Abstract
The present study was undertaken to evaluate the effects of L-DOPA pre-loading on the uptake of BPA using the F98 rat glioma and the murine B16 melanoma models. In vitro pretreatments of F98 glioma and B16 melanoma cells with L-DOPA, followed by exposure to BPA increased boron uptake, as determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Based on this, in vivo studies were initiated in F98 glioma bearing rats. Initially, the LDOPA dosing paradigm was evaluated. Maximum tumor boron uptake was observed following i.p. administration of L-DOPA (50 mg/kg) followed 24 hrs later by BPA (31.8 ± 8.9 vs. 17.2 ± 6.3 μg/g for BPA alone). Next, the effect of L-DOPA pre-loading as a function of the route of administration of BPA was evaluated in F98 glioma bearing rats. The greatest increase in uptake was seen following i.v. administration of BPA, while in contrast no significant increase was seen following intracarotid (i.c.) administration (38.6 ± 12.4 vs. 34.2 ± 10.9). Cellular localization of the F98 glioma, as determined by secondary ion mass spectrometry (SIMS) boron imaging revealed equivalent tumor boron concentrations following L-DOPA pre-loading. In vivo studies in B16 melanoma bearing mice showed equivalent tumor boron values in treated and untreated mice, suggesting that the effects of L-DOPA pre-loading may depend both on the histologic type of tumor and its anatomic site.
Keywords: L-DOPA pre-loading, boronophenylalanine, B16 murine melanoma, F98 rat glioma, SIMS
1. Introduction
We have had a long-standing interest in developing methods to enhance the uptake of boronophenylalanine (BPA) using both glioma (Barth et al., 2000, 1997; Yang et al., 1996) and melanoma tumor models (Barth et al., 1994; Matalka et al., 1993). Using the F98 rat glioma model we have shown that the highest tumor boron concentrations and the most favorable tumor to normal tissue ratios could be achieved by combining i.c. injection with blood-brain barrier disruption (BBB-D) (Barth et al., 2000). This resulted in a fivefold increase in tumor boron concentrations compared to those observed following i.v. injection (56.3 vs. 11.2 μg/g wt tumor). However, this approach is a technically challenging procedure, and for this reason we evaluated an alternative pharmacologic approach for BBB-D using a synthetic nonapeptide, Cereport™ (Receptor Mediated Permeabilizer -7 or RMP-7) to enhance the in vivo uptake of BPA in F98 glioma bearing rats (Yang et al., 2000). Intracarotid administration of RMP-7 combined with i.v. injection of BPA resulted in a doubling of the tumor boron concentrations compared to that observed in rats that received i.v. BPA alone (29.4 vs. 15.4 μg/g wt tumor). Based on these studies, we were especially interested in the publications of Pastore and his research team (Capuani et al., 2008, 2009). They reported that the administration of L-DOPA (L-3, 4-dihydroxyphenylalanine) to C6 glioma bearing rats prior to the i.c. infusion of BPA resulted in a significant increase in the tumor uptake of BPA, as determined by radiowave dielectric spectroscopy. Although the actual amounts of boron were not quantified, the percent changes in cytosol electrical conductivity of C6 cells was equated to enhanced boron uptake. Since L-DOPA preloading is not as technically challenging as i.c. injection combined with BBB-D, we thought that it would be of interest to perform similar studies using the F98 glioma and B16 melanoma models and to quantify boron using two highly sensitive analytical techniques, inductively coupled plasma-optical emission spectroscopy (ICP-OES) and SIMS.
2. Materials and Methods
2.1 In vitro studies on the effects of L-DOPA on the uptake of BPA using the B16 melanoma and F98 glioma cells
Initially, in vitro studies were carried out to determine if L-DOPA (Sigma Aldrich, St. Louis, MO) pre-treatment would increase the uptake of BPA by B16 melanoma and F98 glioma cells. These were grown to 90% confluency in T-75 flasks at which time the cells were exposed to L-DOPA (100 μg/ml) for 4 hrs at 37°C, following which the medium was decanted and medium containing BPA (2 mM or 418 μg/ml) was added and the cells were incubated for an additional 2.5 hrs. Following this, the cells were harvested, and prepared for boron determinations by means of ICP-OES, as previously described (Chandra et al., 2013). Studies identical to the above also were carried out with B16 melanoma cells using the unnatural cyclic amino acid cis isomer of 1-amino-3-boronocyclopentane carboxylic acid (ABCPC) as a racemic mixture of its L- and D- forms, as described in more detail elsewhere (Chandra et al., 2013). Boron uptake was expressed as μg/109 cells, assuming that 109 cells = 1g.
2.2 In vivo studies on the effects of L-DOPA on biodistribution of BPA using the B16 melanoma and F98 glioma models
All of the animal studies described in this report were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and our protocol was approved by the Institutional Animal Care and Use Committee of The Ohio State University (Permit #: A-3261-01 and IACUC protocol number 2011A0000003). B16 melanoma cells were implanted subcutaneously (s.c.) into the right dorsum of syngeneic female C57BL/6 mice. Approximately 10 -12 d later, when tumors had attained a diameter of ~1 cm, biodistribution studies were initiated. BPA (Katchem Ltd, Prague) was converted to a fructose complex as previously described (Yang et al., 1996). The ABCPC compound was dissolved in PBS for dosing in equivalent boron concentration to BPA. L-DOPA was administered intraperitoneally (i.p.) at a dose of 50 mg/kg b.w. Twenty-four hrs later BPA (500 mg/kg b.w., equivalent to 24 mg boron/kg) was administered i.p. Mice were euthanized at 2.5 hr. post injection and various organs and tissue were removed and processed for boron determinations by means of ICP-OES.
The F98 rat glioma (#CRL-2397, American Type Culture Collection, Manasus, VA) has been used in a wide variety of studies in experimental neuro-oncology (Barth et al., 2009). F98 glioma cells (104) were implanted stereotactically into the right caudate nucleus of syngeneic Fischer rats, as previously described (Yang et al., 1996), and ~12 days later biodistribution studies were carried out to determine the optimum dosing paradigm for the combination of L-DOPA and BPA. The BPA was converted to a fructose complex and administered i.p. at a dose of 250 mg/kg b.w. The rats were euthanized 2.5 hrs later and the organs were removed and processed for boron determinations by ICP-OES. Following this, a biodistribution study was initiated in F98 glioma bearing rats. BPA (500 mg/kg b.w.) was administered i.v. or i.c., either alone or in combination with L-DOPA (50 mg/kg b.w.), administered i.p. 24 hrs earlier. Rats were euthanized 2.5 hrs following administration and blood samples were taken, brains were removed, tumors were dissected out, and they, and the tumor (ipsilateral) and non-tumor bearing (contralateral) cerebral hemispheres were removed and processed for boron determinations by means of ICP-OES.
2.3 SIMS imaging studies of BPA in F98 glioma bearing rats
Small portions of tumor and surrounding brain tissue from F98 glioma bearing rats were frozen for correlative SIMS studies from animals that had received BPA, via i.v. infusion, either alone or in combination with L-DOPA preloading. In order to determine the microdistribution of BPA by means of SIMS analysis, 4 μm thick cryosections were attached to silicon wafers (~1 cm2), freeze-dried and sputter coated with a 10 Å layer of Au/Pd for enhancing their electrical conductivity. A CAMECA IMS-3f ion microscope was used for SIMS analyses (Chandra et al., 2013, 2000). Pixel-by-pixel image quantification of boron signals (10B+) was achieved by the secondary ion 12C+ carbon normalization approach and using the relative-sensitivity-factors (RSF) of boron isotopes to the 12C+ tissue matrix signals (Ausserer et al., 1989; Chandra et al., 2013, 2000; Smith et al., 1996). The absolute boron concentrations, determined by this approach, were converted into estimated wet weight concentrations by assuming 85% cellular water content.
2.4 Statistical analysis
Data analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC). The means and standard deviations (SD) were computed for boron concentrations in the tumor, brain around tumor (BAT), ipsilateral (tumor bearing) and contralateral (non-tumor bearing) cerebral hemispheres, and blood and the tumor:brain (T/Br) and tumor:blood (T/Bl) concentration ratios were calculated for each group. One-way analysis of variance followed by Tukey's post hoc test for multiple comparisons between groups. A two-sample t-test was applied to compare concentrations of boron determined by SIMS analysis of glioma of the F98 rats with or without L-DOPA pre-loading. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1 In vitro studies on the effects of L-DOPA pre-exposure on the uptake of BPA and ABCPC on B16 melanoma and F98 glioma cells
Studies carried out with B16 melanoma cells demonstrated that pre-exposure to L-DOPA resulted in a 34% increase in the uptake of BPA (30.1 ± 3.2 vs. 19.7 ± 1.1 μg/ 109 cells). However, studies with the boronated unnatural amino acid (UNAA) ABCPC failed to reveal increased uptake by B16 cells that had been pre-exposed to L-DOPA (34.3 vs. 32.6 μg/109 cells). Studies carried out with BPA using F98 glioma cells showed a 35% increase in uptake, although the absolute quantities taken up were much less (20.7 ± 0.6 vs.13.4 ± 1.2 μg/ 109 cells) (p < 0.001). Based on these data, in vivo studies were initiated for BPA using both tumor models.
3.2 In vivo studies on the effects of L-DOPA pre-loading on the uptake of BPA in B16 melanoma and F98 glioma bearing animals
The mean tumor boron concentration of B16 melanoma bearing mice, which had received LDOPA (50 mg/kg b.w.) followed 24 hrs later by i.p. BPA, was 27 μg/g tumor vs. 23 μg/g for those that did not. These values were not significantly different from each other. Similarly, L-DOPA pre-loading did not have an effect on the uptake of ABCPC, which was concordant with the in vitro data. Studies carried out in F98 glioma bearing rats to optimize the dosing paradigm are summarized in Table 1. The optimum dose of L-DOPA was 50 mg/kg b.w., administered 24 hrs prior to i.v. administration of BPA. This resulted in a 46% increase in the tumor boron concentration (31.8 ± 8.9 μg vs. 17.2 ± 6.3 μg/g). Based on these results, a biodistribution study was initiated in F98 glioma bearing rats to evaluate the effects of L-DOPA preloading on the uptake of BPA following i.v. or i.c. administration (Table 2). Compared to the data obtained following i.v. administration of BPA, only an 11% increase was observed following i.c. administration (38.6 ± 12.4 vs. 34.2 ± 10.9 μg/g), which was not statistically significant.
Table 1.
Optimization of L-DOPA dosing and timing of administration with BPA in F98 glioma bearing rats
| Mean Boron Concentrations ± SD |
|||||
|---|---|---|---|---|---|
| Dose of L-DOPA | Time after administration | Tumor | Brain (Ipsilateral) | Brain (Contralat) | Blood |
| None | N/A | 17.2 ± 6.3 | 5.1 ± 3.2 | 4.7 ± 0.9 | 4.6 ± 0.8 |
| 50 mg/kg | 24 hrs | 31.8 ± 8.9 | 7.8 ± 2.5 | 5.5 ± 0.6 | 6.5 ± 1.4 |
| 100 mg/kg | 24 hrs. | 27.4 ± 4.0 | 8.4 ± 0.5 | 6.0 ± 0.4 | 9.3 ± 1.2 |
| 50 mg/kg | 6 hrs. | 17.5 ± 3.4 | 6.8 ± 1.5 | 5.5 ± 0.5 | 8.8 ± 1.5 |
*L-DOPA was administered i.p. followed at either 6 or 24 hrs. later by i.v. injection of BPA at a dose of 500 mg/kg b.w. and were euthanized 2.5 hrs. later.
Table 2.
Biodistribution of BPA following administration of BPA with or without L-DOPA to F98 glioma bearing rats
|
bBoron Concentration (μg/g)† |
Ratios¶ |
|||||
|---|---|---|---|---|---|---|
| Test Group* | Tumor | Brain (Ipsilateral) | Brain (Contralat) | Blood | T/Br | T/Bl |
| i.v. BPA | 17.2 ± 6.3 | 5.1 ± 3.2 | 4.7 ± 0.9 | 4.6 ± 0.8 | 3.4 | 3.7 |
| i.c. BPA | 34.2 ± 10.9 | 8.2 ± 4.5 | 5.0 ± 2.4 | 6.9 ± 0.8 | 4.2 | 5.0 |
| i.v. BPA+DOPA | 31.8 ± 8.9 | 7.8 ± 3.5 | 5.5 ± 0.6 | 6.5 ± 1.4 | 4.1 | 4.9 |
| i.c. BPA+DOPA | 38.6 ± 12.4 | 7.9 ± 2.6 | 5.2 ± 0.5 | 5.6 ± 1.6 | 4.9 | 6.9 |
BPA at a dose of 500 mg/kg b.w. was administration by either intravenous (i.v.) or intracarotid (i.c.) injection either alone or 24 hrs following intraperitoneally (i.p.) administration of L-DOPA at a dose of 50 mg/kg b.w.
Boron concentrations were determined by means of inductively coupled plasma-atomic emission spectroscopy. Each point represents the arithmetic mean ± SD of 4 rats.
T/Br = tumor to brain and T/Bl = tumor to blood ratios.
SIMS studies were undertaken in order to determine if L-DOPA preloading increased boron concentrations in cells in the main tumor mass and, more importantly, the infiltrating tumor cells in adjacent normal brain tissue of F98 glioma bearing rats 2.5 hr after i.v. injection of BPA. A representative photomicrograph of the SIMS analysis for boron from BPA treatment alone is shown in Fig. 1. Fig. 2 shows an example of SIMS analysis 39K and 10B from BPA following L-DOPA pre-loading. Quantitative SIMS observations revealed that equivalent amounts of boron were detected in the main tumor mass and infiltrating tumor cells in the normal brain of rats that received an i.v. infusion of BPA with or without L-DOPA pre-loading (Table 3). It also was noted that the boron content of infiltrating tumor cells in the normal brain tissue was considerably less than that of tumor cells in the main tumor mass. This most likely was due to the fact that the infiltrating tumor cells in the normal brain are protected by the BBB. As summarized in Table 3, L-DOPA pre-loading did not improve the boron-targeting of infiltrating tumor cells in normal brain tissue.
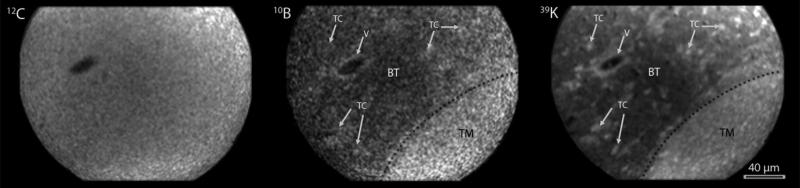
Fig.1.
SIMS imaging of an F98 rat glioma following administration of BPA without L-DOPA pre-loading. The 39K SIMS image shows the boundary between the main tumor mass (TM) and the normal brain tissue (BT) with infiltrating tumor cells (TC) and a vessel (V) in the normal brain tissue. The higher concentrations of 10B from BPA are discernible in the tumor mass (TM) from the adjacent brain tissue in the SIMS 10B image. Furthermore, the vessel wall (V) shows higher 10B than surrounding brain tissue (BT) and some infiltrating tumor cells also can be recognized with slightly higher 10B concentrations than surrounding normal brain tissue. The 12C carbon image is largely homogenous and does not show gradients in these regions. The 12C image is used for pixel-by-pixel registration and quantification of boron images. The 39K SIMS image was integrated on the CCD camera for 0.4 sec. The 10B and 12C images were integrated for 2 min each.
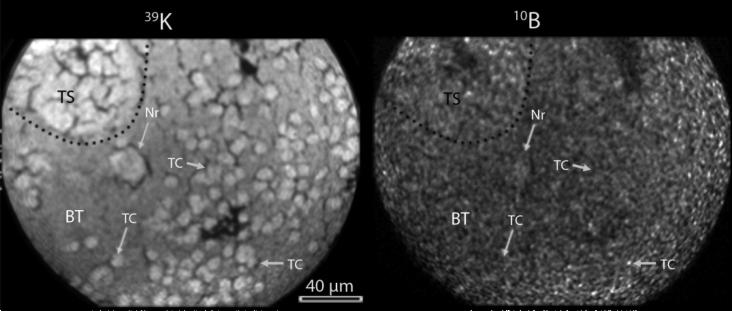
Fig.2.
SIMS imaging of an F98 rat glioma following administration of BPA with L-DOPA pre-loading. This is an example of SIMS analysis 39K and 10B from BPA in the L-DOPA preloading treatment. The tumor satellite cells (TS) and many scattered individual infiltrating tumor cells (TC) in the normal brain tissue (BT) are recognizable in the 39K image. A neuron (Nr) also is recognizable in the 39K image. The corresponding 10B image from this region reveals a minor, but recognizable, gradient of 10B between the tumor satellite cell and the brain tissue. The 39K SIMS image was integrated on the CCD camera for 0.4 sec and the 10B image was for 2 min.
Table 3.
Concentrations of boron determined by SIMS analysis of the F98 rat glioma in tumor cells of the main tumor mass (TM), and infiltrating tumor satellite (TS) and individual tumor cells (TC) in the normal brain tissue (BT) as shown in Fig. 1 and Fig. 2.
| Boron concentrations (μg/g ± SD wet weight) |
|||
|---|---|---|---|
| Treatment | Main tumor mass | Brain Tissue | Infiltration tumor cells |
| BPA | 43 ± 12 | 16 ± 5 | 28 ± 5 |
| DOPA + BPA | 40 ± 15 | 21 ± 7 | 27 ± 8 |
Boron concentrations are expressed in μg/g wet weight by assuming 85% cell tissue water content in tissue. The observations were made in at least 4 SIMS imaging fields in tissue cryosections from 2 rats in each treatment.
4. Discussion and Conclusions
The single most important observation from this study was that L-DOPA pre-loading did indeed increase the tumor uptake of BPA in F98 glioma bearing rats, as determined by ICP-OES. However, SIMS analysis revealed equivalent tumor boron concentrations in both groups. This discrepancy might have been due to the fact that ICP-OES only provides information on the “bulk” tumor boron concentrations, while SIMS provides data with single cell resolution for studying intracellular targeting of boron in tumor cells (Chandra et al., 2013). It is possible that L-DOPA pre-loading increased BPA concentrations in the extracellular fluids alone without significantly affecting the boron concentrations inside the tumor cells. As recently reported by Kawabata, Yang, Barth, et al. (Kawabata et al., 2011), seemingly high tumor boron concentrations, as measured by ICP-OES, following intracerebral convection enhanced delivery (CED) of carboranylporphyrins to F98 glioma bearing rats, did not result in the prolonged mean survival times (MSTs), which would have been predicted based on a very high tumor boron concentration (140.3 μg/g). In fact, the MST was almost identical to that obtained following i.v. administration of BPA, where the tumor boron concentration was 12.5 μg/g (Kawabata et al., 2011). Histopathologic examination of the brains of the tumor bearing rats, which had received the carboranylporphyrins followed by BNCT, revealed tumor-associated, porphyrin-laden macrophages. Clearly, the high “tumor” uptake was spurious and it is possible that this might also have occurred in the rats that received L-DOPA prior to i.v. administration of BPA. The studies in B16 melanoma bearing mice did not reveal any significant increase in tumor boron concentration in those animals that had received L-DOPA prior to the administration of BPA or the UNAA ABCPC (Chandra et al., 2013). Therefore, no definitive conclusions can be made as to the potential utility of L-DOPA pre-loading as a means to enhance BPA uptake. If the bulk tumor boron concentrations in F98 glioma bearing rats, which had received L-DOPA prior to i.v. administration of BPA, were indeed attributable to cellular localization, then based on our previous BNCT studies with the F98 glioma model (Barth et al., 2000, 1997), we would have expected a significant increase in MSTs among those animals that had received L-DOPA. Unfortunately, however, due to funding issues and a cessation of BNCT related research at the Massachusetts Institute of Technology Research Reactor (MITR) such studies are impossible to carryout in the United States. Perhaps investigators in Japan or Argentina, where there are suitable neutron sources, might be able to pursue these studies in the future.
Research Highlights.
Effects of L-DOPA pre-loading on the uptake of BPA were studied using the F98 glioma and B16 melanoma models
In vitro studies using both cell lines revealed that pre-loading resulted in increased uptake of BPA
Enhanced uptake of BPA was seen following i.v. administration to F98 glioma bearing rats (31.8 vs. 17.2 μg/g)
The effects of L-DOPA pre-loading may depend both on the histologic type of tumor and its anatomic site
Acknowledgments
This study was funded by a NIH grant R01 CA129326 to RFB, SC, and GWK. The Kevin J. Mullin Memorial Fund for Brain Tumor Research provided partial support to RFB. The Cornell SIMS Laboratory, under the direction of S. Chandra, is affiliated with New York State Foundation for Science, Technology, and Innovation (NYSTAR). Finally, we thank Mrs. Heidi Bosworth for expert secretarial assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausserer WA, Ling YC, Chandra S, Morrison GH. Quantitative imaging of boron, calcium, magnesium, potassium, and sodium distributions in cultured cells with ion microscopy. Anal Chem. 1989;61:2690–2695. doi: 10.1021/ac00199a002. [DOI] [PubMed] [Google Scholar]
- Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RF, Matalka KZ, Bailey MQ, Staubus AE, Soloway AH, Moeschberger ML, Coderre JA, Rofstad EK. A nude rat model for neutron capture therapy of human intracere bral melanoma. Int J Radiat Oncol Biol Phys. 1994;28:1079–1088. doi: 10.1016/0360-3016(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Barth RF, Yang W, Rotaru JH, Moeschberger ML, Boesel CP, Soloway AH, Joel DD, Nawrocky MM, Ono K, Goodman JH. Boron neutron capture therapy of brain tumors: enhanced survival and cure following blood-brain barrier disruption and intracarotid injection of sodium borocaptate and boronophenylalanine. Int J Radiat Oncol Biol Phys. 2000;47:209–218. doi: 10.1016/s0360-3016(00)00421-1. [DOI] [PubMed] [Google Scholar]
- Barth RF, Yang W, Rotaru JH, Moeschberger ML, Joel DD, Nawrocky MM, Goodman JH, Soloway AH. Boron neutron capture therapy of brain tumors: enhanced survival fol lowing intracarotid injection of either sodium borocaptate or boronophenylalanine with or without blood-brain barrier disruption. Cancer Res. 1997;57:1129–1136. [PubMed] [Google Scholar]
- Capuani S, Gili T, Bozzali M, Russo S, Porcari P, Cametti C, D'Amore E, Colasanti M, Venturini G, Maraviglia B, Lazzarino G, Pastore FS. L-DOPA preloading increases the uptake of borophenylalanine in C6 glioma rat model: a new strategy to improve BNCT efficacy. Int J Radiat Oncol Biol Phys. 2008;72:562–567. doi: 10.1016/j.ijrobp.2008.06.1493. [DOI] [PubMed] [Google Scholar]
- Capuani S, Gili T, Bozzali M, Russo S, Porcari P, Cametti C, Muolo M, D'Amore E, Ma raviglia B, Lazzarino G, Pastore FS. Boronophenylalanine uptake in C6 glioma model is dramatically increased by L-DOPA preloading. Appl Radiat Isot. 2009;67:S34–36. doi: 10.1016/j.apradiso.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Chandra S, Barth RF, Haider SA, Yang W, Huo T, Shaikh AL, Kabalka GW. Bio-distribution and subcellular localization of an unnatural boron-containing amino acid (Cis-ABCPC) by imaging secondary ion mass spectrometry for neutron capture therapy of melanomas and gli omas. PLoS One. 2013;8:e75377. doi: 10.1371/journal.pone.0075377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Smith DR, Morrison GH. Subcellular imaging by dynamic SIMS ion microscopy. Anal Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Yang W, Barth RF, Wu G, Huo T, Binns PJ, Riley KJ, Ongayi O, Gottumukkala V, Vicente MG. Convection enhanced delivery of carboranylporphyrins for neutron capture therapy of brain tumors. J Neurooncol. 2011;103:175–185. doi: 10.1007/s11060-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalka KZ, Bailey MQ, Barth RF, Staubus AE, Soloway AH, Moeschberger ML, Coderre JA, Rofstad EK. Boron neutron capture therapy of intracerebral melanoma using boronophenylalanine as a capture agent. Cancer Res. 1993;53:3308–3313. [PubMed] [Google Scholar]
- Smith DR, Chandra S, Coderre JA, Morrison GH. Ion microscopy imaging of 10B from p-boronophenylalanine in a brain tumor model for boron neutron capture therapy. Cancer Res. 1996;56:4302–4306. [PubMed] [Google Scholar]
- Yang W, Barth RF, Bartus RT, Rotaru JH, Moeschberger ML, Ferketich AK, Nawrocky MM, Coderre JA, Goodman JH. Improved survival after boron neutron capture therapy of brain tumors by Cereport-mediated blood-brain barrier modulation to enhance delivery of boro nophenylalanine. Neurosurgery. 2000;47:189–197. doi: 10.1097/00006123-200007000-00039. discussion 197-188. [DOI] [PubMed] [Google Scholar]
- Yang W, Barth RF, Carpenter DE, Moeschberger ML, Goodman JH. Enhanced deli very of boronophenylalanine for neutron capture therapy by means of intracarotid injection and blood-brain barrier disruption. Neurosurgery. 1996;38:985–992. doi: 10.1097/00006123-199605000-00027. [DOI] [PubMed] [Google Scholar]