Abstract
Oxidative stress can be generated at several sites within the mitochondria. Among these, monoamine oxidases (MAO) have been described as a prominent source. MAO are mitochondrial flavoenzymes responsible for the oxidative deamination of catecholamines, serotonin and biogenic amines, and during this process they generate H2O2 and aldehyde intermediates. The role of MAO in cardiovascular pathophysiology has only recently gathered some attention since it has been demonstrated that both H2O2 and aldehydes may target mitochondrial function and consequently affect function and viability of the myocardium. In the present review, we will discuss the role of MAO in catecholamine and serotonin clearance and cycling in relation to cardiac structure and function. The relevant contribution of each MAO isoform (MAO-A or -B) will be discussed in relation to mitochondrial dysfunction and myocardial injury. Finally, we will examine both beneficial effects of their pharmacological or genetic inhibition along with potential adverse effects observed at baseline in MAO knockout mice, as well as the deleterious effects following their over-expression specifically at cardiomyocyte level.
Keywords: monoamine oxidase, oxidative stress, mitochondrial dysfunction, heart failure, ischemia/reperfusion injury
1 Introduction
Although it is generally accepted that mitochondria are the major source of reactive oxygen species (ROS) in cardiac myocytes [1–4], relevant issues, such as sites at which ROS formation occurs, control mechanisms, relationships between formation and removal reactions and the relative contribution of the various processes to the total accumulation of ROS are far from being elucidated conclusively. Mitochondria contain several enzymes that catalyze ROS formation either as the obligatory product or as the result of an occasional, possibly undesired, reaction.
1.1 Occasional ROS formation within mitochondria
This latter possibility is exemplified by the mitochondrial respiratory chain. A minor fraction of the electrons (about 0.1%) flowing through the transport chain is diverted causing the partial reduction of O2 into superoxide [3]. This process occurs at the level of the first three complexes where flavins or quinones are able to act as single electron donors. In isolated mitochondria the relative contribution of sites IF, IQ, IIF and IIIQo (the roman number indicates the complex and the letter specifies the involvement of flavin or quinone moieties) appears to depend on the substrate utilized [5]. The electron detour at these upstream sites is favored when flow is hampered downstream as a result of either protein alterations in respiratory complexes or inhibitory effects of toxicants.
Superoxide that does not cross the inner mitochondrial membrane is rapidly dismutated into the freely permeable H2O2 by Mn-superoxide dismutase (Mn-SOD). The finding that Mn-SOD deficient mice develop ROS toxicity and dilated cardiomyopathy [6], underlines the importance of ROS in this pathology and mitochondria as their source and target. This concept is further supported by the beneficial effects afforded by targeting catalase expression in mitochondria [7–10].
Besides superoxide generation by respiratory chain complexes, several other mitochondrial enzymes have been described as potential ROS producers. These include (but are not limited to) the flavin containing glycerol-3-phosphate-, proline- and dihydroorotate-dehydrogenase at the outer leaflet of the inner mitochondrial membrane, the electron transfer flavoprotein-ubiquinone (ETF:Q) oxidoreductase system of fatty acid β-oxidation within the inner mitochondrial membrane, and pyruvate- and 2-oxoglutarate dehydrogenase within the mitochondrial matrix [5]. All these enzymes and respiratory complexes normally catalyze reactions other than ROS formation, that are required for energy metabolism, cell function and viability maintenance. Their characterization as ROS forming enzymes has been carried out in isolated mitochondria by means of inhibitors or non-physiological procedures, such as glutathione depletion. Obviously, these approaches can hardly be adopted in living cells or tissues without jeopardizing a wide array of vital functions. This is a major caveat that eventually does not allow obtaining definite evidence that these potential ROS sources contribute to oxidative stress in vivo. If these processes were the only ones responsible for mitochondrial ROS formation, it would be actually impossible to demonstrate that these organelles are primarily involved in oxidative stress in living organs. In fact, oxidative changes within mitochondria could be just the result of alterations caused by ROS formed at other cellular sites. However, this is not the case because mitochondria contain other enzymes that generate hydrogen peroxide (H2O2) as a direct and obligatory product. Inhibition of these enzymes does not affect other energy-linked functions and it provides convincing evidence of mitochondrial ROS formation in vivo and its role in pathophysiology of many organs including the cardiovascular system.
1.2 Obligatory ROS formation within mitochondria
p66Shc is a cytosolic adaptor protein that upon stress translocates to mitochondria where it catalyzes electron transfer from cytochrome c to oxygen [11], a process that can result in the formation of ROS. Indeed, ROS generation is reduced in cells lacking p66Shc and in p66Shc−/− mice, whose lifespan is increased by 30% [11–14] in a protected environment [15]. Furthermore, genetic deletion of p66Shc protects against ischemia/reperfusion (I/R) injury in mice hearts [16] and brain [17] and diabetic complications such as cardiomyopathy, nephropathy, delayed wound healing, and endothelial dysfunction [18–21]. Nicotinamide adenine dinucleotide phosphate oxidase 4 (Nox4) is another ROS generating enzyme that localizes in the plasma membrane but also intracellularly, in the mitochondria, focal adhesions, nucleus, endoplasmic reticulum. Nox4 associates with p22phox for its activation, and, unlike other Noxs, generates H2O2 in preference to superoxide [22]. Nox4/p22phox appears to be constitutively active [23], although several studies have shown that Nox4 activity can be modulated by different stimuli [24–27]. Mice in which Nox4 is targeted in a cardiac-specific manner demonstrate that Nox4 is both protective and injurious in models of cardiac pressure overload [28, 29]. Furthermore, while certain studies reported Nox4 to be deleterious, contributing to mitochondrial dysfunction and several pathologies such as ischemic stroke, diabetic cardiomyopathy, vascular inflammation and remodeling [30–32], others concluded that Nox4 might be vascular-protective rather than vascular-damaging [33]. These controversies may stem from different genetic models in which Nox4 was either silenced or overexpressed, or they may reflect different roles and regulation under pathophysiological conditions. Either way, they warrant further investigation.
Another enzyme localized in the mitochondria is monoamine oxidase (MAO). Activation of this enzyme leads to H2O2 formation and has been shown to contribute to a number of neuronal disorders, such as Parkinson’s or Alzheimer’s disease, most likely due to formation of ROS responsible for oxidative damage to neurons [34]. Although MAO inhibitors are currently used in the clinic for treatment of neurodegenerative diseases, MAO role in cardiac pathophysiology has gained attention only recently. However, charting this territory is likely to be of major pathophysiological relevance because oxidative stress impairs functions in viable cardiac myocytes, leading to contractile failure. In this review we are going to focus mostly on their role in the heart and speculate on the potential use of these compounds for treating cardiovascular diseases.
1.3 Interaction among mitochondrial ROS sources
It is likely that an intense cross-talk exists between different ROS sources in the cell. This is supported by the observation that frequently, inhibition of single ROS source is able to completely abolish oxidative stress and the resulting damage. One way to explain this is to envision that there is an “amplification mechanism’, whereby a single ROS source is activated by an initial stress, starts to generate ROS and triggers other sites in the cell to start producing free radicals leading therefore to oxidative stress. On the other hand, it should not be disregarded that there is significant “buffering” due to cellular antioxidant systems and that ROS formation or oxidative stress may become evident only after a certain threshold has been reached [35]. Either way, inhibition of a single ROS source is able to lower overall ROS levels and, in most cases, to prevent cellular structural and functional derangements. In this regard, it is worth mentioning that inhibitors of p66Shc are not yet available, Nox inhibitors are not isoform-specific or approved for use in clinic, whereas it is inconceivable to think that electron transport chain inhibitors could be used in patients. On the contrary, MAO inhibitors are available and already used in the clinic for the treatment of mood disorders, Parkinson’s and Alzheimer’s disease [34, 36, 37]. Development of a new generation of reversible MAO-A inhibitors, such as moclobemide, makes it worthwhile investigating whether MAO inhibitors could also be used to treat cardiovascular pathologies.
Here, we will discuss the relevant contribution of MAO isoforms to myocardial injury and mitochondrial dysfunction. Next, we will examine the beneficial cardioprotective effects produced by either their pharmacological or genetic inhibition, along with the potential deleterious effects following their over-expression. Finally, we will discuss the role of MAOs in catecholamine and serotonin (5-HT) clearance in relation to cardiac structure and function.
2, MAO: structure, localization and function
Monoamine oxidases are flavoenzymes anchored to the outer mitochondrial membrane through a transmembrane helix located within the carboxyl-terminal domain. They are responsible for the oxidative deamination of neurotransmitters and dietary amines. MAOs exist in two isoforms, MAO-A and -B, distinguished by different substrate specificity and inhibitor sensitivity. These two enzymes present 70% homology in their primary sequence [38, 39] and both contain the obligatory cofactor flavin adenine dinucleotide (FAD) covalently bound through a thioether linkage to Cys406 in MAO-A and Cys397 in MAO-B [40, 41]. This flavin moiety is the only redox-dependent factor necessary for catalysis, since mutations of flavin-linking residues ablate enzymatic activity. Regardless of the similarities, the two isoforms differ considerably in the structures of their active sites opposite the flavin cofactor. In humans, MAO-A has a monopartite cavity, whereas MAO-B exhibits a bipartite cavity structure with an entrance and a substrate cavity. Ile199 functions as a conformational “gate” separating the two cavities. Although the crystal structure of human MAO-A is monomeric whereas MAO-B is dimeric, both enzymes are dimeric in their membrane-bound forms [42, 43].
Besides the covalent attachment of the FAD to the cysteine residues, nascent MAO polypeptidic chains undergo also other posttranslational modifications. The best-characterized processes are the removal of the initiator methionine in MAO-B (but not in MAO-A) and the acetylation of the N-terminus in both molecules (methionine for MAO-A and serine for MAO-B) [44–46]. In addition to the acetylation of the N-terminus, human, rat and mouse MAO-A and -B from several tissues have been shown to contain several acetylated lysine residues [47–49], although whether and how this modification affects their function and ability to generate ROS remains still unclear. Lastly, there are also several serine residues in both MAO-A and -B that can be phosphorylated [50]. Among these, Ser209 phosphorylation within a putative p38 consensus motif results in the inhibition of MAO-A activity in neuronal cell lines [51]. This modification might be tissue-specific and so far no information is available on cardiac myoctes.
The distribution of MAO in various tissues of different species has been investigated by use of specific inhibitors, immunohistochemistry, enzyme autoradiography and in situ hybridization [52–54]. MAO location has been intensively studied in the brain, where MAO-A has been prevalently found in noradrenergic and dopaminergic neurons. Conversely, MAO-B has been detected in cell bodies of serotoninergic neurons, in histaminergic neurons and in glial cells [55–58]. In peripheral tissues, MAO-A has been found in placenta, fibroblasts, liver, intestine and thyroid gland, while platelets, liver, pancreatic islets and kidney contain mainly MAO-B [46, 59, 60]. Human cardiomyocytes contain both enzymes, although MAO-A appears to be the predominant isoform [61, 62].
The subcellular localization of MAOs appears to be variable depending on the tissue and that in some organs MAO may be localized in the norepinephrine storage particles [63]. The majority of MAO is associated with the outer mitochondrial membrane; fractions of the enzyme have, however, been described also in the nuclear envelope, endoplasmic reticulum and even in the plasma membrane. These findings remain controversial due to the lack of specificity of the methodologies employed and thus require more in-depth further investigation. In fact, more recent studies found MAO only in the outer mitochondrial membrane [60]. Interestingly, the use of TEMPO-substituted pargyline analogues (TEMPO or 2,2,6,6-tetramethyl-1-piperidinyloxy is a commonly used structure for making stable spin labels) and MAO inactivation by proteolysis allowed demonstrating that MAO-A and MAO-B are differently oriented in the outer mitochondrial membrane [64]. This finding might be of particular interest for designing novel subtype selective MAO inhibitors.
MAO-A catalyzes preferentially the oxidative deamination of norepinephrine and serotonin. Its activity is inhibited by low concentrations of clorgyline. Conversely, MAO-B has major affinity for phenylethylamine and benzylamine, and is inhibited by selegiline. Both isoforms catalyze the deamination of dopamine, tyramine, octopamine, 3-iodothyronamine and tryptamine and are inhibited by pargyline (for detailed review on MAO inhibitors and their clinical use please see [34, 36, 65]). The reaction of oxidative deamination occurs in several steps [66], ultimately resulting in the formation of the aldehyde from the corresponding amine, ammonia and H2O2. The aldehyde intermediate is rapidly metabolized to the corresponding acid by the action of aldehyde dehydrogenases (ALDH).
Although the roles of MAO in terminating the actions of neurotransmitters/dietary amines in central and peripheral nervous system and in the extraneuronal tissue have been extensively studied for decades, much less attention has been dedicated to the products of their activity. H2O2 is a ROS that could be toxic per se at high concentrations, or it could generate hydroxyl radical in the presence of Fe2+. Ammonia accumulation could also be toxic, although this has not been investigated yet in relation to MAO activity [67, 68]. Aldehyde intermediates are toxic for the biological systems [69, 70] and a decrease in aldehyde dehydrogenase activity, also due to increased oxidative stress [71], might further contribute to the exacerbation of damage. Formation of these byproducts is a salient aspect of MAO biochemical and pharmacological profile that certainly warrants further attention.
3 MAO and neurotransmitters in the heart
MAO and serotonin
MAO substrates catecholamines and serotonin play a major role in the regulation of cardiac function. While catecholamines are mainly released by nerve endings, peripheral serotonin is produced and secreted by extra-neuronal cells. Most of the serotonin in the periphery is produced by intestinal enterochromaffin cells, stored in platelets and released after platelet activation during hemostasis or pathological thrombotic processes. However, serotonin can also be produced in the heart [72] by cardiac endothelial cells through transformation of 5-hydroxytryptophan into 5-HT by L-aromatic amino acid decarboxylase [73]. This finding suggested that serotonin released by activated platelets participates in cardiac injury/repair while that produced within the heart may play an important role in the regulation of physiological cardiac function. Accordingly, serotonin has been involved in a variety of cardiac pathophysiological processes, including ventricular hypertrophy [74], valve fibrosis [75] and I/R injury [76]. These cardiac effects of serotonin have been related to the stimulation of specific serotonin receptors [77, 78].
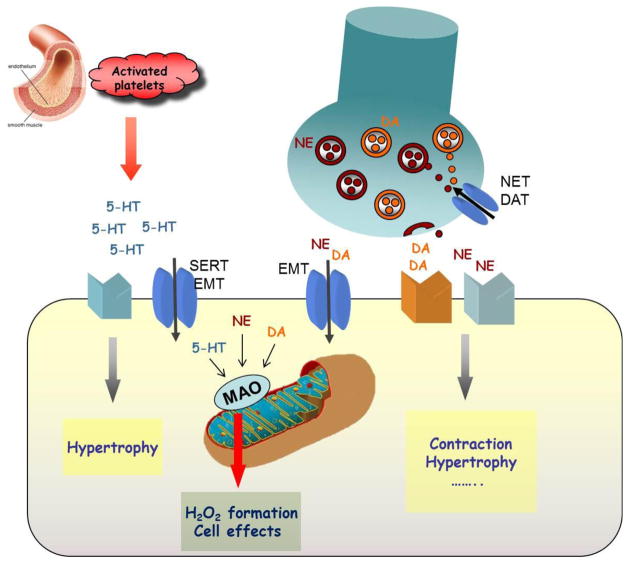
The availability and activity of cardiac serotonin depends not only on its release and/or production but also on its local degradation. Indeed, extracellular serotonin can be internalized into the cardiomyocytes through the plasma membrane serotonin transporter and metabolized by MAO-A [79]. In addition, targeted over-expression of MAO-A in mouse cardiomyocytes led to a significant decrease in cardiac serotonin, concomitant to the rise of its metabolite 5-hydroxyindoleacetic acid [80]. Thus, cardiac degradation of serotonin may be relevant for the regulation of the physiological and/or pathological effects of serotonin in the heart (Fig. 1).
Figure 1. Possible effects of serotonin or catecholamines on cardiac myocytes.
Serotonin (5-HT), released from the activated platelets, and catecholamines (NE, DA) released from the intracardiac nerves, interact with their receptors present at the level of the sarcolemma to exert their effects. Once this interaction is over, the majority of the neurotransmitter is rapidly re-uptaken through the transporter present in the membrane of the nerve terminal (NET, DAT) and only small percent escapes into the circulation or is uptaken through the extraneuronal monoamine transporter (EMT), present in the cardiomyocyte membrane. Once in the cytoplasm, these neurotransmitters are degraded by monoamine oxidases (MAO) and generate H2O2 that, in turn, might affect cellular processes even in the physiological conditions.
MAO and norepinephrine
The myocardium is under the continuous influence of neurohormones, and of sympathomimetic amines in particular. Hence, their turnover and catabolism are likely to have an impact in acute and chronic cardiac conditions, particularly in presence of excessive neurohormonal stimulation, for instance during hypertension and/or early stages of congestive heart failure (CHF). Despite the high pathophysiological potential of abnormalities in catecholamine cycling/catabolism in cardiovascular diseases, investigation in this direction has never been burgeoning.
Impairment of norepinephrine neuronal reuptake and concomitant down-regulation of the β-adrenergic system are well documented in human and experimental CHF [81–83], contributing to the loss of systolic performance in this syndrome. In normal hearts, 92% of the norepinephrine released by sympathetic nerves is recaptured by norepinephrine transporter (NET), 4% is removed by extra-neuronal uptake (through the extra-neuronal monoamine transporter, EMT), and the remaining 4% enters the circulation (Fig. 1). However, NET function declines in CHF contributing to norepinephrine “spillover”, used as a prognostic marker and a therapeutic index in these subjects [82, 84, 85]. Consequently, the extra-neuronal/cardiomyocyte uptake almost doubles in CHF patients [85], leading to enhanced MAO and catechol-O-methyl-transferase (COMT) activity. MAO-A activity is central to this pathogenetic mechanism since, in pressure overloaded hearts, both the pharmacological and genetic ablation of MAO-A activity prevent cardiac norepinephrine depletion, restoring NET expression levels and left ventricle (LV) function to control values. Our findings are fully in keeping with previous evidence showing that systemic infusion of MAO inhibitors increases the number of NET recognition sites through higher norepinephrine availability [86]. Regardless, these findings hint at the intriguing possibility that MAO-A inhibition may lead to increased (or preserved) catecholamine neuronal re-uptake and recycling rather than neosynthesis. This “gearing down” mechanism is strategic in conditions such as exercise in which the maximal rate of catecholamine synthesis (not exceeding 4 fold) cannot match the increased demand (>10 fold) for catecholamine release at the neuro-effector junction [69]. Envisioning a similar situation in human heart failure is plausible because failing heart is a “catecholamine depleted” one [87, 88], an effect that is due in part to deficient neuronal re-uptake (for review, see [89]) and impaired catecholamine neosynthesis, owing to the progressive loss of functional sympathetic fibers [90].
MAO and dopamine
Dopamine is preferentially catabolized by MAO-B, isoform very abundant in species such as mice and humans [61, 91]. Indeed, genetic ablation of MAO-B is accompanied by a rise in cardiac dopamine levels, up-regulation of extracellular signal-regulated kinases (ERK1/2) and lower apoptosis rates after pressure overload [92]. These data fit nicely with previous observations concerning the involvement of mitogen-activated protein kinase kinase 1 (MEK1)-ERK1/2 signaling cascade in maintenance of physiological hypertrophy and preserved cardiac function in stressed hearts [93].
It is tempting to speculate that increased ERK1/2 activity is related to the higher dopamine content in MAO-B−/− mice. Dopamine receptors (D1–4) are expressed in mouse hearts (our unpublished observations), and dopamine levels are elevated in the absence of MAO-B. Therefore, the link between ERK1/2 and dopamine might be supported by the activation of D2 receptors that have been shown to trigger ERK1/2 activation in neuronal and non-neuronal tissue [94]. Thus, endogenous dopamine might be involved in the governance of the plasticity of the adult heart, particularly in myocardial response/protection against chronic stress. Notably, dopamine is still widely used to treat heart failure, particularly after cardiac surgery in infants. The latter use is seemingly due to the fact that newborn myocardium is richer in D1 and β2-adrenergic receptor (AR) mRNA levels as compared to the adult one [95].
4 MAOs in cardiovascular diseases
4.1 MAO inactivation
4.1.1 Baseline phenotypical outcomes of MAO deletion
Deletion of MAO-A and MAO-B genes has proven their important roles in neurotransmitter metabolism and behavior. MAO-A−/− mice have elevated brain levels of serotonin, norepinephrine and, to a lesser extent, dopamine [96–98], whereas only 2-phenylethylamine is increased in MAO-B−/− mice [99]. Interestingly, all monoamines were increased in the brains of combined MAO-A/B−/− mice, to a much greater extent than those observed in either MAO-A−/− or MAO-B−/− mice [100]. This suggests that, in the absence of one isoenzyme, the other can partially overtake its catalytic role [46]. For an in-depth discussion on behavioral outcomes due to MAO deficiency we refer the readers to an excellent review by Bortolato and Shih [46].
The effects of MAO deletion have been characterized also in the cardiovascular setting. Mice lacking MAO-A activity show higher cardiac levels of serotonin, norepinephrine and epinephrine [88, 101] (and unpublished data). This increase in catecholamine levels has its structural and functional consequences on the heart. Lairez and coworkers [101] have shown that MAO-A−/− mice display cardiomyocyte hypertrophy and LV dilation at baseline, although LV dysfunction was absent and no hemodynamic alterations were observed. This effect can most likely be attributed to elevated cardiac 5-HT levels and hyperactivation of 5-HT2A receptors observed in MAO-A−/− mice. Another line of hypomorphic MAO-A mutants (MAO-Aneo) resulting in expression of truncated non-functional variant of MAO-Aneo transcript, displays a slightly different phenotype at baseline [88]. Despite the increase in cardiac catecholamine levels, these mice do not show any structural differences but reveal some hemodynamic differences. For instance, LV systolic pressure, dP/dtmax, and dP/dtmin (indexes of contractility and relaxation, respectively) are all lower in MAO-Aneo compared to wild type (WT) littermates. Contractile function assessed by preload-recruitable stroke work index was also lower in MAO-Aneo mice, so these differences were potentially related to loading changes. However, chamber volume and ejection fraction were similar between the two strains. Regardless of the differences between the two genetic models, it is likely that adaptive changes occur in the MAO-A gene deletion mouse models because these are global and constitutive transgenic mice. Further supporting this contention is the fact that 6 weeks treatment of the mice with MAO-A inhibitor clorgyline does not have any effects on basal cardiac structure or function as assessed by histological and hemodynamic measurements [88].
MAO-B−/− mice instead show higher cardiac dopamine content compared to their WT littermates, and also norepinephrine and epinephrine, although to a lesser extent compared to MAO-Aneo mice [92] (and unpublished observations). At baseline, echocardiography did not show any apparent morphological difference between WT and MAO-B−/− mice. However, a more thorough examination via pressure-volume loops analysis revealed that these null mice have slightly reduced contractility/relaxation at baseline and β-adrenergic desensitization, although fractional shortening and ejection fraction were not significantly different when compared to WT mice [92]. As in the case of MAO-A−/− or mutant mice, it is likely that these changes are due to excessive catecholamine build-up in these mice from birth. This issue could be better evaluated using conditional cardiomyocyte-specific MAO-A−/− or -B−/− mice, but these are currently unavailable.
4.1.2 MAO deletion in cardiovascular disease
MAO knockout and mutant mouse models have proven useful for the evaluation of the MAO role and contribution to cardiovascular disease. In vitro studies using adult mouse cardiomyocytes isolated from both WT and MAO-Aneo mice, showed that NE acts in part independently of α- or β-AR. Norepinephrine-induced hypertrophy is partially mediated by MAO-A generated ROS and stimulation of MAO-A activity, independently of receptor activation, is sufficient to trigger myocyte hypertrophy [88].
The important role of MAO in the transition from compensated hypertrophy to heart failure and I/R injury was confirmed in in vivo studies. MAO-A deficient mice are protected from I/R injury due to lower ceramide/sphingosine 1-phosphate ratio, identified as a critical event in MAO-A-mediated cardiac cell apoptosis [102]. Despite the better characterized role of MAO-A in experimental heart failure, the potential contribution of MAO-B to this syndrome should not be disregarded. The relative expression of each MAO isoform varies greatly across different tissues and species. For instance, MAO-A is the predominant isoform in the rat heart, with little or none of the MAO-B activity, whereas exactly the opposite is true in mice [61, 91]. Recent studies investigated the role of both isoforms employing genetic models [88, 92]. In a mouse model of CHF induced by transverse aortic constriction (TAC), both MAO-Aneo and MAO-B−/− mice displayed reduced cardiac oxidative stress, LV remodeling and apoptosis. Furthermore, absence of MAO activity in those hearts completely prevented LV dilation/pump failure, favoring instead the maintenance of stable concentric hypertrophy in chronically stressed hearts. Thus, genetic ablation of either MAO-A or -B activity benefited TAC hearts by reducing ROS burden and countering LV remodeling, mitochondrial dysfunction and cardiomyocyte apoptosis. On the other hand, catecholamine cycling was also improved in mice lacking MAO-A or MAO-B activity. Indeed, both norepinephrine and dopamine degradation is increased in failing hearts due to MAO activation, and its inhibition leads to improved neuronal pools and availability of these catecholamines.
At variance with previously mentioned findings, a study by Lairez and colleagues [101] observed exacerbated ventricular hypertrophy in MAO-A−/− mice subjected to aortic banding, attributing this effect to the hyperactivation of 5-HT2A receptors in MAO-A−/− mice. As mentioned previously, these knockout mice already showed some adaptive mechanisms at baseline that were further exacerbated by aortic banding. Differences in the genetic model could underlie this apparent contradiction. Indeed, other studies have shown that MAO-A−/− animals show behavioral and morphological alterations distinct from those of MAO-Aneo mice [98]. Furthermore, the severity of aortic banding might also explain the differences because banding of the ascendant aorta (Lairez’ study) results in a less acute model of CHF as compared to the banding of transverse aorta (Kaludercic’ study). In fact, experimental protocol in the first study involved only compensated hypertrophy and the observations were not extended long enough to detect LV dilation and failure. Conversely, the latter study showed that both MAO isoforms play a major role in the transition from compensated hypertrophy to heart failure.
Setting aside these methodological issues, there is no doubt that MAO-A expression and/or activity is a major contributing factor to the development of pathologic hypertrophy and heart failure, at least in rodents. This contention is further supported by the observation that cardiomyocyte-specific over-expression of MAO-A leads to cardiomyocyte necrosis and heart failure whereas pharmacological inhibition of MAO is equally effective in preventing the occurrence of CHF and avoids potential adaptive changes at baseline [80, 88]. It remains to be determined whether myocyte-specific gene deletion would yield the same results.
Brandes’ group recently demonstrated the important role of MAOs in vasculature, showing that MAO expression in mouse aortas was induced through phosphatidylinositide 3-kinase and NFκB pathway following angiotensin II and lipopolysaccharide treatment and greatly contributed to vascular formation of H2O2 [35]. Indeed, pharmacological inhibition of both MAO-A with clorgyline or moclobemide and MAO-B with selegiline completely prevented this increase in oxidative stress and restored normal endothelium-dependent relaxation in vessels. Furthermore, these Authors found that MAO-A limited endothelial cyclic guanosine monophosphate accumulation, suggesting that MAO-generated species limit endothelial nitric oxide formation. Although this is not the first study to highlight the involvement of MAOs in vascular formation of ROS [103, 104], Sturza and colleagues demonstrated that endogenous vascular catecholamines are sufficient to fuel MAO activity, even without supplementing exogenous substrate. The evidence that vascular cells express several enzymes involved in catecholamine synthesis supports this. On the other hand, these observations do not exclude the possibility that there may be still unidentified MAO substrates whose catabolism may be enhanced in pathological states.
4.2 MAO over-expression
As detailed above, MAO-A was identified as a major source of ROS in the heart. As such, any increase in MAO-A activity could lead to enhanced generation of ROS and potential ROS-mediated damage. MAO-A activity can be augmented by means of increased substrate availability, such as serotonin or norepinephrine in the heart. Indeed, hyperactivity of sympathetic drive is evidenced by increased plasma norepinephrine and epinephrine, elevated sympathetic outflow, and heightened norepinephrine spillover in human CHF [105]. Concerning serotonin, one study reported a significant rise in platelet and plasma 5-HT concentrations in CHF [106]. On the other hand, enhanced generation of ROS by MAO-A could result from the enhanced expression of this enzyme at the transcriptional or post-transcriptional level. Such MAO-A up-regulation has been demonstrated in the aging heart, a situation frequently associated with heart failure [107]. In order to better understand whether this increase occurred in cardiac cells, we recently isolated cardiomyocytes from young, middle-age and old rats to measure MAO-A and MAO-B activities independently from other cell types present in the heart. Interestingly, we found that senescent cardiomyocytes displayed strong enhancement of MAO-A activity (95-fold between age of 2-month and 23 month) [80]. Since cardiac aging is a major risk factor for heart failure, we also evaluated the expression of MAO-A in a model of heart failure induced by aortic banding in the rat. We demonstrated that MAO-A activity was enhanced in cardiac hypertrophy and failure in this model [80]. In addition, some studies based on transcriptomic or proteomic analysis identified MAO-A as one of the most up-regulated proteins in different models of rat heart failure induced by volume overload [108], pressure overload [109] or myocardial infarction [110, 111]. At present, the mechanisms involved in MAO-A up-regulation in the heart are unknown.
In order to investigate the consequences of an increase in cardiac MAO-A activity on heart function, we recently developed in vitro and in vivo models of MAO-A over-expression [80]. In vivo, mice with cardiac-specific over-expression of MAO-A (MAO-A Tg) had enhanced levels of H2O2 in the heart and displayed enhanced staining with 8-oxo-2′-deoxyguanosine, a marker of DNA oxidation, which was more prominent in mitochondria. Moreover, norepinephrine and 5-HT concentrations were decreased in the hearts of MAO-A Tg mice, with concomitant increase in 5-hydroxyindoleacetic acid and dihydroxyphenylglycol. Thus, enhancing MAO-A in cardiomyocytes was able to impact global norepinephrine and 5-HT content in the heart. We demonstrated that MAO-A up-regulation was responsible for structural and functional damage of mitochondria accompanied by decreased adenosine triphosphate (ATP) concentrations and mitochondrial biogenesis. Histological analysis of cardiac sections of MAO-A Tg mice demonstrated important cardiomyocyte drop-out (about 50% of cardiomyocytes) between 6 and 12-weeks of age, together with reactive hypertrophy of residual cardiomyocytes and cardiac fibrosis. Progressive dilatation of the heart was measured by echocardiography, and lead to heart failure and premature death at about 30 weeks. By combining in vitro adenoviral transduction of MAO-A and in vivo over-expression in the heart, we found that p53 participated in mitochondrial damage and necrosis linked to MAO-A/H2O2 pathway. Altogether, we provided evidence for the first time that an increase in MAO-A expression could drive spontaneous mitochondrial damage and myocardial degeneration in heart failure and aging [80]. A similar observation has been made in the brain where MAO-B expression is increased during aging. Interestingly, over-expression of MAO-B in astrocytes resulted in oxidative stress, dopaminergic neuron degeneration and a phenotype that mimicked Parkinson disease in mice [112, 113]. Altogether, these studies constitute a direct proof of the deleterious effect of MAO in the generation of ROS and cell death. They provide new models for studying the mechanisms by which pathological conditions occur in relation with monoamine oxidase activation.
5 Mitochondria as a selective target of MAO-mediated injury
Mitochondrial dysfunction is frequently associated with oxidative stress, cell death and cardiac damage under several cardiovascular disease conditions. Considering that MAOs are located in the mitochondria, one might envision that exacerbated MAO activity might lead to damage of these organelles. A recent study by Villeneuve and colleagues [80] showed that cardiac-specific overexpression of MAO-A was accompanied by ultrastructural defects of cardiac mitochondria, ATP depletion and ultimately led to cardiomyocyte necrosis and heart failure. Furthermore, MAO-A over-expression in neonatal cardiomyocytes mimicked these results triggering oxidative stress-dependent p53 activation, leading to peroxisome proliferator-activated receptor-gamma coactivator 1α down-regulation, mitochondrial impairment, and cardiomyocyte necrosis (Fig. 2). Subsequently, we demonstrated the existence of a direct link between MAO activation, mitochondrial ROS formation and mitochondrial dysfunction [92]. Indeed, analyzing ROS formation after MAO activation in a spatiotemporal manner via the genetically-encoded redox sensitive fluorescent probe HyPer enabled us to target it specifically to mitochondria or cytosol. We observed that H2O2 formation occurs much earlier at the mitochondrial level rather than in the cytosol. This is an important finding since it reiterates the issue that mitochondria are “early targets” of endogenously produced oxidative stress that leads to mitochondrial dysfunction. Indeed, MAO activation was associated with the loss of mitochondrial membrane potential, unveiled after incubation with oligomycin. This suggests that ATP synthase was working in the reverse mode, i.e. hydrolyzing glycolytically generated ATP and compensating in this way for the dysfunctional respiratory chain [114].
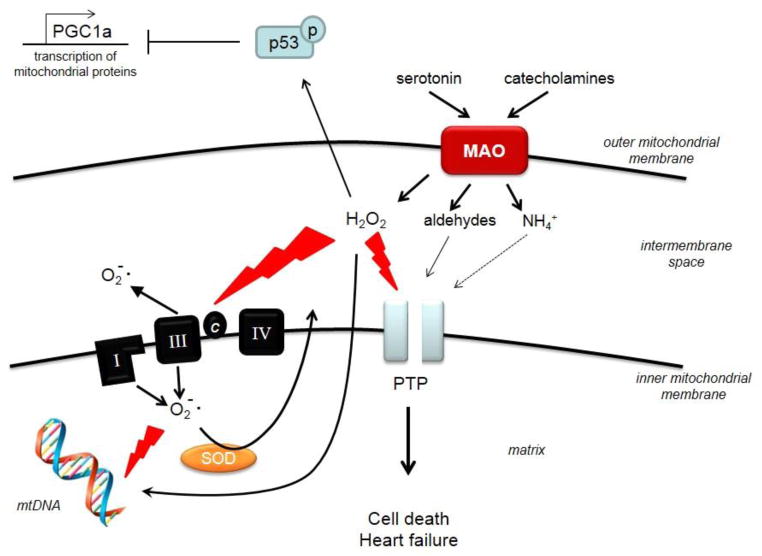
Figure 2. Deleterious effects of MAO activation on mitochondrial function.
Up-regulation of monoamine oxidase activity (MAO) due to higher substrate availability results in enhanced formation of H2O2 and aldehyde intermediates that can directly affect the transfer of the electrons across the respiratory chain and the opening of the permeability transition pore (PTP), leading to cardiomyocyte death, oxidative damage of mitochondrial DNA (mtDNA) and heart failure. Furthermore, MAO-generated oxidative stress triggers p53 activation that, in turn, leads to down-regulation of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis.
Other so far neglected but potentially interesting by-products of MAO activity are aldehydes generated from amines. These aldehydes are toxic species [115] that need to be converted to less harmful metabolites. Thus, MAO is functionally coupled with a NAD(P)+-dependent ALDH, which oxidizes the aldehyde to the corresponding carboxylic acid. Alternatively, depending on the location and the intracellular conditions, aldehydes can be reduced to alcohols or glycols by aldehyde reductase or alcohol dehydrogenase [46]. In the heart, these aldehydes are normally rapidly inactivated and transformed into corresponding acids by ALDH2, the most abundant ALDH isoform expressed in this tissue and localized in the mitochondria [116]. Previous studies have shown the ALDH2 activity is inhibited in conditions of high oxidative stress and that stimulation of its activity reduces myocardial I/R injury in rats [71]. Aldehydes induce inactivation of a number of macromolecules including the proteasome, the electron transport chain in the mitochondria, as well as inactivation of ALDH2 itself [117]. At present, there is only indirect evidence suggesting that ALDH2 activity might be reduced in heart failure, whereas aldehyde formation might be enhanced because malondialdehyde levels are increased in this condition. On the other hand, we recently showed that aldehydes generated by amine catabolism via MAO play a major role in the MAO-mediated mitochondrial dysfunction in cardiac myocytes [92]. In fact, dopamine incubation of siRNA-treated cardiomyocytes against ALDH2 led to high ROS production and accumulation of 3,4-dihydroxyphenylacetaldehyde, an aldehyde deriving from dopamine that is known to be very reactive and a potent neurotoxin [118], resulting ultimately in the loss of mitochondrial membrane potential. Whether degradation of other MAO substrates, such as norepinephrine or serotonin, yields toxic aldehydes that target mitochondrial function still warrants further investigation.
Altogether, this evidence lends supports the contention that mitochondria are early targets not only of extrinsic or endogenously produced ROS, but also of MAO-derived aldehyde intermediates that further fuel mitochondrial and myocardial damage (Fig. 2).
6 Conclusions
Evidence available so far suggests that MAO inhibition is beneficial for treatment of cardiovascular pathologies. From a translational point of view a major hurdle in accepting the use of MAO inhibitors in clinic is the possible occurrence of the so-called “cheese-effect”. In fact, ingestion of food rich in tyramine, such as wine and cheese, has been found to cause hypertensive crises in patients treated with irreversible MAO-A inhibitors. The introduction of a new generation of reversible MAO inhibitors appears to avoid this adverse effect. In fact, a very recent retrospective analysis reported that severe adverse hemodynamic events, such as hypertension and tachycardia, do not occur more frequently in users of both the irreversible MAO inhibitor tranylcypromine and the reversible MAO-A inhibitor moclobemide compared to nonusers [119]. In this investigation, cardiac contractility was not included as an endpoint. Hence, whether taking MAO (-A or -B) inhibitors improves cardiac function in patients with CHF remains a fascinating and potentially important pragmatic question to ask in future studies.
Highlights.
Monoamine oxidases are a major source of H2O2 within the mitochondria.
Their expression and activity is increased in several pathological conditions.
Monoamine oxidase inhibition prevents oxidative stress and cardiac dysfunction.
Monoamine oxidase-generated H2O2 and aldehydes can induce mitochondrial dysfunction.
Acknowledgments
This work was supported by the COST Action “EU-ROS” BM1203 and grants from Fondazione Cariparo and National Research Council of Italy (FDL), NIH (R01HL075265 and R01HL091923 to NP), AHA Grant-in-Aid 17070027 (NP), Agence Nationale pour la Recherche (ANR), Région Midi-Pyrénées, and INSERM, Institut National pour la Santé Et la Recherche Médicale, France(JMP and AP).
Abbreviations
- 5-HT
serotonin
- β-AR
β-adrenergic receptor
- ALDH
aldehyde dehydrogenase
- ATP
adenosine triphosphate
- CHF
congestive heart failure
- COMT
catechol-O-methyl-transferase
- EMT
extraneuronal monoamine transporter
- ERK1/2
extracellular signal-regulated kinases
- FAD
flavin adenine dinucleotide
- H2O2
hydrogen peroxide
- I/R
ischemia/reperfusion
- LV
left ventricle
- MAO
monoamine oxidase
- NET
norepinephrine transporter
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nox
NADPH (nicotinamide adenine dinucleotide phosphate) oxidase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TAC
transverse aortic constriction
- WT
wild type
Footnotes
Disclosures
NP, FDL and NK have a pending patent application entitled “Treatment of heart failure and associated conditions by administration of monoamine oxidase inhibitors”, number: 20090286883.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–72. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 7.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–44. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–74. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 11.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 13.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–95. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 14.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–8. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 15.Giorgio M, Berry A, Berniakovich I, Poletaeva I, Trinei M, Stendardo M, et al. The p66Shc knocked out mice are short lived under natural condition. Aging Cell. 2012;11:162–8. doi: 10.1111/j.1474-9726.2011.00770.x. [DOI] [PubMed] [Google Scholar]
- 16.Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–80. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM, et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2013;34:96–103. doi: 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, et al. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes. 2010;59:2306–14. doi: 10.2337/db09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 20.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–50. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 21.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–22. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touyz RM, Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res. 2012;110:1159–61. doi: 10.1161/CIRCRESAHA.112.269068. [DOI] [PubMed] [Google Scholar]
- 23.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovas Res. 2006;72:447–55. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–29. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–70. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–6. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597–C604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 33.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–25. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 34.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 35.Sturza A, Leisegang MS, Babelova A, Schroder K, Benkhoff S, Loot AE, et al. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension. 2013;62:140–6. doi: 10.1161/HYPERTENSIONAHA.113.01314. [DOI] [PubMed] [Google Scholar]
- 36.Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–32. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riederer P, Lachenmayer L, Laux G. Clinical applications of MAO-inhibitors. Curr Med Chem. 2004;11:2033–43. doi: 10.2174/0929867043364775. [DOI] [PubMed] [Google Scholar]
- 38.Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988;85:4934–8. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edmondson DE, Mattevi A, Binda C, Li M, Hubalek F. Structure and mechanism of monoamine oxidase. Curr Med Chem. 2004;11:1983–93. doi: 10.2174/0929867043364784. [DOI] [PubMed] [Google Scholar]
- 40.Edmondson DE, Binda C, Mattevi A. The FAD binding sites of human monoamine oxidases A and B. Neurotoxicology. 2004;25:63–72. doi: 10.1016/S0161-813X(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 41.Abell CW, Kwan SW. Molecular characterization of monoamine oxidases A and B. Prog Nucleic Acid Res Mol Biol. 2001;65:129–56. doi: 10.1016/s0079-6603(00)65004-3. [DOI] [PubMed] [Google Scholar]
- 42.Binda C, Mattevi A, Edmondson DE. Structural properties of human monoamine oxidases A and B. Int Rev Neurobiol. 2011;100:1–11. doi: 10.1016/B978-0-12-386467-3.00001-7. [DOI] [PubMed] [Google Scholar]
- 43.Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A. 2008;105:5739–44. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Hubalek F, Newton-Vinson P, Edmondson DE. High-level expression of human liver monoamine oxidase A in Pichia pastoris: comparison with the enzyme expressed in Saccharomyces cerevisiae. Protein Expr Purif. 2002;24:152–62. doi: 10.1006/prep.2001.1546. [DOI] [PubMed] [Google Scholar]
- 45.Newton-Vinson P, Hubalek F, Edmondson DE. High-level expression of human liver monoamine oxidase B in Pichia pastoris. Protein Expr Purif. 2000;20:334–45. doi: 10.1006/prep.2000.1309. [DOI] [PubMed] [Google Scholar]
- 46.Bortolato M, Shih JC. Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol. 2011;100:13–42. doi: 10.1016/B978-0-12-386467-3.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–18. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–4. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–31. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, et al. Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun. 2012;3:876. doi: 10.1038/ncomms1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao X, Rui L, Pennington PR, Chlan-Fourney J, Jiang Z, Wei Z, et al. Serine 209 resides within a putative p38(MAPK) consensus motif and regulates monoamine oxidase-A activity. J Neurochem. 2009;111:101–10. doi: 10.1111/j.1471-4159.2009.06300.x. [DOI] [PubMed] [Google Scholar]
- 52.Berry MD, Juorio AV, Paterson IA. The functional role of monoamine oxidases A and B in the mammalian central nervous system. Prog Neurobiol. 1994;42:375–91. doi: 10.1016/0301-0082(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 53.Kitahama K, Maeda T, Denney RM, Jouvet M. Monoamine oxidase: distribution in the cat brain studied by enzyme- and immunohistochemistry: recent progress. Prog Neurobiol. 1994;42:53–78. doi: 10.1016/0301-0082(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 54.Luque JM, Biou V, Nicholls JG. Three-dimensional visualization of the distribution, growth, and regeneration of monoaminergic neurons in whole mounts of immature mammalian CNS. J Comp Neurol. 1998;390:427–38. doi: 10.1002/(sici)1096-9861(19980119)390:3<427::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Arai R, Kimura H, Nagatsu I, Maeda T. Preferential localization of monoamine oxidase type A activity in neurons of the locus coeruleus and type B activity in neurons of the dorsal raphe nucleus of the rat: a detailed enzyme histochemical study. Brain Res. 1997;745:352–6. doi: 10.1016/s0006-8993(96)01239-5. [DOI] [PubMed] [Google Scholar]
- 56.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci U S A. 1982;79:6385–9. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. 1988;25:439–56. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- 58.Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230:181–3. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- 59.Huang YH, Ito A, Arai R. Immunohistochemical localization of monoamine oxidase type B in pancreatic islets of the rat. J Histochem Cytochem. 2005;53:1149–58. doi: 10.1369/jhc.5A6658.2005. [DOI] [PubMed] [Google Scholar]
- 60.Huang YH, Jiang M, Fu BY. Immunocytochemical localization of monoamine oxidase type B in rat liver. Eur J Histochem. 2008;52:11–8. doi: 10.4081/1181. [DOI] [PubMed] [Google Scholar]
- 61.Sivasubramaniam SD, Finch CC, Rodriguez MJ, Mahy N, Billett EE. A comparative study of the expression of monoamine oxidase-A and -B mRNA and protein in non-CNS human tissues. Cell Tissue Res. 2003;313:291–300. doi: 10.1007/s00441-003-0765-6. [DOI] [PubMed] [Google Scholar]
- 62.Saura J, Kettler R, Da PM, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41–1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–99. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Champlain J, Mueller RA, Axelrod J. Subcellular localization of monoamine oxidase in rat tissues. J Pharmacol Exp Ther. 1969;166:339–45. [PubMed] [Google Scholar]
- 64.Wang J, Edmondson DE. Topological probes of monoamine oxidases A and B in rat liver mitochondria: inhibition by TEMPO-substituted pargyline analogues and inactivation by proteolysis. Biochemistry. 2011;50:2499–505. doi: 10.1021/bi101722b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Binda C, Milczek EM, Bonivento D, Wang J, Mattevi A, Edmondson DE. Lights and shadows on monoamine oxidase inhibition in neuroprotective pharmacological therapies. Curr Top Med Chem. 2011;11:2788–96. doi: 10.2174/156802611798184355. [DOI] [PubMed] [Google Scholar]
- 66.Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A. Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry. 2009;48:4220–30. doi: 10.1021/bi900413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinelle K, Haggstrom L. Mechanisms of ammonia and ammonium ion toxicity in animal cells: transport across cell membranes. J Biotechnol. 1993;30:339–50. doi: 10.1016/0168-1656(93)90148-g. [DOI] [PubMed] [Google Scholar]
- 68.Visek WJ. Some aspects of ammonia toxicity in animal cells. J Dairy Sci. 1968;51:286–95. doi: 10.3168/jds.S0022-0302(68)86976-0. [DOI] [PubMed] [Google Scholar]
- 69.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–49. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 70.Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–41. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda K, Tojo K, Otsubo C, Udagawa T, Kumazawa K, Ishikawa M, et al. 5-hydroxytryptamine synthesis in HL-1 cells and neonatal rat cardiocytes. Biochem Biophys Res Commun. 2005;328:522–5. doi: 10.1016/j.bbrc.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Rouzaud-Laborde C, Hanoun N, Baysal I, Rech JS, Mias C, Calise D, et al. Role of endothelial AADC in cardiac synthesis of serotonin and nitrates accumulation. PloS One. 2012;7:e34893. doi: 10.1371/journal.pone.0034893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nebigil CG, Jaffre F, Messaddeq N, Hickel P, Monassier L, Launay JM, et al. Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation. 2003;107:3223–9. doi: 10.1161/01.CIR.0000074224.57016.01. [DOI] [PubMed] [Google Scholar]
- 75.Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease--it was meant 2B. Pharmacol Ther. 2011;132:146–57. doi: 10.1016/j.pharmthera.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajesh KG, Suzuki R, Maeda H, Murio Y, Sasaguri S. 5-HT2 receptor blocker sarpogrelate prevents downregulation of antiapoptotic protein Bcl-2 and protects the heart against ischemia-reperfusion injury. Life Sci. 2006;79:1749–55. doi: 10.1016/j.lfs.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 77.Levy RJ. Serotonin transporter mechanisms and cardiac disease. Circulation. 2006;113:2–4. doi: 10.1161/CIRCULATIONAHA.105.593459. [DOI] [PubMed] [Google Scholar]
- 78.Nebigil CG, Maroteaux L. Functional consequence of serotonin/5-HT2B receptor signaling in heart: role of mitochondria in transition between hypertrophy and heart failure? Circulation. 2003;108:902–8. doi: 10.1161/01.CIR.0000081520.25714.D9. [DOI] [PubMed] [Google Scholar]
- 79.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J. 2005;19:641–3. doi: 10.1096/fj.04-2518fje. [DOI] [PubMed] [Google Scholar]
- 80.Villeneuve C, Guilbeau-Frugier C, Sicard P, Lairez O, Ordener C, Duparc T, et al. p53-PGC-1alpha pathway mediates oxidative mitochondrial damage and cardiomyocyte necrosis induced by monoamine oxidase-a upregulation: role in chronic left ventricular dysfunction in mice. Antioxid Redox Signal. 2013;18:5–18. doi: 10.1089/ars.2011.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Himura Y, Felten SY, Kashiki M, Lewandowski TJ, Delehanty JM, Liang CS. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- 82.Liang CS, Fan TH, Sullebarger JT, Sakamoto S. Decreased adrenergic neuronal uptake activity in experimental right heart failure. A chamber-specific contributor to beta-adrenoceptor downregulation. J Clin Invest. 1989;84:1267–75. doi: 10.1172/JCI114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nozawa T, Igawa A, Yoshida N, Maeda M, Inoue M, Yamamura Y, et al. Dual-tracer assessment of coupling between cardiac sympathetic neuronal function and downregulation of beta-receptors during development of hypertensive heart failure of rats. Circulation. 1998;97:2359–67. doi: 10.1161/01.cir.97.23.2359. [DOI] [PubMed] [Google Scholar]
- 84.Backs J, Haunstetter A, Gerber SH, Metz J, Borst MM, Strasser RH, et al. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation. J Mol Cell Cardiol. 2001;33:461–72. doi: 10.1006/jmcc.2000.1319. [DOI] [PubMed] [Google Scholar]
- 85.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, et al. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–76. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 86.Lee CM, Javitch JA, Snyder SH. Recognition sites for norepinephrine uptake: regulation by neurotransmitter. Science. 1983;220:626–9. doi: 10.1126/science.6301013. [DOI] [PubMed] [Google Scholar]
- 87.Chidsey CA, Sonnenblick EH, Morrow AG, Braunwald E. Norepinephrine stores and contractile force of papillary muscle from the failing human heart. Circulation. 1966;33:43–51. doi: 10.1161/01.cir.33.1.43. [DOI] [PubMed] [Google Scholar]
- 88.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273–82. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- 90.Pool PE, Covell JW, Levitt M, Gibb J, Braunwald E. Reduction of cardiac tyrosine hydroxylase activity in experimental congestive heart failure. Its role in the depletion of cardiac norepinephrine stores. Circ Res. 1967;20:349–53. doi: 10.1161/01.res.20.3.349. [DOI] [PubMed] [Google Scholar]
- 91.Dorris RL. A simple method for screening monoamine oxidase (MAO) inhibitory drugs for type preference. J Pharmacol Methods. 1982;7:133–7. doi: 10.1016/0160-5402(82)90025-0. [DOI] [PubMed] [Google Scholar]
- 92.Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, et al. Monoamine Oxidase B Prompts Mitochondrial and Cardiac Dysfunction in Pressure Overloaded Hearts. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shioda N, Yamamoto Y, Watanabe M, Binas B, Owada Y, Fukunaga K. Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J Neurosci. 2010;30:3146–55. doi: 10.1523/JNEUROSCI.4140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding G, Wiegerinck RF, Shen M, Cojoc A, Zeidenweber CM, Wagner MB. Dopamine increases L-type calcium current more in newborn than adult rabbit cardiomyocytes via D1 and beta2 receptors. Am J Physiol Heart Circ Physiol. 2008;294:H2327–35. doi: 10.1152/ajpheart.00993.2007. [DOI] [PubMed] [Google Scholar]
- 96.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–6. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport. 2008;19:739–43. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, et al. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–88. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, et al. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 1997;17:206–10. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 100.Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem. 2004;279:39645–52. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C, et al. Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J Mol Cell Cardiol. 2009;46:587–95. doi: 10.1016/j.yjmcc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 102.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, et al. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–9. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 103.Poon CC, Seto SW, Au AL, Zhang Q, Li RW, Lee WY, et al. Mitochondrial monoamine oxidase-A-mediated hydrogen peroxide generation enhances 5-hydroxytryptamine-induced contraction of rat basilar artery. Br J Pharmacol. 2010;161:1086–98. doi: 10.1111/j.1476-5381.2010.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, et al. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic Biol Med. 2007;43:80–9. doi: 10.1016/j.freeradbiomed.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 105.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nigmatullina RR, Kirillova VV, Jourjikiya RK, Mukhamedyarov MA, Kudrin VS, Klodt PM, et al. Disrupted Serotonergic and Sympathoadrenal Systems in Patients with Chronic Heart Failure May Serve as New Therapeutic Targets and Novel Biomarkers to Assess Severity, Progression and Response to Treatment. Cardiology. 2009;113:277–86. doi: 10.1159/000205962. [DOI] [PubMed] [Google Scholar]
- 107.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, et al. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1460–H7. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 108.Petrak J, Pospisilova J, Sedinova M, Jedelsky P, Lorkova L, Vit O, et al. Proteomic and transcriptomic analysis of heart failure due to volume overload in a rat aorto-caval fistula model provides support for new potential therapeutic targets - monoamine oxidase A and transglutaminase 2. Proteome Sci. 2011;9:69. doi: 10.1186/1477-5956-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, et al. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 110.Jin H, Yang R, Awad TA, Wang F, Li W, Williams SP, et al. Effects of early angiotensin-converting enzyme inhibition on cardiac gene expression after acute myocardial infarction. Circulation. 2001;103:736–42. doi: 10.1161/01.cir.103.5.736. [DOI] [PubMed] [Google Scholar]
- 111.Strom CC, Kruhoffer M, Knudsen S, Stensgaard-Hansen F, Jonassen TE, Orntoft TF, et al. Identification of a core set of genes that signifies pathways underlying cardiac hypertrophy. Comp Funct Genomics. 2004;5:459–70. doi: 10.1002/cfg.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siddiqui A, Mallajosyula JK, Rane A, Andersen JK. Ability to delay neuropathological events associated with astrocytic MAO-B increase in a Parkinsonian mouse model: implications for early intervention on disease progression. Neurobiol Dis. 2011;43:527–32. doi: 10.1016/j.nbd.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 113.Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PloS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–71. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 115.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–62. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 116.Alnouti Y, Klaassen CD. Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci. 2008;101:51–64. doi: 10.1093/toxsci/kfm280. [DOI] [PubMed] [Google Scholar]
- 117.Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–7. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–15. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 119.van Haelst IM, van Klei WA, Doodeman HJ, Kalkman CJ, Egberts TC, Group MS. Antidepressive treatment with monoamine oxidase inhibitors and the occurrence of intraoperative hemodynamic events: a retrospective observational cohort study. J Clin Psychiatry. 2012;73:1103–9. doi: 10.4088/JCP.11m07607. [DOI] [PubMed] [Google Scholar]