6, but not CRP concentration, over time may have an impact on healthy aging, but risk stratification by relative levels was not explored.(15) Findings from the Atherosclerosis Risk in Communities (ARIC) study confirm that high levels of hsCRP predict stroke risk (adjusted HR=1.97, 95%CI, 1.14–3.39)(16) and congruently, increasing log-IL6 levels are associated with increased risk of coronary heart disease (adjusted HR=1.28, p-value=0.03).(17) Relative levels of IL6 to CRP were not evaluated in ARIC. Findings from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort found that hsCRP measured using the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial cut-offs (hsCRP ≥2 mg/L) was not associated with risk of incident CVD events (adjusted HR=1·08, 95%CI, 0·71–1·64) and IL6 was not been evaluated in relation to stroke in this trial.(18)
Pure reductionist approaches to use of a single biomarker to characterize the “state” of systemic inflammation may fail to accurately measure complex immunological processes and resultantly may not find associations with clinical endpoints. Research developments have sought to identify new biomarkers and novel analytic ways of combining information from multiple biomarkers to better characterize vascular risk. Some cardiovascular studies have incorporated as many as 30 biomarkers in cluster analyses to identify chronic inflammation and subclinical cardiovascular risk.(19) However, combined information from as few as two strategically selected biomarkers may also provide a novel characterization of risk. Integrated data from hsCRP and interleukin-6 (IL6) serum concentrations, such as the ratio of hsCRP to IL6, has been shown to vary among high-risk vascular patients receiving statins.(5)
Clinical evidence of IL6 anti-inflammatory associations are also derived from (i) cancer patients administered recombinant IL6 and (ii) cytokine monitoring in healthy patients engaging in strenuous physical activity. A study that examined serial measurements of inflammatory biomarkers in cancer patients that received a continuous intravenous IL6 infusion (30 ug/kg/24-hour) for a 120-hour period, observed an initial and rapid peak increase in interleukin-1 receptor antagonist (IL-1Ra) and a sustained gradual increase in TNFsRp55.(20) IL-1Ra has been shown to have anti-inflammatory protective associations when administered through exogenous infusion.(21) TNFsRp55 is believed to be a soluble inhibitory factor of pro-inflammatory cytokine tumor necrosis factor alpha.(22) The elevation of these anti-inflammatory cytokines in response to IL6 highlights the complexity of normal homeostatic self-regulating immunologic responses. Interestingly, exogenously administered IL6 in animal models has been shown to prevent ischemia and reperfusion injury in experimentally induced intestinal transplantation, with markedly lower serum TNF-alpha and IL1-B, as compared to animals that did not receive IL6 infusion.(23) This data is suggestive that IL6 anti-inflammatory pathways could potentially play a protective role in ischemic events by reducing pro-inflammatory cytokine up-regulation.
Exercise has also been shown to endogenously induce high levels of serum IL6 concentrations. While the acute associations of exercise such as muscle fiber tears may be pro-inflammatory, the longer-term associations of physical activity are generally regarded as anti-inflammatory. (24) Cytokine monitoring of subjects engaging in strenuous physical activity show that individuals experience marked elevations of serum IL6 that is temporally followed by increases in serum anti-inflammatory compounds, such as IL-10 in healthy individuals.(25) IL-10 functions to inhibit cytokine and chemokine synthesis in macrophage populations, and reduces responses to Toll-like receptor (TLR) activation from pathogen stimulation.(26) These anti-inflammatory associations are believed to occur in response to exercising muscle tissue secretion of IL6, and are believed to be partially responsible for conferring cardiovascular protective associations of physical activity. (25, 27) In addition, there is consensus in the immunology literature that the ability to return from an inflammatory “effector phase” back to homoeostasis is predominantly regulated through IL6-mediated pathways: locally, as can be the case in vascular lesions, through change of leukocyte infiltrates from polymorphonuclear neutrophils (acute inflammation) to macrophages (chronic inflammation); and globally, through broad systemic associations.(28) (29) Taken together, this body of research may suggest a plausible mechanism whereby IL6 may elicit protective vascular associations. Further evidence supporting the role of IL6 in chronic inflammation could be derived from serial measures of disease progression and biomarkers, but repeated cytokine measures are not currently available in the NOMAS population. Studies have noted mild to moderate invariance of categorical levels of IL6 and hsCRP over time, possibly indicative of extended duration in inflammatory states identified in this paper.(30)
CRP is a non-specific acute phase protein, meaning that levels may be elevated in response to various immunologic factors such as infection, injury, or stress responses. It is well known that serum IL6 induces CRP secretion in the liver(31) and mechanisms have been elucidated that show CRP-gene expression to be regulated through IL6-independent pathways, as well. Murine models (with transgenic human CRP protein) have shown CRP transcription requires IL6 proteins for wounding and LPS stimuli, but CRP transcription is readily stimulated by other cytokines such as interleukin-1, interleukin-11, leukemia inhibitory factors, and oncostatin M and are IL6 independent. (32) Inflammation characterized by elevated CRP but not elevated IL6 may reflect these alternative etiologies, and may be consistent with vascular or chronic inflammation rather than stimulation induced by transient or acute causes.
The trichotemized inflammatory construct is a novel approach to parse associations of inversely related biomarkers, and although non-dynamic, attempt to capture chronic immune system inflammatory states. The associations of ischemic stroke risk of those individuals with inversely related hsCRP and IL6 levels are not explicitly tested using typical interaction terms in generalized linear models, as the standard interaction term would capture the effect of jointly elevated or decreased hsCRP and IL6 levels. Instead, probing the association requires either a ratio (a form of an interaction term) or nominal categorization. A continuous form of the ratio was not used as a predictor in order to avoid any possibilities of spurious associations due to violations of the marginality principle, i.e., a problem that occurs when modeling an interaction term without including the interacting sub-components in the regression model.(33) The inflammation state variables were shown to be predictive of vascular events even after adjustment for trichotomized CRP and IL6 variables, demonstrating unique risk information above modeling CRP and IL6 independently. Based on existing literature, we a priori hypothesized that individual membership in the upper-triangular area corresponds with chronic inflammation, where individuals are unable to return to normal cytokine levels (hsCRP-dominant) and the lower-triangular area corresponds to reduced risk, with an increased ability to return to homeostasis (IL6-dominant). The diagonal cells (the referent group), corresponds to joint elevation (or declination) of hsCRP and IL6 inflammation, likely a response to tissue injury or infection, and greater capacity to return to homeostasis after inflammation, as compared to hsCRP-dominant group.
We found that the effects of IL6 dominance on ischemic stroke were modified by insurance status. Compared to co-dominance, the inverse associations of IL6 dominance with the risk of IS were present only among those with Medicare or private insurance. Insurance status may be a proxy for multiple factors that can influence stroke risk, including many elements of socio-economic position, as well as health care utilization and quality of care. (34) Protective associations of IL6 dominance in the private insurance and Medicare group may be related to modifiable elements associated with medical care. Treatment with statins has been shown in some studies to reduce relative CRP and IL6 levels to varying degrees in patients with cardiovascular risk factors.(35) (36) (37) Obesity and central adiposity have been linked to elevated inflammatory cytokine levels, with a strong dose response between degree of abdominal adiposity and CRP, but not IL6.(38) (39) Interventional evidence linking obesity reduction and low hsCRP relative to IL6, comes from among patients who experienced weight-loss after bariatric surgery, where concentrations of CRP were reduced but the levels of IL6 did not change after weight loss. (6, 40) Recurrent contact with primary care providers may lead to better health behaviors and no insurance or Medicaid-only may lead to poor management of comorbid conditions, increasing risk for adverse vascular outcomes. (41)
Compared to co-dominance, the hsCRP dominant status indicates increased risk of ischemic stroke among white and Hispanics, but not among blacks. The inverse association of IL6 dominance was present only among blacks. The effect modification may be due to the variable distributions of CRP and IL6 values across race-ethnic groups (blacks had the highest upper quartile ranges for CRP and IL6), but use of race-specific quartiles to create the inflammatory construct (as opposed to cohort specific quartiles) did not change the significant overall associations or the presence of effect modification by race-ethnicity (data not shown). The incidence of IS in the co-dominance group was higher in blacks as compared to whites or Hispanics, suggesting heterogeneity by race-ethnic subgroup may be driven by our referent category. This effect modification needs further investigation to understand if variable ischemic stroke risk is due to data distributions by race, homogenous effects in reference category after certain thresholds, or social factor mediators of race-ethnic status that modify the association of inflammation and stroke risk.(42)
The main implication of this research is that we identify a novel characterization of systemic inflammation and ischemic stroke risk from readily available biomarkers. The risk prediction of this construct appears to be consistent over long-term periods. Attention to hsCRP and IL6 dominant inflammation could potentially be used to ascertain anti-inflammatory responses to intervention on modifiable risk factors as a means to ascertain efficacy in reducing overall inflammation and cerebrovascular risk.
There are several potential limitations of this analysis. First, we are limited to CRP and IL6 values on only half of the NOMAS cohort. We attempted to address our missing data using standard approaches and our results remained qualitatively similar and robust. Second, serum levels of hsCRP and IL6 were measured at a single time point and this can limit our ability to investigate the stability of hsCRP dominant or IL6-dominant inflammation status over time. Establishing that hsCRP-dominance is consistent with chronic inflammation would require repeated measures of inflammatory biomarkers to further establish construct validity by correlating our proposed chronic inflammation category with invariance of group status over time. Third, our data is observational and requires validation of this measure in different populations to strengthen construct validity. Our next steps will to be to collaborate with other cardiovascular research groups to evaluate the generalizability of the relative hsCRP-to-IL6 construct to predict ischemic stroke risk, examine race-ethnic cut-offs, and also further investigate the lack of association between this construct and MI or vascular mortality. Finally, our records of hyperlipidemia medication at baseline do not provide sufficient resolution to identify drug class or adjust for potential anti-inflammatory associations of medication.
Conclusion
In this multi-ethnic cohort, the stroke risk associated with the inflammatory biomarkers hsCRP and IL6 depended on the relationship to one another. When hsCRP quartile dominated the IL6 quartile, risk was increased, and when IL6 dominated hsCRP, risk was decreased. Combined panels of inflammatory biomarkers may predict ischemic events, even when individual measures do not. This construct may be useful in assessing chronic inflammation, ischemic stroke risk prediction over time, and possibly the evaluation of effective anti-inflammatory clinical interventions. Stroke risk prediction using this construct is influenced by insurance status and possibly race-ethnic status. Further studies are needed to evaluate this risk categorization in other populations and to further assess construct validity by investigating the relationship between hsCRP-dominant inflammation and mediators of ischemic stroke, such as carotid plaque thickness. Finally, we believe that incorporating system science approaches – repeated measures over time, measuring multiple pathways in complex biological system, and assessing possible feedback dynamics – may improve a clinician’s ability to identify and treat individuals at high risk for future stroke events.
Acknowledgments
Funding Sources: NIH/National Institute of Neurological Disorders and Stroke (R37 NS 29993 [RLS/MSVE])
Footnotes
Disclosures: None
References
- 1.Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, et al. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. 2010;17:1–11. doi: 10.5551/jat.2600. [DOI] [PubMed] [Google Scholar]
- 2.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, et al. High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan Study. Neurology. 2009;73:1300–7. doi: 10.1212/WNL.0b013e3181bd10bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marz W, Winkler K, Nauck M, Bohm BO, Winkelmann BR. Effects of statins on C-reactive protein and interleukin-6 (the Ludwigshafen Risk and Cardiovascular Health study) The American journal of cardiology. 2003;92:305–8. doi: 10.1016/s0002-9149(03)00633-7. [DOI] [PubMed] [Google Scholar]
- 6.Pardina E, Ferrer R, Baena-Fustegueras J, Rivero J, Lecube A, Fort J, et al. Only C-Reactive Protein, but not TNF-α or IL6, Reflects the Improvement in Inflammation after Bariatric Surgery. Obesity Surgery. 2012;22:131–139. doi: 10.1007/s11695-011-0546-3. [DOI] [PubMed] [Google Scholar]
- 7.Elkind MSV, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, et al. High-sensitivity C-reactive protein predicts mortality but not stroke: The Northern Manhattan Study. Neurology. 2009;73:1300–1307. doi: 10.1212/WNL.0b013e3181bd10bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborators NMSS, Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, et al. Stroke Incidence among White, Black, and Hispanic Residents of an Urban Community: The Northern Manhattan Stroke Study. American Journal of Epidemiology. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 9.Greene HL, Richardson DW, Barker AH, Roden DM, Capone RJ, Echt DS, et al. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study) The American journal of cardiology. 1989;63:1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Chen HY. Augmented inverse probability weighted estimator for Cox missing covariate regression. Biometrics. 2001;57:414–9. doi: 10.1111/j.0006-341x.2001.00414.x. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, Paik MC, Rundek T, Elkind MS, Sacco RL. Reweighting estimators for Cox regression with missing covariate data: analysis of insulin resistance and risk of stroke in the Northern Manhattan Study. Stat Med. 2011;30:3328–40. doi: 10.1002/sim.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39:97–106. doi: 10.1093/ije/dyp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, et al. Association of Carotid Artery Intima-Media Thickness, Plaques, and C-Reactive Protein With Future Cardiovascular Disease and All-Cause Mortality: The Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 14.Zakai NA, Katz R, Jenny NS, Psaty BM, Reiner AP, Schwartz SM, et al. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. Journal of Thrombosis and Haemostasis. 2007;5:1128–1135. doi: 10.1111/j.1538-7836.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PHM, et al. Long-term Assessment of Inflammation and Healthy Aging in Late Life: The Cardiovascular Health Study All Stars. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, et al. Lipoprotein-associated phospholipase a2, high-sensitivity c-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the atherosclerosis risk in communities (aric) study. Archives of Internal Medicine. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, et al. An assessment of incremental coronary risk prediction using c-reactive protein and other novel risk markers: The atherosclerosis risk in communities study. Archives of Internal Medicine. 2006;166:1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 18.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. The Lancet. 378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Ham HJ, de Jager W, Bijlsma JW, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology. 2009;48:899–905. doi: 10.1093/rheumatology/kep125. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–8. [PubMed] [Google Scholar]
- 21.Zhu RZ, Xiang D, Xie C, Li JJ, Hu JJ, He HL, et al. Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World J Gastroenterol. 2010;16:2771–9. doi: 10.3748/wjg.v16.i22.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westlund KN, Zhang L, Ma F, Oz HS. Chronic inflammation and pain in a tumor necrosis factor receptor (TNFR) (p55/p75−/−) dual deficient murine model. Transl Res. 2012;160:84–94. doi: 10.1016/j.trsl.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimizuka K, Nakao A, Nalesnik MA, Demetris AJ, Uchiyama T, Ruppert K, et al. Exogenous IL-6 inhibits acute inflammatory responses and prevents ischemia/reperfusion injury after intestinal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:482–94. doi: 10.1111/j.1600-6143.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 24.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 25.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2006;57 (Suppl 10):43–51. [PubMed] [Google Scholar]
- 26.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8686–91. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:884–6. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 28.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. IL-6 and Its Soluble Receptor Orchestrate a Temporal Switch in the Pattern of Leukocyte Recruitment Seen during Acute Inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 29.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. The Journal of Clinical Investigation. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-Year Change in Inflammatory Markers in Elderly People and Mortality: The Invecchiare in Chianti Study. Journal of the American Geriatrics Society. 2007;55:1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shine B, de Beer FC, Pepys MB. Solid phase radioimmunoassays for human C-reactive protein. Clinica chimica acta; international journal of clinical chemistry. 1981;117:13–23. doi: 10.1016/0009-8981(81)90005-x. [DOI] [PubMed] [Google Scholar]
- 32.Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. The Biochemical journal. 1997;327:425–9. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronmal RA. Spurious Correlation and the Fallacy of the Ratio Standard Revisited. Journal of the Royal Statistical Society Series A (Statistics in Society) 1993;156:379–392. [Google Scholar]
- 34.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579–84. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y, Tong G, Xu W, Pan J, Ryan K, Yang R, et al. Anti-inflammatory effects of simvastatin on adipokines in type 2 diabetic patients with carotid atherosclerosis. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2009;6:262–8. doi: 10.1177/1479164109339966. [DOI] [PubMed] [Google Scholar]
- 36.Devaraj S, Chan E, Jialal I. Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2006;91:4489–96. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- 37.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–5. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 38.Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32:1734–6. doi: 10.2337/dc09-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galcheva SV, Iotova VM, Yotov YT, Bernasconi S, Street ME. Circulating proinflammatory peptides related to abdominal adiposity and cardiometabolic risk factors in healthy prepubertal children. Eur J Endocrinol. 2011;164:553–8. doi: 10.1530/EJE-10-1124. [DOI] [PubMed] [Google Scholar]
- 40.Barnard EE. JUPITER: Observation of The Great Red Spot. Science. 1881;2:9–10. doi: 10.1126/science.os-2.29.9-a. [DOI] [PubMed] [Google Scholar]
- 41.Fowler-Brown A, Corbie-Smith G, Garrett J, Lurie N. Risk of cardiovascular events and death--does insurance matter? J Gen Intern Med. 2007;22:502–7. doi: 10.1007/s11606-007-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–90. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]